Abstract
Asthma is characterized by T helper cell 2 (Th2) type inflammation, leading to airway hyperresponsiveness and tissue remodeling. Th2 cell-driven inflammation is likely to represent an abnormal response to harmless airborne particles. These reactions are normally suppressed by regulatory T cells, which maintain airway tolerance. The anti-inflammatory cytokine IL-10 is likely to play a central role. The role of the cytokine transforming growth factor β (TGF-β) is more complex, with evidence for immune suppression and remodeling in the airways. In asthmatic individuals there is a breakdown in these regulatory mechanisms. There is emerging evidence that early life events, including exposure to allergen and infections, are critical in programming effective regulatory pathways to maintain pulmonary homeostasis. In this review we examine the clinical and experimental evidence for T regulatory cell function in the lung and discuss the events that might influence the functioning of these cells. Ultimately, the ability to enhance regulatory function in affected individuals may represent an effective treatment for asthma.
The Lung as a Unique Mucosal Site
The lung represents a unique mucosal environment, which is specialized to meet the requirements for its primary function of gaseous exchange. This includes a large surface area exposed to the external environment, which is continuously exposed to airborne antigens, many of which although immunogenic do not usually pose a threat to the host, e.g., allergens. In order to prevent the continual induction of de novo immune responses and stimulation of memory effector cells, which would support chronic inflammation in the airways and damage to the epithelial barrier and would impair gaseous exchange, a number of specialized control mechanisms exist. An absolute requirement is that these mechanisms discriminate harmless airborne antigens entering via the airways from pathogens.
Given these risks, it is not surprising that multiple interactions between leukocytes and cells of the lung stroma are central to this process of homeostatic pulmonary regulation. The epithelium plays a central role via the production of molecules such as mucins, surfactants, complement products, and antimicrobial peptides (reviewed in Holt et al., 2008). In addition, a unique population of macrophages termed alveolar macrophages, which reside outside the body in the airway spaces, are likely to play an important role. These exhibit potent phagocytic and antimicrobial functions (Holt et al., 2008). They are likely to play a major role in sequestering antigens from dendritic cells, but have also been proposed to be immunosuppressive, dampening down local dendritic and T cell activation (Holt et al., 2008).
Regulatory T cells (Treg cells) are essential in the maintenance of immunological tolerance to “self” and in the regulation of the immune response to infectious organisms, both pathogens and commensals. Furthermore, Treg cells represent a major pathway proposed to contribute to the maintenance of immune homeostasis in the airways. Major populations of regulatory T cells studied in the context of pulmonary health and asthma are the natural thymic-derived CD4+Foxp3+ Treg cells and peripherally antigen-induced adaptive CD4+ Treg cells, which comprise both Foxp3-positive and -negative populations (Hawrylowicz and O’Garra, 2005; Roncarolo et al., 2006). Peripheral induction of Treg cells may represent an important mechanism to generate Treg cells with specificity for exogenous antigens, such as allergens, as well as to maintain Treg cell populations with age. A number of different mechanisms by which antigen-specific Treg cells inhibit the function of effector T cells, antigen-presenting cells, and innate cells have been described (reviewed in Vignali et al., 2008). A prominent inhibitory mechanism appears to be via anti-inflammatory cytokines such as IL-10 and TGF-β (Li et al., 2006; O’Garra et al., 2008), but inhibitory molecules such as CTLA-4 and PD1 are also likely to contribute (Vignali et al., 2008). Although considerable focus has been placed on CD4+ Treg cells, any cell with the capacity to secrete inhibitory cytokines may have “regulatory” potential, including CD8+ T cells, NK cells, γδ T cells, B cells, mast cells, and various APC populations (O’Garra et al., 2008). The different Treg cell populations, their lineage development, and mechanisms of actions have been discussed in a recent series of reviews in this journal (May 2009). Instead of these topics, the present article will highlight regulatory T cell function with relevance to the respiratory environment. It seems probable that primary functions of Treg cells in the airways are to limit the inflammatory consequences of infection and to maintain tolerance to harmless, inhaled aeroallergens.
Asthma
The incidence of asthma has increased dramatically in recent decades, with the greatest prevalence observed in developed countries. Currently there are an estimated 300 million asthmatics worldwide, with 5 million asthmatics in the UK alone, one of the highest rates worldwide (Masoli et al., 2004). Asthma is a chronic inflammatory disease of the airways associated with airway hyperresponsiveness (AHR), coupled with wheezing, breathlessness, chest tightening, and coughing. Characteristically, the obstruction of the airways is reversible, either spontaneously or with treatment. There is a strong genetic association with atopy, the predisposition to produce IgE antibodies to environmental allergens. Major cellular components driving asthmatic reactions include mast cells, eosinophils, and T cells, with a prominent role for CD4+ Th2 cells. More recently, roles for basophils, iNKT cells, Th17 cells, and a number of soluble mediators, including TSLP, IL-25, and IL-33, have also been proposed (reviewed in Barrett and Austen, 2009, this issue of Immunity). Even in patients, often older individuals, with nonatopic or intrinsic disease there is good evidence for involvement of similar biological mechanisms and Th2 cells. Asthma is also associated with structural changes in the airways that include hyperplasia of the epithelium, mucus metaplasia, and increased airway smooth muscle mass and increased deposition of extracellular matrix proteins. Importantly, these structural abnormalities can be observed even in preschool children with wheeze (Martinez et al., 1995; Saglani et al., 2007).
Currently, the major therapies used to control asthma are glucocorticoids, with or without beta 2 agonists. These have broad-ranging anti-inflammatory actions in controlling disease symptoms but fail to provide a cure. Importantly they do not prevent long-term decline in lung function. The reasons for the therapeutic interest in Treg cells for more effective treatment of asthma are the subject of the present review and arise from evidence that Treg cells are important in maintaining immune homeostasis in the airways and that their function may be altered in asthmatic disease. Furthermore, certain therapies associated with the amelioration of disease symptoms, including allergen immunotherapy, glucocorticoids, and beta 2 agonists, may promote or enhance Treg cell activity. These data highlight the therapeutic interest in promoting Treg cell function for patient benefit in asthma. Treg cell-directed therapies currently under investigation encompass adoptive cell transfer therapies, strategies investigated in the context of autoimmune disease, and transplantation and strategies to boost endogenous Treg cells, which represents a major focus of research for clinical application of Treg cells in allergic diseases, including in asthma (Riley et al., 2009).
Importance of Early Life Events in Programming the Immune System and Effect on Disease Development
Ninety percent of asthmatics are diagnosed by 6 years of age. Although asthma is difficult to diagnose before this age, atopic diseases and viral wheezing illnesses in infancy synergistically increase the risk for and are predictive of subsequent childhood asthma (Morgan et al., 2005; Sly et al., 2008). These data imply that early life events are highly predictive for the development of protective regulatory mechanisms within the pulmonary immune system. Indeed, evidence exists that Treg cells are already impaired in the cord blood of neonates at hereditary risk of allergy (Haddeland et al., 2005; Smith et al., 2008).
Recurrent wheezing is a common symptom during infancy and early childhood. Those with persistent wheezing develop abnormal lung function during their early years, which persists through to early adulthood (Morgan et al., 2005). Biopsies from asthmatic children show evidence of inflammation and structural abnormalities. Although not every child with wheezing will develop asthma, recurrent wheezing during the first 3 years of life is considered a major risk factor, particularly if there is a parental history of asthma. The majority of the wheezing lower respiratory tract illnesses of childhood are caused by viruses, so it has been postulated that early exposure to viruses has lasting effects on the shaping of pulmonary immune responses and are thus a risk factor for developing asthma (Martinez et al., 1995; Sly et al., 2008). In contrast, the “hygiene hypothesis” proposed that early childhood infections inhibit the tendency to develop allergic disease and early infection is thus protective. Epidemiologic evidence seems to support this with living in a developing country, having several older siblings, early attendance at day care, and exposure to livestock all being associated with a lower incidence of allergic disease and this has been linked with the development of regulatory pathways (Bach, 2002). However, most of the evidence relates to protection against atopy and atopic diseases rather than asthma itself. Whether infection is beneficial or harmful for the development of asthma, it is clear that its influence is likely to be highly dependent on both the timing and nature of the infection with critical implications in the programming of the immune system.
There is evidence that prenatally, and even preconception, environmental factors may influence the development of the neonatal immune system, because prenatal exposure to a farming environment influences innate immune patterning. Maternal exposure during pregnancy to an environment rich in microbial compounds was associated with higher expression of Toll-like receptors 2 and 4 (TLR2 and TLR4) and CD14 on peripheral blood cells, implying that exposure might prevent sensitization of the children (Ege et al., 2006). More directly, farm exposure during pregnancy is associated with increased number and function of regulatory T cells within cord blood as well as reduced Th2 cytokine production and lymphocyte proliferation after innate restimulation (Schaub et al., 2008). Studies in mice have shown that exposure of mothers to endotoxin prevents subsequent allergen-induced sensitization and airway inflammation in the pups (Gerhold et al., 2006). Moreover, immunologic tolerance can be transferred from mother to her offspring if the mother is tolerized before pregnancy, implying that even before conception the immune status of the mother is critical in defining the immune response of the offspring to allergens (Polte et al., 2008).
In addition to infection and exposure to aeroallergens, environmental pollution and diet (Hollingsworth et al., 2008; Miller, 2008; Litonjua and Weiss, 2007) have recently been highlighted to influence the development of disease in early life. Reduced maternal intake of vitamins D and E and zinc during pregnancy have all been associated with enhanced asthma symptoms in children (Litonjua and Weiss, 2007; Willers et al., 2008). Vitamin D in particular has been implicated in the development or maintenance of both Foxp3+ and IL-10+ Treg cells in humans and mice (Penna et al., 2005; Urry et al., 2009; Adorini and Penna, 2008). Furthermore, a recent study has shown that dietary factors can also modify the risk of allergic airway disease via epigenetic mechanisms (Hollingsworth et al., 2008). Mice given a diet rich in methyl donors, such as folic acid or vitamin B12, resulted in enhanced allergic airway disease that was inherited over multiple generations. This prenatal methyl-rich diet was postulated to promote DNA methylation and reduce transcriptional activity of genes associated with downregulation of allergic immune responses, such as Runx3. Prenatal maternal exposure to diets high in folates, vitamin B12, choline, and methionine–all of which provide methyl donors–as well as to cigarette smoke may repress gene transcription and promote asthma phenotypes (Miller, 2008). Although these studies have implicated regulatory factors such as IL-10 and TGF-β in the altered immune programming of the lung, there is little direct evidence that specific regulatory T cell populations are actually affected. In contrast, a recent elegant study examined neonatal diet, asking whether exposure of lactating mice to an airborne allergen affected development of allergic airway disease in their progeny (Verhasselt et al., 2008). Airborne antigens were transferred efficiently via breast milk and this transfer resulted in tolerance and protection from allergic asthma. Moreover, this breastfeeding-induced tolerance was dependent on the presence of TGF-β during lactation and was mediated by regulatory CD4+ T cells, which signaled via TGF-β. These data provide a mechanism underlying tolerance provided by breastfeeding neonates and underpins the importance of maternal influences on the development of regulatory mechanisms in the neonate, which ultimately affect the development of allergic symptoms.
