Abstract
With the gradual increase of cases using fillers, cases of patients treated by non-medical professionals or inexperienced physicians resulting in complications are also increasing. We herein report 2 patients who experienced acute complications after receiving filler injections and were successfully treated with adipose-derived stem cell (ADSCs) therapy. Case 1 was a 23-year-old female patient who received a filler (Restylane) injection in her forehead, glabella, and nose by a non-medical professional. The day after her injection, inflammation was observed with a 3×3 cm skin necrosis. Case 2 was a 30-year-old woman who received a filler injection of hyaluronic acid gel (Juvederm) on her nasal dorsum and tip at a private clinic. She developed erythema and swelling in the filler-injected area A solution containing ADSCs harvested from each patient's abdominal subcutaneous tissue was injected into the lesion at the subcutaneous and dermis levels. The wounds healed without additional treatment. With continuous follow-up, both patients experienced only fine linear scars 6 months postoperatively. By using adipose-derived stem cells, we successfully treated the acute complications of skin necrosis after the filler injection, resulting in much less scarring, and more satisfactory results were achieved not only in wound healing, but also in esthetics.
Keywords: Hyaluronic acid, Humans, Mesenchymal stem cell transplantation, Necrosis
INTRODUCTION
The use of facial fillers to provide adequate volume on depressed areas of the skin and maintain a youthful rejuvenating appearance has been increasing remarkably in recent years. Fillers have become very popular due to their safety profiles and the low invasiveness, reversibility, and easy-to-handle characteristics.
When administered by experienced and licensed professionals, filler injections rarely result in complications. Frequently, patients who experienced acute and chronic complications have been treated by unlicensed individuals, and these complications, such as vascular compromise and skin necrosis, are often significant and permanent. Patients with skin necrosis referred to our department for treatment after filler injection have been treated with skin grafts, local flaps, surgical debridement, and dressings with different materials. However, the treatments have often resulted in unsightly skin loss, scarring, and asymmetry.
Stem cells are known to self-renew and differentiate in order to regenerate multiple tissues [1]. They have also been known to promote angiogenic processes and wound healing by secreting angiogenic factors, differentiating into different cells contributing to neovascular formation and stimulating cells that perform significant roles in wound healing through mechanisms not yet fully understood.
We herein report 2 patients treated with adipose-derived stem cells (ADSCs) for skin necrosis and acute inflammatory reaction after receiving filler injections for soft tissue augmentation in the nose. To the best of our knowledge, there are no published reports on the use of ADSCs for the treatment of acute complications after a filler injection.
