Abstract
India leads the world with largest number of diabetics earning the dubious distinction of “the diabetes capital of the world.” Diabetes is associated with maternal and perinatal morbidity and mortality. The number of pregnant women with pre-existing diabetes is increasing, mainly from an increase in type 2 diabetes, but also an increase in type 1 diabetes. Overall, type 1 diabetes accounts for approximately 5% to 10% of all diabetes outside of pregnancy, and in pregnancy put together with type 2 account for 10% of diabetic pregnancies. Management of the pregnant diabetic woman is a complex task that ideally begins before conception. Specific attention is required for diabetic pregnancies in different trimesters of pregnancy. Diabetes, especially type 1 diabetes, can be a challenge in pregnancy, but with education, close monitoring, and latest therapeutic modalities, these women can have healthy newborns. Close attention to diet, glycemic control, metabolic stresses, and early diagnosis and monitoring of complications can make pregnancy a successful experience for women with diabetes. A MedLine search was done to review relevant articles in English literature on diabetes and pregnancy, and specific issues related to pregnancy in type 1 diabetes were reviewed.
Keywords: Gestational diabetes mellitus, Insulin, Pregnancy, Type 1 diabetes mellitus
Introduction
India leads the world with largest number of diabetics earning the dubious distinction of “the diabetes capital of the world.” It was estimated to have had 31.7 million people having diabetes in year 2000, which is projected to be 79.4 million in 2030. Bothersome is a 151% projected increase in number of people with diabetes vis a vis just a 40% projected increase in population of India during the same period.[1] According to the Diabetes Atlas 2009 published by the International Diabetes Federation, the number of people with diabetes in India in year 2010 was reported to be around 50.8 million, which is expected to rise to 69.9 million by 2025 unless urgent preventive steps are taken.[2]
Diabetes mellitus (DM) has long been associated with maternal and perinatal morbidity and mortality. Before insulin was discovered in 1921, diabetic women rarely reached reproductive age or survived pregnancy. In fact, pregnancy termination was routinely recommended for women with diabetes because of high mortality rates. But with advent of insulin, scenario changed. Diabetes and pregnancy are of utmost importance as this is the phase, which would decide whether diabetes would span the generations or can be limited. There are genetic factors that pass from generation to generation but there is also environmental component, the metabolic environment that women create for their fetuses in utero.
Approximately 87.5% of pregnancies complicated by diabetes are estimated to be due to gestational diabetes (which may or may not resolve after pregnancy), with 7.5% being due to type 1 diabetes and the remaining 5% being due to type 2 diabetes. The number of pregnant women with pre-existing diabetes is increasing, mainly from an increase in type 2 diabetes, but also an increase in type 1 diabetes.[3,4] Thus, the knowledge and management of this condition in pregnancy has become important. Reasons for the increase in type 1 diabetes are somewhat unclear but may be related to harmful environmental conditions. This review shall ponder upon certain issues unique to pregnancies on women with type 1 diabetes. However, specific management of diabetes shall be out of preview of present review and shall be dealt elsewhere.
Diabetes and Pregnancy
Diabetes during pregnancy is still generally classified using the original system proposed by Priscilla White almost 60 years ago.[5] White's classification relates the onset of diabetes, its duration, and the degree of vasculopathy to the outcome of pregnancy. Because there were differences and some confusion in the interpretation of class A diabetes, particularly when the patient required insulin for therapy, a revision made by Hare and White proposed that class A diabetes should include women known to have diabetes before pregnancy and who are treated with diet alone.[6] Thus, White's class ‘A’ classification includes only patients with pre-gestational diabetes and defines gestational diabetes as a completely separate group.
Practically speaking, women with pregnancies complicated by diabetes mellitus may be separated into one of two groups:
Gestational diabetes: women with carbohydrate intolerance of variable severity, with onset or first recognition during the present pregnancy.
Pre-gestational diabetes: women known to have diabetes before pregnancy.
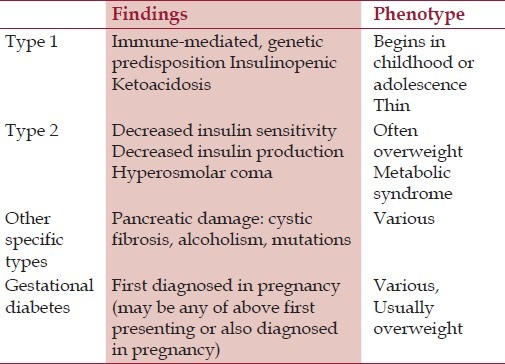
Although the White's classification is still valuable, the more recent diabetes classification from the Expert Committee on the Diagnosis and Classification of Diabetes,[7] summarized in Table 1, may be more useful in patient management because it alerts clinicians to the type of diabetes, which may have somewhat different treatment strategies. Overall, type 1 diabetes accounts for approximately 5% to 10% of all diabetes outside of pregnancy, and type 2 diabetes for 90% to 95%. Ninety percent of all pregnant diabetic patients have gestational diabetes mellitus (GDM), whereas type 1 (formally also called insulin dependent diabetes) and type 2 (formally also called noninsulin-dependent diabetes) account for the remaining 10%.[8] The clinical distinction between the ketosis-prone type 1 and the non-ketosis prone type 2 has been recognized by clinicians for many years, but the two types were thought to represent differences in the expression of a single disease. Later, it was discovered that type 1 is a human leukocyte antigen (HLA)-linked disorder, whereas type 2 is not, and thus they are two different diseases genetically.[9,10] In general, type 1 and type 2 can be distinguished from each other using clinical criteria or islet cell antibody (ICA) studies. Also, whereas for most of the patients of GDM, non-insulin management in form of medical nutrition therapy (MNT) and oral hypoglycemic agents (OHAs) shall suffice,[11] a substantial number of patients with type-1 DM shall require antenatal insulin therapy (AIT).