Evidence that Treg Cells Influence Pulmonary Homeostasis
A primary requisite of the pulmonary tract is to maintain tolerance in the face of continuous exposure to potential antigens. Early experimental studies have highlighted the fact that antigens entering via the respiratory route generally induce tolerance or weak Th2 cell responses (Holt et al., 2008). Although many of these studies do not specifically highlight the induction of Treg cells, it is now commonly accepted that Treg cells represent a major mechanism of peripheral tolerance. An important role for the anti-inflammatory cytokine IL-10, from both innate cells and Treg cells, is also increasingly believed to play a central role (Hawrylowicz and O’Garra, 2005).
In the early 1980s, Holt et al. (1981) demonstrated that exposure of mice to aerosolized ovalbumin (OVA) intranasally in the absence of adjuvant elicited transient IgE responses, which subsequently declined. When the animals were challenged intra-peritoneally with OVA, their subsequent IgE responses were markedly suppressed relative to controls. The authors postulated a role for relatively long-lived antigen-specific suppressor cells that could transfer inhibition of the IgE response to other animals (Holt et al., 1981). More recently, tolerance induction in the airways in animal models has been correlated more specifically with the induction of regulatory T cells. Repeated exposure of mice to low-dose allergen promoted the development of a regulatory CD4+ T cell population that expressed membrane-bound TGF-β and Foxp3. Adoptive transfer of these cells to naive mice prevented allergic sensitization (Ostroukhova et al., 2004). A higher dose of inhaled allergen stimulated the development of a T regulatory cell population that secreted high amounts of IL-10 (Akbari et al., 2002). This particular study highlights the involvement of respiratory accessory cells in promoting tolerance and/or Treg cell induction. Repeated exposure of mice to inhaled antigen stimulated pulmonary DCs to produce IL-10, which were then able to induce the development of IL-10-producing Treg cells. Critically, transfer of the IL-10-producing dendritic cells prevented development of allergic inflammation after subsequent allergen challenge of recipient mice. It is however important to note that peptide inhalation studies in humans after sensitization had already occurred failed to promote respiratory tolerance (Ali et al., 2004).
Structural cells in the airways are likely to play an active role in mediating pulmonary immune responses to inhaled antigens. Alveolar epithelial cells can present antigen and promote development of Foxp3+ cells via a TGF-β-dependant mechanism in mice (Gereke et al., 2009). Moreover, airway epithelial cells are able to initiate specific tolerogenic mechanisms on exposure to proteinase allergen. Retinal dehydrogenase 1 (RALDH-1), an enzyme involved in the production of retinoic acid, is induced and promotes the development of immunosuppressive regulatory T cells (Goswami et al., 2009). In vivo this enzyme is regulated by epithelial-derived MMP-7, and a deficiency in MMP-7 results in enhanced T regulatory cells in the lung accompanied by an attenuated response to allergen challenge. Although there is less direct evidence of a role for alveolar macrophages (AM) in specifically influencing T regulatory cell pathways, their role in suppression of over-exuberant immune responses in the lung has been postulated (Holt et al., 2008). In vitro evidence suggests that AM can actively tolerise CD4+ T cells in an antigen-specific manner, implying that they mediate a form of immune privilege in the lungs that effectively limits immune responses in the pulmonary compartment but has little effect on systemic immunity (Blumenthal et al., 2001). Resident dendritic cells are also vital in controlling pulmonary immune responses. In particular, plasmacytoid dendritic cells limit immune responses to harmless inhaled allergens in mice (see Lambrecht, 2009, review in this issue). It seems likely that there is cooperation between these different populations of lung-resident cells as well as those of the immune system in order to promote pulmonary immune homeostasis.
Delivery of foreign antigens to the airways, in the presence of TLR signaling, has been shown to preferentially induce Th2 cell-mediated responses in humans and in mice. Whereas at other sites such signaling drives Th1 cell responses, only high amounts of LPS with antigen results in Th1 cell responses (Eisenbarth et al., 2002). Conversely, TLR signaling, both directly and indirectly, can impair Treg cell function and is postulated to allow the more efficient clearance of pathogens (Sutmuller et al., 2006; Urry et al., 2009). These data imply that in susceptible individuals the combination of exposure of the lungs to pathogens in conjunction with allergens affects the programming of the pulmonary immune system, which in turn might precipitate an inappropriate response to allergen.
Immune homeostasis in the lung is achieved by the balance in inflammatory and modulatory cytokines (Figure 1). IL-10 is a potent anti-inflammatory cytokine and its production by a wide range of cell types has been described, including B cells, macrophages, dendritic cells, mast cells, and eosinophils (O’Garra et al., 2008). Many T cell subsets synthesize IL-10, including CD8+ T cells, CD25+Foxp3+ Treg cells, and effector CD4+ T cell populations, namely Th1 cells, Th2 cells, and Th17 cells. Of note, two subsets of Th2 cells have been described, IL-10-producing regulatory Th2 cells, and TNF-α-producing inflammatory Th2 cells (Ito et al., 2005). IL-10 production by effector T cells is likely to be important in limiting their inflammatory potential (O’Garra et al., 2008). A wealth of studies in animal models has proposed a protective role for IL-10 in the maintenance of respiratory homeostasis. IL-10 production by innate cells and antigen-specific T cells in the respiratory tract has been shown to limit inflammation in response to both viral and bacterial pathogens (Higgins et al., 2003; Sun et al., 2009). Studies in adults demonstrate that rhinovirus-induced lower respiratory tract illness, a virus associated with asthma-promoting wheeze in infants and exacerbations in adults, is associated with reduced IL-10 production and augmented Th2 cell immunity (Message et al., 2008). Il10−/− outbred mice exhibited exaggerated airway inflammation and heightened expression of IL-5 and IFN-γ in bronchoalveolar lavage (BAL) fluids in allergic bronchopulmonary aspergillosis (Grunig et al., 1997). Interleukin-10 gene transfer to the airway prevents allergic mucosal sensitization in mice suppressing cellular recruitment and inflammation (Stampfli et al., 1999). Intranasal instillation of IL-10 into airways at time of allergen challenge inhibited leukocyte recruitment (Zuany-Amorim et al., 1995), and T cells expressing IL-10 inhibit allergic airway disease (Oh et al., 2002). As discussed below, many human and mouse studies propose a prominent role for T cell-derived IL-10 as an important mediator of immunological tolerance in the airways.
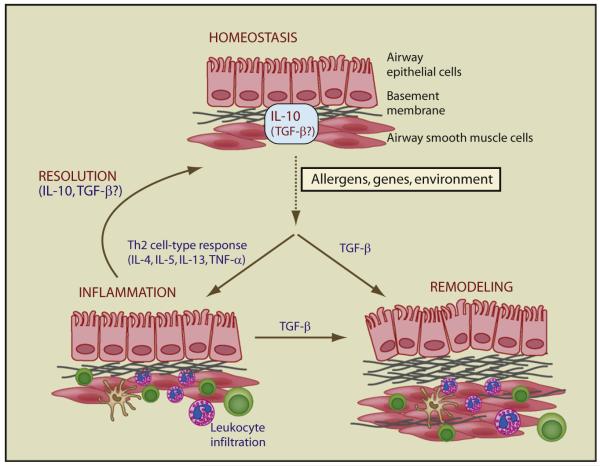
Figure 1. Regulatory Pathways Maintain Lung Homeostasis.
The lung is maintained in a state of homeostasis by a complex network of cells and molecules. IL-10 plays a central role in this process, but the role of TGF-β is less clear at present. Exposure of susceptible individuals to allergen is associated with a Th2 cell type immune response characterized by IL-4, IL-5, IL-13, and TNF, which culminates in leukocyte infiltration of the lungs. The development of this inflammation is influenced by multiple factors, but early childhood infection, diet, vitamin D, and TLR ligand expression all affect the initiation and development of the allergic response. In a proportion of individuals, inflammation is chronic and associated with significant remodeling of the airways. IL-10 and TGF-β are thought to be able to promote resolution of inflammation whereas TFG-β family members initiate tissue repair and remodeling. Induction of TGF-β by Th2 cytokines during acute inflammation may also contribute to lung remodeling. Whether chronic asthma develops as a result of ineffective inflammatory resolution or as an aberrant wound healing response is the subject of investigation, but it is likely that IL-10 and TGF-β are key mediators.
Experimental Evidence for Treg Cell Function in Allergic Airway Disease
Mouse models of allergic airway disease have long been used to dissect the immunological mechanisms underlying the pathophysiological features of asthma (Lloyd, 2007; Hawrylowicz and O’Garra, 2005). Adoptive transfer of antigen-specific CD4+CD25+ T regulatory cells was found to suppress allergic inflammation and hyperreactivity via a mechanism dependent upon IL-10 (Kearley et al., 2005). Moreover, although IL-10 was vital for suppression to occur, the IL-10 was induced from bystander CD4+ cells rather than the CD4+CD25+ T regulatory cells themselves. Importantly, when delivered after the onset of disease, T regulatory cells were able to downregulate established inflammation and prevent airway remodeling (Kearley et al., 2008). Conversely, depletion of CD4+CD25+ T regulatory cells before sensitization is enough to enhance the severity of inflammation and AHR in the lung (Lewkowich et al., 2005). Depletion of CD25+ T cells resulted in increased numbers of airway dendritic cells with higher expression of activation markers and enhanced potential to promote effector T cell proliferation. Airway regulatory cells do not need to be antigen specific for in vivo suppression (Leech et al., 2007); however, they appear rapidly after antigen exposure (Strickland et al., 2006). Collectively, these data suggest that T regulatory cells restrain dendritic cell function in the lung, resulting in suppression of inappropriate immune responses.