CASES
Case 1
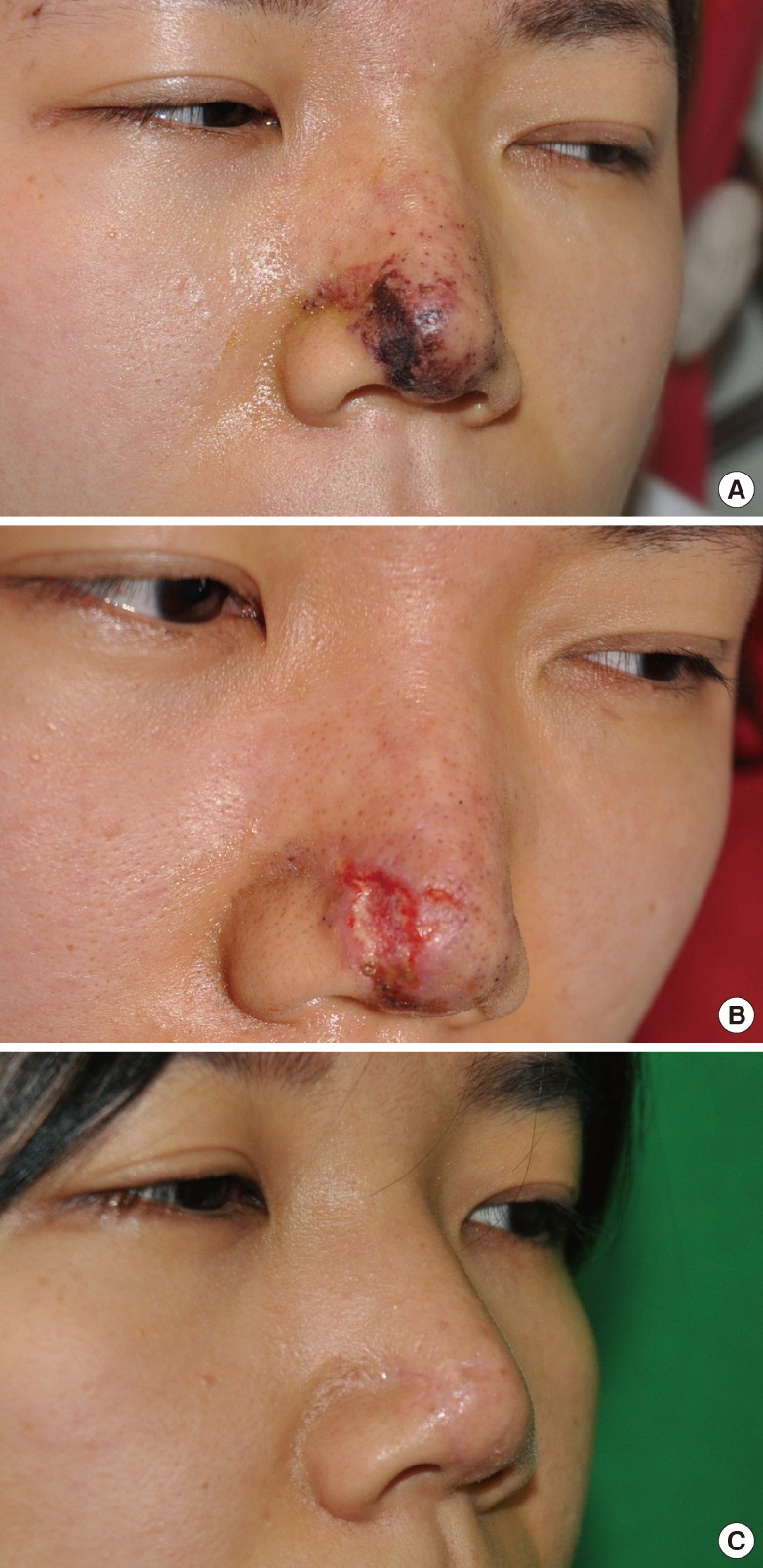
A 25-year-old female received filler injection (Restylane, Q-med, Uppsala, Sweden) on her forehead, glabella, and nose by a non-medical professional. She reported tenderness and redness on the injected areas the day after the injection, which were treated with intravenous antibiotics and hydrocolloid dressings. However, the wound continued to aggravate, and when she was referred on the fifth day after injection, her wound exhibited pus-like discharge, severe inflammation, and a 3×3 cm skin necrosis over her nasal tip, lateral wall, and dorsum. Following her hospital admission, she underwent an empirical intravenous antibiotic therapy and a debridement of necrotic tissues. On the third day of admission, she received adipose-derived stem cell therapy on her nose. Her postoperative course was uneventful, and she was discharged on postoperative day 8. Her wound was completely re-epithelialized 10 days after injection and during 6 months of follow-up; there remained an unnoticeable linear scar on the skin and soft tissue defect site without evidence of asymmetry or disfigurement due to scar contraction on the nasal tip and nostril (Fig. 1).
Fig. 1.
Case 1
(A) A 23-year-old female patient. Inflammation and necrosis with swelling, erythema, and pus-like discharge in the nose dorsum and tip area. (B) After foreign body and necrotic tissue removal. The nasal tip shows skin necrosis. (C) View 6 months postoperatively after adipose-derived stem cell therapy. A linear scar remains only in the nose tip area without scar contracture deformity.
Case 2
A 30-year-old woman underwent filler hyaluronic acid (HA) injection (Juvederm, Allergan, Irvine, CA, USA) on her nasal dorsum and tip at a private clinic. Erythema and painful swelling developed on the injected area from the nasal tip to the dorsum and lateral wall on the day after injection, for which she received hyaluronidase (1,000 U) and a steroid injection by the physician who had performed the original filler injection. When she was referred to us on the fifth day after filler injection, the wound showed multiple pustules, eschars, and regional necrosis on the nasal tip and dorsum. Debridement of the necrotic tissue was performed and intravenous empiric antibiotics were administered. On the eleventh day after the filler injection, she was admitted to our hospital and adipose-derived stem cell therapy was administered to the wound. She was discharged 4 days after the stem cell treatment and the wound was completely reepithelialized 8 days after the injection without any evidence of complications. On follow-up, the wound had healed with satisfactory results and only a slightly noticeable scar remained (Pictures of this case were not included because the patient did not want them to be publicly available).