IL-10 has been found to be essential for effective suppression of allergic responses in the lung (Joetham et al., 2007; Kearley et al., 2005; Leech et al., 2007). This dependence on IL-10 highlights an important feature of T regulatory cell control of pulmonary immune responses. Although IL-10 is not required for control of systemic autoimmunity, it is absolutely required for restraint of immune responses at mucosal surfaces such as the gut or lung (Rubtsov et al., 2008). Mice with a targeted deletion of IL-10 specifically in regulatory (Foxp3+) T cells develop spontaneous colitis and showed enhanced AHR and inflammation after exposure to inhaled allergen. These data highlight the unique and complex interplay of regulatory systems functioning at mucosal sites with the effector T cells and indicate that T regulatory cells utilize multiple pathways to control inflammatory responses and the location and environment dictate which particular pathway is employed.
The appropriate localization of regulatory cells is important for their function because they may exert their suppressive activity via cell-cell contact as well as via cytokine secretion. In vivo homing studies have determined that the chemokine [C-C motif] receptor 4 (CCR4) is essential for the recruitment of CD4+Foxp3+ regulatory cells. Recruitment of Treg cells to the lung is impaired in the absence of CCR4 (Sather et al., 2007). Moreover, mice with a complete loss of CCR4 in the Treg cell compartment develop lymphocytic infiltration and severe inflammatory disease of the airways. This finding is intriguing because CCR4 is also the primary chemokine receptor responsible for recruitment of allergen-specific Th2 cells to the lung (Lloyd et al., 2000). CD4+CD25+ Treg cells from human donors have been shown to preferentially express CCR4 and migrate to the CCR4 ligands CCL17 and CCL22 (Iellem et al., 2001). Adoptive transfer studies have also highlighted a role for CCR4 ligands CCL17 and CCL22 in the recruitment and retention of CD4+CD25+ Treg cells to the lung during chronic allergen challenge (Kearley et al., 2008). Retention in the airways is also thought to be dependent upon continued antigen exposure. Maintenance of protective T regulatory cell activity was absolutely dependent upon continued allergen exposure because interruption of allergen challenge resulted in a reduction in Treg cell activity concomitant with resurgence in Th2 cell type pathology (Strickland et al., 2006). This occurs in human systems too, with beekeepers showing an in vivo switch to a IL-10-secreting phenotype during the beekeeping season, which wanes during periods of nonexposure (Meiler et al., 2008).
Prevention of inappropriate immune responses to harmless antigens is one of the primary functions of regulatory T cells, and the decision as to whether an immune response is initiated or not is critical to maintain host defense. Recent evidence suggests that effector and Treg cell function is closely linked. Naturally occurring Treg cells are thought to be able to suppress inflammatory responses during the later stages of infection in order to prevent collateral tissue damage. However, the absence of Treg cells during mucosal viral infection results in uncontrolled inflammation and early death, because of delayed migration of effector cells to the inflammatory site (Lund et al., 2008). These data suggest that Treg cells control recruitment of effector cells to sites of inflammation, including the airways and coordinate effective immune responses at early as well as later stages. Treg cells may restrain effector responses in other ways too. In mouse Treg cells, high amounts of interferon regulatory factor-4 (IRF4), a transcription factor essential for Th2 effector cell differentiation, is dependent on Foxp3 expression. Ablation of a conditional Irf4 allele in Treg cells resulted in selective dysregulation of Th2 cell responses, suggesting that expression of IRF4 in some, as yet undefined, way endows Treg cells with the capacity to inhibit Th2 cell responses (Zheng et al., 2009). These data further highlight a close link between Treg and T effector cells.
Genetic and Clinical Evidence for the Role of Treg Cells in Promoting Lung Health
Some of the most persuasive evidence for the importance of Treg cells in prevention of allergic diseases in early life in humans, prior to the onset of asthma, comes from genetic mutation studies. Foremost among these are studies of children with immune dysregulation polyendocrinopathy enteropathy X-linked (IPEX) syndrome. Treg cells stably expressing the forkhead box protein P3 transcription factor, Foxp3, are generated in the thymus and released into the circulation as lineage-specific CD4+CD25+Foxp3+ T cells. Many children with IPEX exhibit mutations in Foxp3 and have absent or reduced numbers of CD4+CD25hi Treg cells or lack functional CD4+CD25hi Treg cells. At least 22 different mutations in the Foxp3 gene have been described to date with many in the DNA binding region (Bacchetta et al., 2006; reviewed in Zheng and Rudensky, 2007; Torgerson and Ochs, 2007). IPEX presents at very early age and can be detected within a few days of birth, as a syndrome associated with autoimmunity, allergy, and failure to thrive. It is generally fatal, in many cases within the first year of life resulting from recurrent infection, without bone marrow transplantation or profound immunosuppression (Chatila et al., 2000; Torgerson and Ochs, 2007). The only curative approach for IPEX is allogeneic hematopoietic stem cell transplantation (HSCT), and a recent study suggests that a patient remaining healthy at 5 to 6 years after nonmyeloablative HSCT demonstrated Foxp3 expression and numbers of CD4+CD25hiCD127loFoxp3-expressing cells of donor origin at low-normal range (Rao et al., 2007; Seidel et al., 2009). Although autoimmune manifestations have been most prominently reported, these boys suffer from severe eczema, elevated IgE titers, eosinophilia, and food allergy (Chatila et al., 2000). Both resting PBMC and cells stimulated in culture are skewed toward a Th2 phenotype with high amounts of IL-4, IL-5, and IL-13. These studies suggest that Foxp3+CD25hi Treg cells play a prominent role in the prevention of allergic sensitization in early life.
The Scurfy (sf) mouse lacks CD4+Foxp3+ regulatory T cells, caused by a mutation of the X-linked transcription factor Foxp3 resulting in aggressive fatal autoimmune disease (reviewed in Zheng and Rudensky, 2007; Torgerson and Ochs, 2007). However, mice with a targeted loss-of-function mutation in the murine Foxp3 gene by targeted mutagenesis may provide a better model of the human disease. They demonstrate an intense multiorgan inflammatory response associated with allergic airway inflammation, a striking hyperimmunoglobulinemia E, eosinophilia, and dysregulated Th1 and Th2 cytokine production, albeit in the absence of overt Th2 cell skewing, recapitulating the human condition, IPEX, associated with Foxp3 mutation (Lin et al., 2005).
Up to one third of patients with IPEX may not have mutations in Foxp3 (Gambineri et al., 2003). At least two studies (Roifman, 2000; Caudy et al., 2007) have reported a variant of IPEX, where patients presented with IL-2 receptor alpha chain (CD25) deficiency, and certainly in the later study a normal Foxp3 gene. That study directly compared two patients, one with CD25 deficiency and the other with a Foxp3 mutation, concluding that both presented with an IPEX-like syndrome. CD4+ T cells from the CD25 subject demonstrated defective IL-10 production after in vitro stimulation (Caudy et al., 2007) and went on to develop, among a range of abnormalities, recurrent pulmonary infections and asthma. Somewhat unexpectedly, the Foxp3-deficient patient showed normal expression of IL-10. These studies imply essential and nonredundant roles for both Foxp3+ Treg cells and IL-10+ Treg cells in the prevention of allergic and asthmatic disease.
A number of studies in humans have investigated evidence for impaired Foxp3 Treg cell function in allergic and asthmatic disease (Ryanna et al., 2009; Ling et al., 2004). The interpretation of many of these studies, however, is hampered by the fact that Treg cells were defined on the basis of CD4 and CD25 expression and not Foxp3 expression. In some cases, the data could also be interpreted as an increase of activated T effector cells, as for example where Treg cells are defined on the basis of CD25 expression and the greatest degree of impairment is seen in active disease. Similarly, because most studies assessed Treg cell numbers and function in the peripheral blood, they failed to account for the possible migration of Treg cells into recently activated, inflamed tissues. A study in pediatric lung and blood in children demonstrated impaired CD4+CD25hi T cell numbers and function as well as reduced Foxp3 mRNA in the lung (but not peripheral blood) in the asthmatic subjects in comparison to children with cough but not asthma (Hartl et al., 2007). More recently, a study suggests that asthmatic patients have normal peripheral blood numbers of CD4+CD25hi and CD4+CD25hi Foxp3+ Treg cells compared to healthy donors, but Foxp3 protein expression is decreased (Provoost et al., 2009), although the functional consequences of this were not explored. Conversely, evidence to suggest that Treg cells actively prevent Th2 cell responses to allergen in healthy donors also exists. For example increased responses to allergen, both proliferative and Th2 cytokine responses, are observed in the peripheral blood of healthy nonatopic donors after the depletion of CD4+CD25+ T cells from PBMC as compared to undepleted PBMC cultures (Ling et al., 2004). These data highlight the need for further studies in human pediatric and adult respiratorytissues.
IL-10–A Critical Controller in Asthma
IL-10 has broad immunosuppressive and anti-inflammatory actions relevant to the inhibition of asthma pathology (O’Garra et al., 2008). It is a potent inhibitor of proinflammatory cytokine production and acts on antigen-presenting cells to dampen T cell activation, including Th2 cells. It inhibits effector cells associated with both the early- and late-phase asthmatic response, mast cells, and eosinophils. IL-10 promotes IgG4 production, an immunoglobulin isotope generally believed to be protective in the context of allergic responses. It also inhibits IgE and results in favorable ratios of IgG4 to IgE; this Ig balance is associated with health and tolerance induced after allergen immunotherapy (Till et al., 2004).
A number of studies in humans have investigated IL-10 synthesis in allergic and asthmatic patients as compared to healthy individuals. A substantial reduction in IL-10 mRNA and protein and increased amounts of proinflammatory cytokines was reported in the bronchoalveolar lavage fluid and in alveolar macrophages of patients with asthma as compared to control healthy subjects (John et al., 1998). A polymorphism in the IL10 gene promoter resulting in reduced IL-10 expression is associated with more severe disease (Lim et al., 1998). Similar inverse correlations of reduced IL-10 synthesis by CD4+ T lymphocytes have been reported in relation to atopic status (Akdis et al., 2004). An important study by Akdis et al. demonstrated that there was a substantial increase in the frequency of allergen-responsive IL-10-positive T cells in the peripheral blood of healthy nonatopic individuals as compared to allergic patients, who demonstrated reduced IL-10+ and increased IL-4+ allergen-responsive T cells (Akdis et al., 2004). Conversely, natural immune tolerance in nonatopic individuals, specifically beekeepers in whom seasonal exposure to bee stings is important to maintain tolerance, is associated with increased venom-allergen-specific IL-10+ CD4+ T cells (Meiler et al., 2008).
Importance and Complexity of TGF-β
TGF-β is a pleiotropic cytokine that regulates lymphocyte homeostasis, inhibits Th2 and Th1 cell responses, promotes the differentiation of certain T cell lineages, inhibits IgE, and promotes IgA production (Li et al., 2006). The complete absence of TGF-β in mice results in early death from multiorgan inflammation (Shull et al., 1992), highlighting a crucial role in peripheral tolerance. However, heterozygous mice express lower amounts of TGF-β1 and are viable. After allergen sensitization and challenge, they exhibit exacerbated airway disease compared to wild-type animals, suggesting a role for endogenous TGF-β in suppressing the development of allergic airway disease (Scherf et al., 2005).
Intratracheal delivery of TGF-β suppresses allergen-induced inflammation (Joetham et al., 2007) whereas CD4+ T cells engineered to secrete latent TGF-β efficiently suppress allergen-specific airway inflammation and hyperresponsiveness (Hansen et al., 2000). Blocking TGF-β signaling specifically in T cells also results in enhanced airway hypersensitivity, airway inflammation, and increased Th2 cytokine production (Nakao et al., 2000). Collectively, these data reinforce the idea that TGF-β acts to regulate immune responses in the lung and that perturbations in the degree of expression of either the cytokine, its receptor, or even molecules within its signaling pathway have severe consequences for maintenance of pulmonary homeostasis.
TGF-β also induces the peripheral expression of the transcription factor FoxP3, which promotes the generation of CD4+CD25+ Treg cells able to inhibit allergic airway disease (Chen et al., 2003). Although TGF-β has been implicated in CD4+Foxp3+ Treg cell control of allergic airway disease in at least two studies (Ostroukhova et al., 2004; Joetham et al., 2007), the majority of studies have described a central role for IL-10, with or without TGF-β in the control of allergic airway inflammation. This contrasts with studies in the gut where naturally occurring TGF-β-secreting Treg cells are present and play a vital nonredundant role in regulation mucosal immune responses (Barnes and Powrie, 2009, review in this edition) and may reflect the fact that the lower airways represent a sterile environment whereas the gut is not. Cooperation between IL-10 and TGF-β is likely to be important in the regulation of pulmonary mucosal immune responses.
TGF-β influences the lineage specificity of effector T cell subsets. It is instrumental in driving the RORγT-dependent differentiation pathway in CD4+ T cells, resulting in either Th17 or Treg cells depending on the concurrent presence of maturation and polarization factors such as IL-6, IL-21, retinoic acid, IL-23, and IL-10. TGF-β has the potential to reprogram apparently differentiated Th cells into a new functional subset producing IL-9 (Veldhoen et al., 2008; Dardalhon et al., 2008). Thus it is possible that effector T cells within mucosal tissues exhibit a degree of plasticity that is influenced by TGF-β. In the context of allergic pulmonary inflammation, it may mean that it is possible to redirect allergen-specific T cell responses with immunotherapy in order to alleviate symptoms.
Although TGF-β exerts a suppressive activity on many immune processes, it also plays a vital role in promoting the structural changes of tissue remodeling. Some of the functions of TGF-β that contribute to subepithelial fibrosis include amplification of fibroblast proliferation and differentiation as well as induction of the expression of collagen and other ECM proteins (Makinde et al., 2007). TGF-β induces apoptosis of airway epithelial cells and is potentially involved in the regulation of the adhesion properties of epithelial cells leading to damage of the epithelial layer (Szefler, 2005). TGF-β has been shown to play a role in enhancement of goblet cell proliferation and mucus secretion (Makinde et al., 2007; McMillan et al., 2005). It also causes airway smooth muscle (ASM) proliferation and contributes to increased ASM cell mass (Makinde et al., 2007). Asthmatic patients show increased TGF-β expression in both bronchial biopsy sections and BAL in comparison to normal subjects, and expression correlated with the depth of subepithelial fibrosis (Doherty and Broide, 2007). Neutralization of TGF-β in two different models of chronic allergen challenge reduced airway remodeling in one study where sensitization occurred via the peritoneum (McMillan et al., 2005), but did not affect remodeling and worsened inflammation and AHR in a second (Fattouh et al., 2008) in which unsensitized mice inhaled HDM. The differing outcome of these studies highlights the use of a model where the initial encounter with allergen is via the lung epithelium, and therefore the first encounter with the immune system is via the pulmonary dendritic cell network. Furthermore, they highlight the complexity of targeting TGF-β with the potential for differing outcomes on remodeling versus immune function.
TGF-β is a member of a complex superfamily, and the potential of other key members of the family to control immune responses versus repair and remodeling in the lung have yet to be explored. The TGF superfamily of molecules incorporates three TGF-β isoforms, the bone morphogenic proteins (BMPs), as well as activins, all of which have a diverse array of functions including organogenesis, immune regulation, and wound repair. Activin A has been postulated to provide a link between acute allergen-specific T cell responses and chronic TGF-β1-mediated airway remodeling in asthma (Karagiannidis et al., 2006). A recent study has determined that activin-A suppresses antigen-specific Th2 cell responses and protects against AHR and allergic inflammation in mice (Semitekolou et al., 2009). Interestingly, activin-A was shown to exert this suppressive function via induction of antigen-specific regulatory T cells that suppressed Th2 cell responses in vitro and upon transfer in vivo. At present there is little information regarding the role of the BMP family in regulation. However, there is evidence that allergen provocation activates BMP signaling and receptor expression in allergic asthmatics, implying that this arm of the TGF-β family may also exert influence over regulation and repair in the lung (Kariyawasam et al., 2008). Collectively, the data on TGF-β and family members may imply that asthma is associated with aberrant wound repair or failure to effectively resolve inflammation. Further investigation into the relationship between IL-10 and TGF-β-secreting regulatory cells may provide some clues.
Evidence of the Impact of Therapies on Treg Cell Numbers and Function
Certain treatments that ameliorate allergic and asthmatic symptoms are associated with increased or restored Treg cell function (Figure 2). A striking example of this is allergen immunotherapy, which involves the administration of increasing doses of allergen to which an individual is sensitized, under carefully controlled clinical conditions, in order to induce a state of immune tolerance to the allergen (Akdis and Akdis, 2007; Till et al., 2004). It requires several years of administration for maximal efficacy, is only effective in certain patient groups, and can be associated with adverse events, including systemic anaphylaxis, and so is not without serious risk in asthmatic patients. Importantly, allergen immunotherapy in allergic children has been shown to reduce the subsequent incidence of asthma (Moller et al., 2002). Immunological studies in allergic patients have demonstrated that immunotherapy inhibits allergen-specific Th2 cell responses and promotes an increased frequency of IL-10-secreting Treg cells that occurs quite rapidly after immunotherapy and is followed by an increase in circulating IgG4, known to be regulated by IL-10 (Akdis and Akdis, 2007; Till et al., 2004). This IL-10 phenotype is analogous to that seen in healthy nonatopic donors upon exposure to allergen (Akdis et al., 2004) and in individuals who demonstrate natural tolerance to allergen (Meiler et al., 2008). At least two recent studies now also demonstrate that allergen immunotherapy is associated with changes in Foxp3+ Treg cells. In one study of rush venom immunotherapy, a progressive increase in CD4+CD25hiFoxp3+ T cells was reported that was positively correlated with allergen-specific IgG4/IgE ratio (Pereira-Santos et al., 2008). A second study looked directly in the nasal mucosa of hay fever patients, demonstrating that after grass pollen immunotherapy, Foxp3+CD25+CD3+ cells were increased, some of which also coexpressed IL-10 (Radulovic et al., 2008). Additional reports of changes in IFN-γ and a report of increased TGF-β after immunotherapy also exist (reviewed in Akdis and Akdis, 2007).
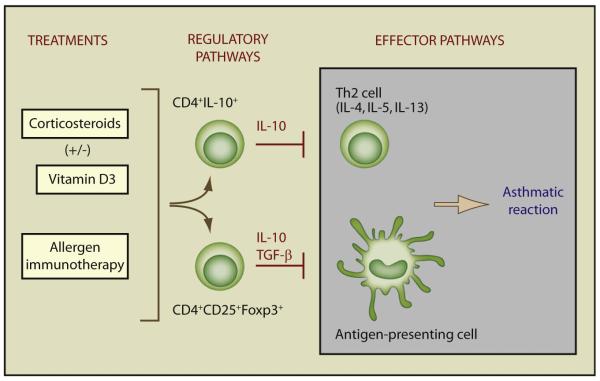
Figure 2. Effect of Current Asthma Treatments on Regulatory Pathways.
Regulatory T cells inhibit effector T cells, antigen-presenting cells, and cells of the innate immune response associated with the asthmatic reaction. Corticosteroids, a key treatment for asthma, are associated with the upregulation of both Foxp3+ Treg cells and IL-10 production by CD4+ T cells. Allergen immunotherapy has also been associated with an increase in allergen-specific IL-10-secreting Treg cells. More recent data suggest that Foxp3+ Treg cells may also be increased by this treatment. Vitamin D may both directly increase IL-10 and also enhance steroid-induced IL-10 production. Both Foxp3+ and IL-10+ (Foxp3-negative and -positive) Treg cell populations are likely to use additional inhibitory mechanisms including cell-contact-dependent pathways and cytotoxicity.
A number of strategies are being investigated to improve the safety and efficacy of allergen IT, which are likely to enhance its applicability to asthma. One approach has been to use allergen-derived peptides containing T cell epitopes, which lack IgE binding sequences in order to reduce adverse reactions mediated via IgE cross linking of mast cells. Although intradermal administration of these peptides still unexpectedly elicited isolated late asthmatic reactions (LAR) in around 25% of patients, this was followed by a period of bronchial hyporesponsiveness to peptide (Haselden et al., 1999). These studies have highlighted that peptide immunotherapy in cat-allergic, asthmatic patients is associated with downregulation of Th2 cell responses to whole allergen and the induction of antigen-specific T regulatory cell pathways associated with increased IL-10 (Verhoef et al., 2005). A mouse model designed specifically to mimic the human protocol determined that peptide immunotherapy generated CD4+IL-10+ cells and tolerance to subsequent allergen challenge was IL-10 dependent (Campbell et al., 2009). Future initiatives are likely to include the use of lower doses of peptide to minimize adverse events (reviewed in Larche, 2007). Other approaches include the use of an anti-IgE, omalizumab, to reduce circulating IgE titers with evidence for increased safety of immunotherapy. Anti-IgE cross linking can provoke IL-10 synthesis by human blood monocytes (Novak et al., 2001), which although unproven might contribute to greater efficacy. Other strategies have utilized Toll-like receptor ligands to skew the immunogenicity of allergen away from a Th2 cell response. For example, the conjugation of immunostimulatory DNA to the short ragweed allergen Amb a 1 enhanced its immunogenicity and reduced its allergenicity in mice, rabbits, and monkeys (Tighe et al., 2000), and this has now been tested in patients with allergic rhinitis with evidence of improved efficacy over placebo (Creticos et al., 2006). Considerable research interest remains in identifying safer and more effective protocols for immunotherapy likely to involve modified allergens, alternative routes of administration, and novel adjuvants, which would increase their applicability to severe asthmatics.