Method
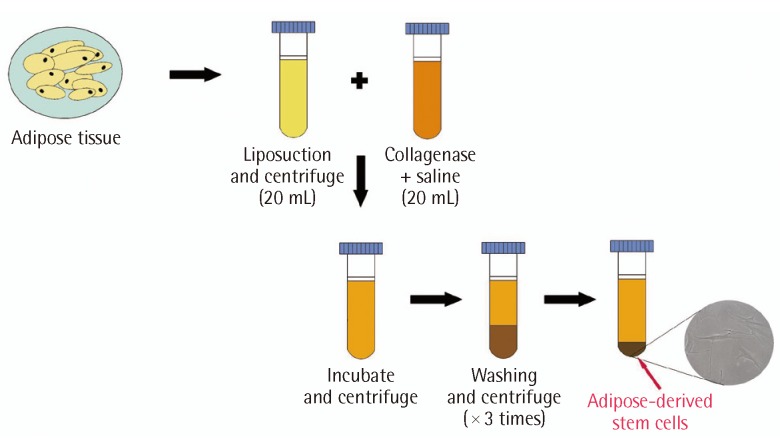
Adipose tissues were harvested from the patients' abdomens by using a Lipokit (Medikan Inc., Seoul, Korea) for the lipotransplant and centrifuge. Fat tissues harvested by liposuction were divided into 50 mL syringes and centrifuged for 4 minutes at 3,500 rpm. They were subsequently mixed with collagenase type II (Worthington industries, Columbus, OH, USA) and liquefied by saline (20 mL) in the syringe. A mixture of harvested fat and enzymes was incubated for 30 minutes at 37℃ with Maxstem (Medikan Inc.) and centrifuged for 3 minutes at 3,500 rpm. After mixing the washing solution composed of gentamicin, Hartmann solution, and 5% dextrose saline, the syringe was centrifuged again for 3 minutes at 200 relative centrifugal force. The centrifuging and washing procedure was repeated 3 times and the solution containing adipose-derived stem cells, which amounted to 3 mL, was divided into 1 mL syringes (Fig. 2). Two mL of the solution were injected into the lesion at the subcutaneous and dermis levels, and a wet dressing with the remaining 1 mL of solution was performed. Four days after the procedure, the first dressing was applied with foam material and repeated every 2 days.
Fig. 2.
Schematic procedure showing isolation of adipose-derived stem cell
DISCUSSION
Paraffin was the first injectable material used by Gersuny in 1899 as a testicular prosthesis in a man whose testicles had been resected [2]. Since then, many different materials such as paraffin, silicone, collagen, and more recently HA products have been developed for soft tissue augmentation. Ideal facial augmentation materials should be host-compatible to avoid inflammation and easy to handle in order to avoid additional scars, thereby maintaining healthy and natural skin. Among all the materials for soft tissue augmentation, HA fillers have become the most popular.
HA was first named by Karl Meyer, who isolated an unknown substance from a cow's vitreous in 1934, and it was subsequently demonstrated to exist in all species of animals, including humans. HA is a naturally existing glycosaminoglycan that constitutes the extracellular matrix of the connective tissues, supports structures, and provides volume while binding water. Injecting HA fillers into the skin immediately suppliess volume and rejuvenates the appearance of the skin. Moreover, they have good safety profiles and reversibility, and do not require allergy tests. Complications of injectable fillers are known to be uncommon clinically, but mild complications such as erythema, swelling, tenderness, bruising, and lumps can develop temporarily. More serious complications, which are rare, include the Tyndall effect, allergic reaction, nodule and granuloma formation, and skin necrosis [3]. Skin necrosis is recognized as the most severe complication, and occurs secondary to vascular compromise in the areas with direct or indirect interruption of blood vessels. Hydrophilic actions of HA fillers can sometimes compress the facial artery, angular artery, supratrochlear artery or branches that supply the nasal tip, alar, and glabellar area. Hydrophilic actions are thought to be the main cause of skin necrosis of the nasal area [4]. Although skin necrosis is very rare, it results in scarring, asymmetry, and permanent disfigurement. In the early stage of necrosis, conservative management including topical nitroglycerin and a heat lamp can be applied to stimulate vasodilatation. Hyaluronidase is also known to be able to resolve HA with successful outcomes [5]. If not managed properly, necrosis can be aggravated, making wounds wider and deeper without showing any improvement with conventional dressings. They may require more invasive treatment modalities, such as surgical debridement and different types of local flaps or grafts.