Asthma is routinely treated with broad-ranging anti-inflammatory mediators, classically corticosteroids, which control symptoms in most patients. Several studies have demonstrated that glucocorticoid treatment correlates with increased IL-10 and Foxp3 gene and and/or protein expression in patients (reviewed by Ryanna et al., 2009). Inhaled corticosteroids increase IL-10 synthesis and reduce proinflammatory cytokine production by alveolar macrophages from adult asthma patients (John et al., 1998), whereas a triamcinolone aerosol given to children with moderate asthma doubled serum titers of IL-10 in parallel with clinical improvement (Stelmach et al., 2002). Both inhaled and systemic glucocorticoid treatment in moderate and severe asthmatics have been shown to increase mRNA for Foxp3 and IL-10 in peripheral blood CD4+ T cells tested directly ex vivo (Karagiannidis et al., 2004). A further study importantly addressed this at the active site of disease, demonstrating that isolated CD4+CD25hi T cells from the BAL fluid (but not peripheral blood) of asthmatic children were reduced in number and failed to suppress proliferation and Th2 cytokine and chemokine production by CD4+CD25− cells, as compared to children with cough or controls. Importantly, inhaled corticosteroids reversed the defect in CD4+CD25hi numbers and suppressive function (Hartl et al., 2007).
IL-10 may contribute to the clinical efficacy of gluocorticoids in asthma as suggested by a study where two groups of severe asthma patients were compared, those responding well to high-dose oral steroid trreatment for improved lung function and those with no improvement. CD4+ T cells from the steroid-refractory asthma patients showed an impaired in vitro response to steroids for induction of IL-10 in comparison to cells from the steroid-sensitive asthmatics (Hawrylowicz et al., 2002). Glucocorticoids, in conjunction with 1α,25-dihydroxyvitmain D3 (calcitriol), the active form of vitamin D, induce a population of IL-10-secreting regulatory T cells in mice and humans (Barrat et al., 2002). Calcitriol was subsequently shown to restore the defective steroid-induced IL-10 synthesis of CD4+ T cells from steroid refractory asthma patients (Xystrakis et al., 2006). This occurred not only in vitro, but also after patient ingestion of calcitriol at standard formulary doses, raising the possibility that combined drug treatment might improve asthma control in this important patient cohort. There is a growing awareness of the prevalence of vitamin D insufficiency worldwide and its association with poor pulmonary function including asthma (Holick, 2007). This has fuelled a lively debate about the importance of vitamin D sufficiency in maintaining, and possibly programming, Treg cell populations important for pulmonary health, because low dietary intake of vitamin D by pregnant women has been associated with an increased incidence of wheeze in the offspring at 3 and 5 years in 2 of 3 recent studies (Litonjua and Weiss, 2007). In vitro studies demonstrate the capacity of vitamin D to induce a tolerogenic dendritic cell phenotype with increased IL-10, which promotes the generation of Foxp3+ Treg cells (Penna et al., 2005), and also acts directly on human CD4+ T cells to induce IL-10+ Treg cells (Urry et al., 2009). Vitamin D supplementation in multiple sclerosis patients increased serum TGF-β (Mahon et al., 2003) and enhanced IL-10 and TNF/IL-10 ratios in a placebo-controlled study of vitamin D supplementation in patients with congestive heart failure (Schleithoff et al., 2006). Administration of the pharmacologically active form calcitriol to steroid-refractory asthma patients increased IL-10 mRNA in CD3+CD4+ T cells tested pre- and postcalctriol ingestion, directly ex vivo (Urry et al., 2009). In vivo studies in animal models also identify a role for vitamin D in the induction of therapeutic tolerance through the induction of both Foxp3 and IL-10 (reviewed in Adorini and Penna, 2008; Taher et al., 2008). These data have led to the suggestion that vitamin D may contribute to pulmonary health via the induction and/or maintenance of essential Treg cell populations, which, coupled with the capacity of vitamin D to promote antimicrobial pathways, might promote homeostasis required for the unique pulmonary environment (Adams and Hewison, 2008).
These data imply the capacity of broad-acting inhibitory mediators such as corticosteroids or vitamin D to maintain, restore, or enhance Treg cell function in asthma. Nevertheless, the capacity to combine such treatments with an antigen-specific regimen such as allergen immunotherapy may offer even greater benefit and safer application for the treatment of asthma. One example of this is a study in allergic airway disease in mice, which showed that 1α,25-dihydroxyvitamin D3 potentiated the beneficial effects of allergen immunotherapy, facilitating the use of a lower dose of antigen for the induction of inhalational tolerance after sensitization, with a role for IL-10 and TGF-β (Taher et al., 2008). Combining immunotherapy with glucocorticoids, vitamin D, anti-IgE, or even microbial compounds offers the promise to both increase the safety and improve the efficacy of IT in asthma, an area that is being and will be addressed through clinical trials.
Future Issues
Complex immunological mechanisms exist to maintain homeostasis within the lung, with overwhelming evidence to suggest that Treg cells are key players in this process. We need to increase our understanding of these different immunological mechanisms utilized by Treg cells in order to manipulate these pathways for more effective therapy to treat asthmatic individuals. This is likely to involve both novel strategies and improvement of existing therapies such as allergen immunotherapy.
In addition to CD4+CD25+Foxp3+ Treg cells and IL-10-secreting T cells, there is emerging evidence for other regulatory cell populations in the lung. In humans, IL-10-secreting NK cells can suppress antigen-specific effector function at least in vitro (Deniz et al., 2008). CD8+ T cells have been shown to provide effective, long-lasting memory responses in the lung, enhancing Th1 over Th2 cell type immunity and preventing allergic sensitization in a mouse model in vivo (Leggat et al., 2008). IL-17-producing γδ and NK cells have also been shown to have regulatory activity during pulmonary infections, although their potential in asthma has not yet been explored. Similarly, the regulatory potential of human pulmonary γδ cells and NK T cells has yet to be investigated.
Regulatory cells in the lung do not always share characteristics with those in the periphery, highlighting the importance of studying lung-derived cells. Moreover, analysis of antigenspecific Treg cells is likely to be far more informative and emerging technology will facilitate this. T cell lineage commitment may be less rigid than previously believed. Studies now show that existing Th2 cells may be converted into alternative CD4+ T helper cells expressing IL-9 and IL-10, depending on the cytokine milieu (Dardalhon et al., 2008; Veldhoen et al., 2008). This flexibility in T cell differentiation may explain down-regulation of Th2 cytokine production in vivo but it remains to be seen whether this is a transient change in phenotype or if these T cells are truly “reprogrammed.”
Immune programming in the lung occurs early in life and exposure to pathogens as well as dietary factors affect the TLR patterning of the lung and development of pulmonary regulatory pathways. Going forward it will be important to study these pathways in utero, in children, and in adults in order to boost host regulatory pathways, particularly in individuals who have a genetic susceptibility for asthma. Immune reprogramming in early life remains an exciting prospect. Ultimately, a thorough understanding of the nature of pulmonary immune homeostasis will enable us to exploit these regulatory mechanisms to develop novel and improved therapies for immune diseases such as asthma.
REFERENCES
- Adams JS, Hewison M. Unexpected actions of vitamin D: New perspectives on the regulation of innate and adaptive immunity. Nat. Clin. Pract. Endocrinol. Metab. 2008;4:80–90. doi: 10.1038/ncpendmet0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adorini L, Penna G. Control of autoimmune diseases by the vitamin D endocrine system. Nat. Clin. Pract. Rheumatol. 2008;4:404–412. doi: 10.1038/ncprheum0855. [DOI] [PubMed] [Google Scholar]
- Akbari O, Freeman GJ, Meyer EH, Greenfield EA, Chang TT, Sharpe AH, Berry G, DeKruyff RH, Umetsu DT. Antigen-specific regulatory T cells develop via the ICOS-ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat. Med. 2002;8:1024–1032. doi: 10.1038/nm745. [DOI] [PubMed] [Google Scholar]
- Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy. J. Allergy Clin. Immunol. 2007;119:780–791. doi: 10.1016/j.jaci.2007.01.022. [DOI] [PubMed] [Google Scholar]
- Akdis M, Verhagen J, Taylor A, Karamloo F, Karagiannidis C, Crameri R, Thunberg S, Deniz G, Valenta R, Fiebig H, et al. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. J. Exp. Med. 2004;199:1567–1575. doi: 10.1084/jem.20032058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali FR, Oldfield WL, Higashi N, Larche M, Kay AB. Late asthmatic reactions induced by inhalation of allergen-derived T cell peptides. Am. J. Respir. Crit. Care Med. 2004;169:20–26. doi: 10.1164/rccm.200305-690OC. [DOI] [PubMed] [Google Scholar]
- Bacchetta R, Passerini L, Gambineri E, Dai M, Allan SE, Perroni L, Dagna-Bricarelli F, Sartirana C, Matthes-Martin S, Lawitschka A, et al. Defective regulatory and effector T cell functions in patients with FOXP3 mutations. J. Clin. Invest. 2006;116:1713–1722. doi: 10.1172/JCI25112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N. Engl. J. Med. 2002;347:911–920. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- Barnes MJ, Powrie F. Regulatory T cells reinforce intestinal homeostasis. Immunity. 2009;31(this issue):401–411. doi: 10.1016/j.immuni.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Barrat FJ, Cua DJ, Boonstra A, Richards DF, Crain C, Savelkoul HF, de Waal-Malefyt R, Coffman RL, Hawrylowicz CM, O’Garra A. In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J. Exp. Med. 2002;195:603–616. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett N, Austen KF. Airway inflammation in asthma: Th2 cell immunity and beyond. Immunity. 2009;31(this issue):425–437. doi: 10.1016/j.immuni.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal RL, Campbell DE, Hwang P, DeKruyff RH, Frankel LR, Umetsu DT. Human alveolar macrophages induce functional inactivation in antigen-specific CD4 T cells. J. Allergy Clin. Immunol. 2001;107:258–264. doi: 10.1067/mai.2001.112845. [DOI] [PubMed] [Google Scholar]
- Campbell JD, Buckland KF, McMillan SJ, Kearley J, Oldfield WL, Stern LJ, Gronlund H, van Hage M, Reynolds CJ, Boyton RJ, et al. Peptide immunotherapy in allergic asthma generates IL-10-dependent immunological tolerance associated with linked epitope suppression. J. Exp. Med. 2009;206:1535–1547. doi: 10.1084/jem.20082901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudy AA, Reddy ST, Chatila T, Atkinson JP, Verbsky JW. CD25 deficiency causes an immune dysregulation, polyendocrinopathy, enteropathy, X-linked-like syndrome, and defective IL-10 expression from CD4 lymphocytes. J. Allergy Clin. Immunol. 2007;119:482–487. doi: 10.1016/j.jaci.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Chatila TA, Blaeser F, Ho N, Lederman HM, Voulgaropoulos C, Helms C, Bowcock AM. JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity-allergic disregulation syndrome. J. Clin. Invest. 2000;106:R75–R81. doi: 10.1172/JCI11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25-naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creticos PS, Schroeder JT, Hamilton RG, Balcer-Whaley SL, Khattignavong AP, Lindblad R, Li H, Coffman R, Seyfert V, Eiden JJ, Broide D. Immunotherapy with a ragweed-toll-like receptor 9 agonist vaccine for allergic rhinitis. N. Engl. J. Med. 2006;355:1445–1455. doi: 10.1056/NEJMoa052916. [DOI] [PubMed] [Google Scholar]
- Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, Mitsdoerffer M, Strom TB, Elyaman W, Ho IC, et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(—) effector T cells. Nat. Immunol. 2008;9:1347–1355. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deniz G, Erten G, Kucuksezer UC, Kocacik D, Karagiannidis C, Aktas E, Akdis CA, Akdis M. Regulatory NK cells suppress antigen-specific T cell responses. J. Immunol. 2008;180:850–857. doi: 10.4049/jimmunol.180.2.850. [DOI] [PubMed] [Google Scholar]
- Doherty T, Broide D. Cytokines and growth factors in airway remodeling in asthma. Curr. Opin. Immunol. 2007;19:676–680. doi: 10.1016/j.coi.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Ege MJ, Bieli C, Frei R, van Strien RT, Riedler J, Ublagger E, Schram-Bijkerk D, Brunekreef B, van Hage M, Scheynius A, et al. Prenatal farm exposure is related to the expression of receptors of the innate immunity and to atopic sensitization in school-age children. J. Allergy Clin. Immunol. 2006;117:817–823. doi: 10.1016/j.jaci.2005.12.1307. [DOI] [PubMed] [Google Scholar]
- Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J. Exp. Med. 2002;196:1645–1651. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattouh R, Midence NG, Arias K, Johnson JR, Walker TD, Goncharova S, Souza KP, Gregory RC, Jr., Lonning S, Gauldie J, Jordana M. Transforming growth factor-beta regulates house dust mite-induced allergic airway inflammation but not airway remodeling. Am. J. Respir. Crit. Care Med. 2008;177:593–603. doi: 10.1164/rccm.200706-958OC. [DOI] [PubMed] [Google Scholar]
- Gambineri E, Torgerson TR, Ochs HD. Immune dysregulation, polyendocrinopathy, enteropathy, and X-linked inheritance (IPEX), a syndrome of systemic autoimmunity caused by mutations of FOXP3, a critical regulator of T-cell homeostasis. Curr. Opin. Rheumatol. 2003;15:430–435. doi: 10.1097/00002281-200307000-00010. [DOI] [PubMed] [Google Scholar]
- Gereke M, Jung S, Buer J, Bruder D. Alveolar type II epithelial cells present antigen to CD4(+) T cells and induce Foxp3(+) regulatory T cells. Am. J. Respir. Crit. Care Med. 2009;179:344–355. doi: 10.1164/rccm.200804-592OC. [DOI] [PubMed] [Google Scholar]
- Gerhold K, Avagyan A, Seib C, Frei R, Steinle J, Ahrens B, Dittrich AM, Blumchen K, Lauener R, Hamelmann E. Prenatal initiation of endotoxin airway exposure prevents subsequent allergen-induced sensitization and airway inflammation in mice. J. Allergy Clin. Immunol. 2006;118:666–673. doi: 10.1016/j.jaci.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Goswami S, Angkasekwinai P, Shan M, Greenlee KJ, Barranco WT, Polikepahad S, Seryshev A, Song LZ, Redding D, Singh B, et al. Divergent functions for airway epithelial matrix metalloproteinase 7 and retinoic acid in experimental asthma. Nat. Immunol. 2009;10:496–503. doi: 10.1038/ni.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunig G, Corry DB, Leach MW, Seymour BW, Kurup VP, Rennick DM. Interleukin-10 is a natural suppressor of cytokine production and inflammation in a murine model of allergic bronchopulmonary aspergillosis. J. Exp. Med. 1997;185:1089–1099. doi: 10.1084/jem.185.6.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddeland U, Karstensen AB, Farkas L, Bo KO, Pirhonen J, Karlsson M, Kvavik W, Brandtzaeg P, Nakstad B. Putative regulatory T cells are impaired in cord blood from neonates with hereditary allergy risk. Pediatr. Allergy Immunol. 2005;16:104–112. doi: 10.1111/j.1399-3038.2005.00250.x. [DOI] [PubMed] [Google Scholar]
- Hansen G, McIntire JJ, Yeung VP, Berry G, Thorbecke GJ, Chen L, DeKruyff RH, Umetsu DT. CD4(+) T helper cells engineered to produce latent TGF-beta1 reverse allergen-induced airway hyperreactivity and inflammation. J. Clin. Invest. 2000;105:61–70. doi: 10.1172/JCI7589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl D, Koller B, Mehlhorn AT, Reinhardt D, Nicolai T, Schendel DJ, Griese M, Krauss-Etschmann S. Quantitative and functional impairment of pulmonary CD4+CD25hi regulatory T cells in pediatric asthma. J. Allergy Clin. Immunol. 2007;119:1258–1266. doi: 10.1016/j.jaci.2007.02.023. [DOI] [PubMed] [Google Scholar]
- Haselden BM, Kay AB, Larche M. Immunoglobulin E-independent major histocompatibility complex-restricted T cell peptide epitope-induced late asthmatic reactions. J. Exp. Med. 1999;189:1885–1894. doi: 10.1084/jem.189.12.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawrylowicz CM, O’Garra A. Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nat. Rev. Immunol. 2005;5:271–283. doi: 10.1038/nri1589. [DOI] [PubMed] [Google Scholar]
- Hawrylowicz C, Richards D, Loke TK, Corrigan C, Lee T. A defect in corticosteroid-induced IL-10 production in T lymphocytes from corti costeroid-resistant asthmatic patients. J. Allergy Clin. Immunol. 2002;109:369–370. doi: 10.1067/mai.2002.121455. [DOI] [PubMed] [Google Scholar]
- Higgins SC, Lavelle EC, McCann C, Keogh B, McNeela E, Byrne P, O’Gorman B, Jarnicki A, McGuirk P, Mills KH. Toll-like receptor 4-mediated innate IL-10 activates antigen-specific regulatory T cells and confers resistance to Bordetella pertussis by inhibiting inflammatory pathology. J. Immunol. 2003;171:3119–3127. doi: 10.4049/jimmunol.171.6.3119. [DOI] [PubMed] [Google Scholar]
- Holick MF. Vitamin D deficiency. N. Engl. J. Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- Hollingsworth JW, Maruoka S, Boon K, Garantziotis S, Li Z, Tomfohr J, Bailey N, Potts EN, Whitehead G, Brass DM, Schwartz DA. In utero supplementation with methyl donors enhances allergic airway disease in mice. J. Clin. Invest. 2008;118:3462–3469. doi: 10.1172/JCI34378. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Holt PG, Batty JE, Turner KJ. Inhibition of specific IgE responses in mice by pre-exposure to inhaled antigen. Immunology. 1981;42:409–417. [PMC free article] [PubMed] [Google Scholar]