Stem cells are known to undergo self-renewal and can differentiate into multiple cell phenotypes. Due to their reproducibility and multi-potency, they have been considered to play a significant role in many clinical and preclinical fields. Stem cells can be harvested from various mesenchymal sources, most commonly bone marrow, but harvesting stem cells from the bone marrow causes pain and discomfort to patients and only a relatively small number of cells can be harvested. In 2002, Zuk et al. [6] isolated stem cells from human adipose tissues capable of differentiating into adipogenic, chondrogenic, myogenic, and osteogenic cells as an alternative source to bone marrow-derived stem cells. ADSCs leave less donor site morbidity, yield a greater number of mesenchymal stem cells, and are relatively easier to harvest than bone marrow-derived stem cells.
Among the diverse properties of adipose-derived stem cells, many studies are focused on their angiogenic effects in ischemic models. These include models of myocardial infarction, heart failure, limb ischemia, diabetic foot, and arteriosclerosis obliterans. Local injection and topical administration of ADSCs are also found to be effective in enhancing the healing of ischemic skin flaps in animal models. ADSCs are thought to affect ischemic tissues by secreting angiogenic factors that stimulate angiogenesis, differentiation of ADSCs into vascular cells as functional components of neovasculatures, and secretion of factors that enhance progenitor cell availability [7]. These angiogenic effects are thought to improve necrosis and inflammation of wounds that are believed to be caused by vascular compromises after filler injection into the nasal area. In addition to these angiogenic properties, Kim et al. [8] suggested that ADSCs promote human fibroblast proliferation via direct cell-to-cell contact and paracrine activation of secretory factors that result in significant reduction of the wound size and acceleration of re-epithelialization. The potential of ADSCs that differentiate into multiple cell phenotypes, promote angiogenesis, and secrete growth factors that have been thought to enhance wound repair could be applied to acute complications of skin necrosis by vascular compromise developed after filler injection.
In rare cases, dermal filler injection produces skin necrosis that leads to undesirable results if not treated in a timely manner. The cases we present herein describe acute complications of skin necrosis on the nasal area after filler injection. Usually, skin defects developed after debridement of necrotic and inflammed tissues can be reconstructed with different grafts or flaps, but donor site morbidity and scarring can remain a permanent problem.
We herein report 2 cases of patients who achieved satisfactory results with successful reconstruction of the inflamed and necrotized area. Following treatment with adipose-derived stem cells, they healed with barely noticeable linear scars without complications like, asymmetry, disfigurement, or pigmentation that would require further management. This report suggests a potential therapeutic use of ADSCs for managing skin necrosis that may occur after filler injection.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Utsunomiya T, Shimada M, Imura S, et al. Human adiposederived stem cells: potential clinical applications in surgery. Surg Today. 2011;41:18–23. doi: 10.1007/s00595-010-4415-9. [DOI] [PubMed] [Google Scholar]
- 2.Klein AW, Elson ML. The history of substances for soft tissue augmentation. Dermatol Surg. 2000;26:1096–1105. [PubMed] [Google Scholar]
- 3.Weinberg MJ, Solish N. Complications of hyaluronic acid fillers. Facial Plast Surg. 2009;25:324–328. doi: 10.1055/s-0029-1243081. [DOI] [PubMed] [Google Scholar]
- 4.Grunebaum LD, Bogdan Allemann I, Dayan S, et al. The risk of alar necrosis associated with dermal filler injection. Dermatol Surg. 2009;35(Suppl 2):1635–1640. doi: 10.1111/j.1524-4725.2009.01342.x. [DOI] [PubMed] [Google Scholar]
- 5.Brody HJ. Use of hyaluronidase in the treatment of granulomatous hyaluronic acid reactions or unwanted hyaluronic acid misplacement. Dermatol Surg. 2005;31:893–897. doi: 10.1097/00042728-200508000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hong SJ, Traktuev DO, March KL. Therapeutic potential of adipose-derived stem cells in vascular growth and tissue repair. Curr Opin Organ Transplant. 2010;15:86–91. doi: 10.1097/MOT.0b013e328334f074. [DOI] [PubMed] [Google Scholar]
- 8.Kim WS, Park BS, Sung JH, et al. Wound healing effect of adipose-derived stem cells: a critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci. 2007;48:15–24. doi: 10.1016/j.jdermsci.2007.05.018. [DOI] [PubMed] [Google Scholar]