- Holt PG, Strickland DH, Wikstrom ME, Jahnsen FL. Regulation of immunological homeostasis in the respiratory tract. Nat. Rev. Immunol. 2008;8:142–152. doi: 10.1038/nri2236. [DOI] [PubMed] [Google Scholar]
- Iellem A, Mariani M, Lang R, Recalde H, Panina-Bordignon P, Sinigaglia F, D’Ambrosio D. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J. Exp. Med. 2001;194:847–853. doi: 10.1084/jem.194.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, Qin FX, Yao Z, Cao W, Liu YJ. TSLP-activated dendritic cells induce an inflammatory T helper type 2 response through OX40 ligand. J. Exp. Med. 2005;20:1213–1223. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joetham A, Takada K, Taube C, Miyahara N, Matsubara S, Koya T, Rha YH, Dakhama A, Gelfand EW. Naturally Occurring Lung CD4+CD25+ T Cell Regulation of Airway Allergic Responses Depends on IL-10 Induction of TGF-beta. J. Immunol. 2007;178:1433–1442. doi: 10.4049/jimmunol.178.3.1433. [DOI] [PubMed] [Google Scholar]
- John M, Lim S, Seybold J, Jose P, Robichaud A, O’Connor B, Barnes PJ, Chung KF. Inhaled corticosteroids increase interleukin-10 but reduce macrophage inflammatory protein-1alpha, granulocyte-macrophage colony-stimulating factor, and interferon-gamma release from alveolar macrophages in asthma. Am. J. Respir. Crit. Care Med. 1998;157:256–262. doi: 10.1164/ajrccm.157.1.9703079. [DOI] [PubMed] [Google Scholar]
- Karagiannidis C, Akdis M, Holopainen P, Woolley NJ, Hense G, Ruckert B, Mantel PY, Menz G, Akdis CA, Blaser K, Schmidt-Weber CB. Glucocorticoids upregulate FOXP3 expression and regulatory T cells in asthma. J. Allergy Clin. Immunol. 2004;114:1425–1433. doi: 10.1016/j.jaci.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Karagiannidis C, Hense G, Martin C, Epstein M, Ruckert B, Mantel PY, Menz G, Uhlig S, Blaser K, Schmidt-Weber CB. Activin A is an acute allergen-responsive cytokine and provides a link to TGF-beta-mediated airway remodeling in asthma. J. Allergy Clin. Immunol. 2006;117:111–118. doi: 10.1016/j.jaci.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Kariyawasam HH, Xanthou G, Barkans J, Aizen M, Kay AB, Robinson DS. Basal expression of bone morphogenetic protein receptor is reduced in mild asthma. Am. J. Respir. Crit. Care Med. 2008;177:1074–1081. doi: 10.1164/rccm.200709-1376OC. [DOI] [PubMed] [Google Scholar]
- Kearley J, Barker JE, Robinson DS, Lloyd CM. Resolution of airway inflammation and hyperreactivity after in vivo transfer of CD4+CD25+ regulatory T cells is interleukin 10 dependent. J. Exp. Med. 2005;202:1539–1547. doi: 10.1084/jem.20051166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearley J, Robinson DS, Lloyd CM. CD4+CD25+ regulatory T cells reverse established allergic airway inflammation and prevent airway remodeling. J. Allergy Clin. Immunol. 2008;122:617–624. doi: 10.1016/j.jaci.2008.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrecht BN. Biology of lung dendritic cell subsets at the origin of asthma. Immunity. 2009;31(this issue):412–424. doi: 10.1016/j.immuni.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Larche M. Update on the current status of peptide immunotherapy. J. Allergy Clin. Immunol. 2007;119:906–909. doi: 10.1016/j.jaci.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Leech MD, Benson RA, De Vries A, Fitch PM, Howie SE. Resolution of Der p1-induced allergic airway inflammation is dependent on CD4+CD25+Foxp3+ regulatory cells. J. Immunol. 2007;179:7050–7058. doi: 10.4049/jimmunol.179.10.7050. [DOI] [PubMed] [Google Scholar]
- Leggat JA, Gibbons DL, Haque SF, Smith AL, Wells JW, Choy K, Lloyd CM, Hayday AC, Noble A. Innate responsiveness of CD8 memory T-cell populations nonspecifically inhibits allergic sensitization. J. Allergy Clin. Immunol. 2008;122:1014–1021. doi: 10.1016/j.jaci.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewkowich IP, Herman NS, Schleifer KW, Dance MP, Chen BL, Dienger KM, Sproles AA, Shah JS, Kohl J, Belkaid Y, Wills-Karp M. CD4+CD25+ T cells protect against experimentally induced asthma and alter pulmonary dendritic cell phenotype and function. J. Exp. Med. 2005;202:1549–1561. doi: 10.1084/jem.20051506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu. Rev. Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- Lim S, Crawley E, Woo P, Barnes PJ. Haplotype associated with low interleukin-10 production in patients with severe asthma. Lancet. 1998;352:113. doi: 10.1016/S0140-6736(98)85018-6. [DOI] [PubMed] [Google Scholar]
- Lin W, Truong N, Grossman WJ, Haribhai D, Williams CB, Wang J, Martin MG, Chatila TA. Allergic dysregulation and hyperimmunoglobulinemia E in Foxp3 mutant mice. J. Allergy Clin. Immunol. 2005;116:1106–1115. doi: 10.1016/j.jaci.2005.08.046. [DOI] [PubMed] [Google Scholar]
- Ling EM, Smith T, Nguyen XD, Pridgeon C, Dallman M, Arbery J, Carr VA, Robinson DS. Relation of CD4+CD25+ regulatory T-cell suppression of allergen-driven T-cell activation to atopic status and expression of allergic disease. Lancet. 2004;363:608–615. doi: 10.1016/S0140-6736(04)15592-X. [DOI] [PubMed] [Google Scholar]
- Litonjua AA, Weiss ST. Is vitamin D deficiency to blame for the asthma epidemic? J. Allergy Clin. Immunol. 2007;120:1031–1035. doi: 10.1016/j.jaci.2007.08.028. [DOI] [PubMed] [Google Scholar]
- Lloyd CM. Building better mouse models of asthma. Curr. Allergy Asthma Rep. 2007;7:231–236. doi: 10.1007/s11882-007-0077-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd CM, Delaney T, Nguyen T, Tian J, Martinez AC, Coyle AJ, Gutierrez-Ramos JC. CC chemokine receptor (CCR)3/eotaxin is followed by CCR4/monocyte-derived chemokine in mediating pulmonary T helper lymphocyte type 2 recruitment after serial antigen challenge in vivo. J. Exp. Med. 2000;191:265–274. doi: 10.1084/jem.191.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund JM, Hsing L, Pham TT, Rudensky AY. Coordination of early protective immunity to viral infection by regulatory T cells. Science. 2008;320:1220–1224. doi: 10.1126/science.1155209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon BD, Gordon SA, Cruz J, Cosman F, Cantorna MT. Cytokine profile in patients with multiple sclerosis following vitamin D supplementation. J. Neuroimmunol. 2003;134:128–132. doi: 10.1016/s0165-5728(02)00396-x. [DOI] [PubMed] [Google Scholar]
- Makinde T, Murphy RF, Agrawal DK. The regulatory role of TGF-beta in airway remodeling in asthma. Immunol. Cell Biol. 2007;85:348–356. doi: 10.1038/sj.icb.7100044. [DOI] [PubMed] [Google Scholar]
- Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N. Engl. J. Med. 1995;332:133–138. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004;59:469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- McMillan SJ, Xanthou G, Lloyd CM. Manipulation of Allergen-Induced Airway Remodeling by Treatment with Anti-TGF-{beta} Antibody: Effect on the Smad Signaling Pathway. J. Immunol. 2005;174:5774–5780. doi: 10.4049/jimmunol.174.9.5774. [DOI] [PubMed] [Google Scholar]
- Meiler F, Zumkehr J, Klunker S, Ruckert B, Akdis CA, Akdis M. In vivo switch to IL-10-secreting T regulatory cells in high dose allergen exposure. J. Exp. Med. 2008;205:2887–2898. doi: 10.1084/jem.20080193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Message SD, Laza-Stanca V, Mallia P, Parker HL, Zhu J, Kebadze T, Contoli M, Sanderson G, Kon OM, Papi A, et al. Rhinovirus-induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL-10 production. Proc. Natl. Acad. Sci. USA. 2008;105:13562–13567. doi: 10.1073/pnas.0804181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RL. Prenatal maternal diet affects asthma risk in offspring. J. Clin. Invest. 2008;118:3265–3268. doi: 10.1172/JCI37171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller C, Dreborg S, Ferdousi HA, Halken S, Host A, Jacobsen L, Koivikko A, Koller DY, Niggemann B, Norberg LA, et al. Pollen immunotherapy reduces the development of asthma in children with seasonal rhinoconjunctivitis (the PAT-study) J. Allergy Clin. Immunol. 2002;109:251–256. doi: 10.1067/mai.2002.121317. [DOI] [PubMed] [Google Scholar]
- Morgan WJ, Stern DA, Sherrill DL, Guerra S, Holberg CJ, Guilbert TW, Taussig LM, Wright AL, Martinez FD. Outcome of asthma and wheezing in the first 6 years of life: follow-up through adolescence. Am. J. Respir. Crit. Care Med. 2005;172:1253–1258. doi: 10.1164/rccm.200504-525OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao A, Miike S, Hatano M, Okumura K, Tokuhisa T, Ra C, Iwamoto I. Blockade of transforming growth factor beta/Smad signaling in T cells by overexpression of Smad7 enhances antigen-induced airway inflammation and airway reactivity. J. Exp. Med. 2000;192:151–158. doi: 10.1084/jem.192.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak N, Bieber T, Katoh N. Engagement of Fc epsilon RI on human monocytes induces the production of IL-10 and prevents their differentiation in dendritic cells. J. Immunol. 2001;167:797–804. doi: 10.4049/jimmunol.167.2.797. [DOI] [PubMed] [Google Scholar]
- O’Garra A, Barrat FJ, Castro AG, Vicari A, Hawrylowicz C. Strategies for use of IL-10 or its antagonists in human disease. Immunol. Rev. 2008;223:114–131. doi: 10.1111/j.1600-065X.2008.00635.x. [DOI] [PubMed] [Google Scholar]
- Oh JW, Seroogy CM, Meyer EH, Akbari O, Berry G, Fathman CG, Dekruyff RH, Umetsu DT. CD4 T-helper cells engineered to produce IL-10 prevent allergen-induced airway hyperreactivity and inflammation. J. Allergy Clin. Immunol. 2002;110:460–468. doi: 10.1067/mai.2002.127512. [DOI] [PubMed] [Google Scholar]
- Ostroukhova M, Seguin-Devaux C, Oriss TB, Dixon-McCarthy B, Yang L, Ameredes BT, Corcoran TE, Ray A. Tolerance induced by inhaled antigen involves CD4(+) T cells expressing membrane-bound TGF-beta and FOXP3. J. Clin. Invest. 2004;114:28–38. doi: 10.1172/JCI20509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penna G, Roncari A, Amuchastegui S, Daniel KC, Berti E, Colonna M, Adorini L. Expression of the inhibitory receptor ILT3 on dendritic cells is dispensable for induction of CD4+Foxp3+ regulatory T cells by 1,25-dihydroxyvitamin D3. Blood. 2005;106:3490–3497. doi: 10.1182/blood-2005-05-2044. [DOI] [PubMed] [Google Scholar]
- Pereira-Santos MC, Baptista AP, Melo A, Alves RR, Soares RS, Pedro E, Pereira-Barbosa M, Victorino RM, Sousa AE. Expansion of circulating Foxp(3+)D25bright CD4+ T cells during specific venom immunotherapy. Clin. Exp. Allergy. 2008;38:291–297. doi: 10.1111/j.1365-2222.2007.02887.x. [DOI] [PubMed] [Google Scholar]
- Polte T, Hennig C, Hansen G. Allergy prevention starts before conception: maternofetal transfer of tolerance protects against the development of asthma. J. Allergy Clin. Immunol. 2008;122:1022–1030. doi: 10.1016/j.jaci.2008.09.014. [DOI] [PubMed] [Google Scholar]
- Provoost S, Maes T, van Durme YM, Gevaert P, Bachert C, Schmidt-Weber CB, Brusselle GG, Joos GF, Tournoy KG. Decreased FOXP3 protein expression in patients with asthma. Allergy. 2009 doi: 10.1111/j.1398-9995.2009.02056.x. in press. Published online April 9, 2009. 10.1111/j.1398-9995.2009.02056.x. [DOI] [PubMed] [Google Scholar]
- Radulovic S, Jacobson MR, Durham SR, Nouri-Aria KT. Grass pollen immunotherapy induces Foxp3-expressing CD4+ CD25+ cells in the nasal mucosa. J. Allergy Clin. Immunol. 2008;121:1467–1472. doi: 10.1016/j.jaci.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Rao A, Kamani N, Filipovich A, Lee SM, Davies SM, Dalal J, Shenoy S. Successful bone marrow transplantation for IPEX syndrome after reduced-intensity conditioning. Blood. 2007;109:383–385. doi: 10.1182/blood-2006-05-025072. [DOI] [PubMed] [Google Scholar]
- Riley JL, June CH, Blazar BR. Human T regulatory cell therapy: take a billion or so and call me in the morning. Immunity. 2009;30:656–665. doi: 10.1016/j.immuni.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roifman CM. Human IL-2 receptor alpha chain deficiency. Pediatr. Res. 2000;48:6–11. doi: 10.1203/00006450-200007000-00004. [DOI] [PubMed] [Google Scholar]
- Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol. Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR, Jr., et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- Ryanna K, Stratigou V, Safinia N, Hawrylowicz C. Regulatory T cells in bronchial asthma. Allergy. 2009;64:335–347. doi: 10.1111/j.1398-9995.2009.01972.x. [DOI] [PubMed] [Google Scholar]
- Saglani S, Payne DN, Zhu J, Wang Z, Nicholson AG, Bush A, Jeffery PK. Early detection of airway wall remodeling and eosinophilic inflammation in preschool wheezers. Am. J. Respir. Crit. Care Med. 2007;176:858–864. doi: 10.1164/rccm.200702-212OC. [DOI] [PubMed] [Google Scholar]
- Sather BD, Treuting P, Perdue N, Miazgowicz M, Fontenot JD, Rudensky AY, Campbell DJ. Altering the distribution of Foxp3(+) regulatory T cells results in tissue-specific inflammatory disease. J. Exp. Med. 2007;204:1335–1347. doi: 10.1084/jem.20070081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub B, Liu J, Hoppler S, Haug S, Sattler C, Lluis A, Illi S, von Mutius E. Impairment of T-regulatory cells in cord blood of atopic mothers. J. Allergy Clin. Immunol. 2008;121:1491–1499. doi: 10.1016/j.jaci.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Scherf W, Burdach S, Hansen G. Reduced expression of transforming growth factor beta 1 exacerbates pathology in an experimental asthma model. Eur. J. Immunol. 2005;35:198–206. doi: 10.1002/eji.200425209. [DOI] [PubMed] [Google Scholar]
- Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am. J. Clin. Nutr. 2006;83:754–759. doi: 10.1093/ajcn/83.4.754. [DOI] [PubMed] [Google Scholar]
- Semitekolou M, Alissafi T, Aggelakopoulou M, Kourepini E, Kariyawasam HH, Kay AB, Robinson DS, Lloyd CM, Panoutsakopoulou V, Xanthou G. Activin-A induces regulatory T cells that suppress T helper cell immune responses and protect from allergic airway disease. J. Exp. Med. 2009;206:1769–1785. doi: 10.1084/jem.20082603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel MG, Fritsch G, Lion T, Jurgens B, Heitger A, Bacchetta R, Lawitschka A, Peters C, Gadner H, Matthes-Martin S. Selective engraftment of donor CD4+25high FOXP3-positive T cells in IPEX syndrome after nonmyeloablative hematopoietic stem cell transplantation. Blood. 2009;113:5689–5691. doi: 10.1182/blood-2009-02-206359. [DOI] [PubMed] [Google Scholar]
- Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, et al. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sly PD, Boner AL, Bjorksten B, Bush A, Custovic A, Eigenmann PA, Gern JE, Gerritsen J, Hamelmann E, Helms PJ, et al. Early identification of atopy in the prediction of persistent asthma in children. Lancet. 2008;372:1100–1106. doi: 10.1016/S0140-6736(08)61451-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M, Tourigny MR, Noakes P, Thornton CA, Tulic MK, Prescott SL. Children with egg allergy have evidence of reduced neonatal CD4(+)CD25(+)CD127(lo/-) regulatory T cell function. J. Allergy Clin. Immunol. 2008;121:1460–1466. doi: 10.1016/j.jaci.2008.03.025. [DOI] [PubMed] [Google Scholar]
- Stampfli MR, Cwiartka M, Gajewska BU, Alvarez D, Ritz SA, Inman MD, Xing Z, Jordana M. Interleukin-10 gene transfer to the airway regulates allergic mucosal sensitization in mice. Am. J. Respir. Cell Mol. Biol. 1999;21:586–596. doi: 10.1165/ajrcmb.21.5.3755. [DOI] [PubMed] [Google Scholar]
- Stelmach I, Jerzynska J, Kuna P. A randomized, double-blind trial of the effect of glucocorticoid, antileukotriene and beta-agonist treatment on IL-10 serum levels in children with asthma. Clin. Exp. Allergy. 2002;32:264–269. doi: 10.1046/j.1365-2222.2002.01286.x. [DOI] [PubMed] [Google Scholar]
- Strickland DH, Stumbles PA, Zosky GR, Subrata LS, Thomas JA, Turner DJ, Sly PD, Holt PG. Reversal of airway hyperresponsiveness by induction of airway mucosal CD4+CD25+ regulatory T cells. J. Exp. Med. 2006;203:2649–2660. doi: 10.1084/jem.20060155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Madan R, Karp CL, Braciale TJ. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat. Med. 2009;15:277–284. doi: 10.1038/nm.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutmuller RP, Morgan ME, Netea MG, Grauer O, Adema GJ. Toll-like receptors on regulatory T cells: expanding immune regulation. Trends Immunol. 2006;27:387–393. doi: 10.1016/j.it.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Szefler SJ. Airway remodeling: therapeutic target or not? Am. J. Respir. Crit. Care Med. 2005;171:672–673. doi: 10.1164/rccm.2501001. [DOI] [PubMed] [Google Scholar]
- Taher YA, van Esch BC, Hofman GA, Henricks PA, van Oosterhout AJ. 1alpha,25-dihydroxyvitamin D3 potentiates the beneficial effects of allergen immunotherapy in a mouse model of allergic asthma: role for IL-10 and TGF-beta. J. Immunol. 2008;180:5211–5221. doi: 10.4049/jimmunol.180.8.5211. [DOI] [PubMed] [Google Scholar]
- Tighe H, Takabayashi K, Schwartz D, Van Nest G, Tuck S, Eiden JJ, Kagey-Sobotka A, Creticos PS, Lichtenstein LM, Spiegelberg HL, Raz E. Conjugation of immunostimulatory DNA to the short ragweed allergen amb a 1 enhances its immunogenicity and reduces its allergenicity. J. Allergy Clin. Immunol. 2000;106:124–134. doi: 10.1067/mai.2000.107927. [DOI] [PubMed] [Google Scholar]
- Till SJ, Francis JN, Nouri-Aria K, Durham SR. Mechanisms of immunotherapy. J. Allergy Clin. Immunol. 2004;113:1025–1034. doi: 10.1016/j.jaci.2004.03.024. [DOI] [PubMed] [Google Scholar]
- Torgerson TR, Ochs HD. Immune dysregulation, polyendocrinopathy, enteropathy, X-linked: forkhead box protein 3 mutations and lack of regulatory T cells. J. Allergy Clin. Immunol. 2007;120:744–750. doi: 10.1016/j.jaci.2007.08.044. [DOI] [PubMed] [Google Scholar]
- Urry Z, Xystrakis E, Richards D, McDonald J, Sattar Z, Cousins D, Corrigan C, Hickman E, Brown Z, Hawrylowicz C. Ligation of TLR9 induced on human IL-10-secreting Tregs by 1a,25-dihydroxyvitamin D3 abrogates regulatory function. J. Clin. Invest. 2009;119:387–398. doi: 10.1172/JCI32354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen M, Uyttenhove C, van Snick J, Helmby H, Westendorf A, Buer J, Martin B, Wilhelm C, Stockinger B. Transforming growth factor-beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat. Immunol. 2008;9:1341–1346. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- Verhasselt V, Milcent V, Cazareth J, Kanda A, Fleury S, Dombrowicz D, Glaichenhaus N, Julia V. Breast milk-mediated transfer of an antigen induces tolerance and protection from allergic asthma. Nat. Med. 2008;14:170–175. doi: 10.1038/nm1718. [DOI] [PubMed] [Google Scholar]
- Verhoef A, Alexander C, Kay AB, Larche M. T cell epitope immunotherapy induces a CD4+ T cell population with regulatory activity. PLoS Med. 2005;2:e78. doi: 10.1371/journal.pmed.0020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat. Rev. Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willers SM, Wijga AH, Brunekreef B, Kerkhof M, Gerritsen J, Hoekstra MO, de Jongste JC, Smit HA. Maternal food consumption during pregnancy and the longitudinal development of childhood asthma. Am. J. Respir. Crit. Care Med. 2008;178:124–131. doi: 10.1164/rccm.200710-1544OC. [DOI] [PubMed] [Google Scholar]
- Xystrakis E, Kusumakar S, Boswell S, Peek E, Urry Z, Richards DF, Adikibi T, Pridgeon C, Dallman M, Loke TK, et al. Reversing the defective induction of IL-10-secreting regulatory T cells in glucocorticoid-resistant asthma patients. J. Clin. Invest. 2006;116:146–155. doi: 10.1172/JCI21759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Rudensky AY. Foxp3 in control of the regulatory T cell lineage. Nat. Immunol. 2007;8:457–462. doi: 10.1038/ni1455. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Chaudhry A, Kas A, deRoos P, Kim JM, Chu TT, Corcoran L, Treuting P, Klein U, Rudensky AY. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature. 2009;458:351–356. doi: 10.1038/nature07674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuany-Amorim C, Haile S, Dumarey C, Huerre M, Vargaftig BB, Pretolani M. Interleukin-10 inhibits antigen-induced cellular recruitment into the airways of sensitized mice. J. Clin. Invest. 1995;95:2644–2651. doi: 10.1172/JCI117966. [DOI] [PMC free article] [PubMed] [Google Scholar]