Table 1.
Diabetes Classification[7]
Genetics of type 1 diabetes mellitus
In the past few years, it has become increasingly clear that autoimmunity plays a key role in type 1 diabetes.[12,13] It is currently believed that type 1 diabetes mellitus is actually a slow process in which insulin-secreting cells are gradually destroyed, leading to islet cell failure and hyperglycemia. The exact mechanism of the inheritance of type 1 diabetes is not known. Formerly, it was suggested that the risk of inheriting diabetes in offspring with one affected parent was in the range of 1–6%.[14] Based on recent information,[15] it has become clear that type 1 diabetes is transmitted less frequently to the offspring of diabetic mothers (1%) than to children of diabetic fathers (6%). This preferential paternal transmission rate may be related to greater transfer of DR4 alleles to the offspring of DR4 fathers than to the offspring of DR4 mothers.[16] Family studies have shown that the estimated risk of type 1 diabetes in offspring in a family with one affected sibling but unaffected parents is 5–6%.[17]
Metabolism in a diabetic pregnancy
Islet cells’ function is different in type 1 than in type 2 diabetes. There is a difference in insulin secretion between the two forms of diabetes. C-peptide, which is the connecting peptide between the A and B chains of insulin, has been found to be higher in pregnant women with type 2 diabetes than in normal control subjects. However, in type 1 diabetes, C-peptide levels have been found to be very low or almost undetectable, which indicates that there is no residual B-cell function.[18] It has also been shown that in patients with type 1 diabetes, the increase in insulin requirement is almost 40%, whereas in type 2 it can be much higher, reaching as high as 100%.[19] It is also known that maternal fuel levels other than that of glucose can be abnormal in diabetic pregnant patients. Plasma triglyceride concentrations in pregnant patients with type 1 diabetes during the third trimester have been reported higher than normal.[20] FFA levels have also been found to be elevated in pregnant patients with type 1 diabetes. There seems to be a correlation between these concentrations and neonatal birth weight.[21] In summary, the metabolic disturbances in diabetic pregnant patients are expressed in increased concentrations of circulating metabolic fuels, including carbohydrate, protein, and fat. This increased circulating maternal level can be transferred to the fetus and may contribute to the development of fetal macrosomia.[20,22]
Fetal Complications
Women with diabetes are at an increased risk for first trimester miscarriage, congenital malformations, IUGR, macrosomia, birth trauma, stillbirth, and iatrogenic preterm delivery. The neonate is at risk for hypoglycemia, hypocalcemia, hyperbilirubinemia, polycythemia, and morbidity and mortality from congenital malformations or severe prematurity. Children of mothers with diabetes are at risk for obesity, glucose intolerance, and cardiovascular disease later in life.
Congenital anomalies in infants of mothers with type 1 diabetes
The frequency of major congenital anomalies among infants of diabetic mothers has been estimated as 6–10%, which represents a two- to five-fold increase over the frequency observed in the general population.[23] The risk for malformations in a fetus of a mother with a normal HbA1c level is only slightly greater than that for the general population; newborns of women with a conception HbA1c greater than 10% have an approximately 22% probability of having congenital malformations. These malformations usually involve multiple organ systems, with cardiac anomalies being the most common, followed by central nervous system and skeletal malformations.[24] Studies indicate that diabetes associated birth defects occur after disruption of developmental processes during organogenesis and are associated with abnormal metabolism, which is thought to be related mostly to hyperglycemia.[25] In vivo studies in rats suggest a cause and effect relationship between altered glucose metabolism and congenital anomalies; however, the target site of action remains unknown.[26] Few studies have suggested that that the yolk sac is the primary target site for the adverse metabolic effect of diabetes and that embryonic malformations occur as a secondary phenomenon to the primary yolk sac damage.[26] Factors other than hyperglycemia have been implicated in diabetes-associated birth defects, including ketone bodies, hypoglycemia, low levels of trace metals, and somatomedin-inhibiting factors.[27,28] A correlation between maternal oxygen-free radicals produced in excess in patients with diabetes mellitus and the induction of fetal anomalies has also been suggested. Clinical studies suggest that euglycemia during organogenesis is critical in the prevention of congenital anomalies.[29]
Still-birth
The stillbirth rate in women with diabetes is approximately 5.8 of every 1000 births.[30] Approximately half of these stillbirth or fetal deaths are related to hyperglycemia, and the remainder caused by infection or congenital anomalies.[31] Studies with fetal blood sampling confirm that hyperglycemia has been associated with fetal hypoxia and acidosis. A recent meta-analysis compared type 1 and 2 diabetes and found that women with type 2 had lower HA1c at the first visit but a higher rate of perinatal mortality.[32] Despite a milder glycemic disturbance, women with type 2 diabetes had no better perinatal outcomes than those with type 1.
Maternal Complications
Medical complications of diabetes and pregnancy include those specifically related to diabetes and an increased risk for pre-eclampsia.
Diabetic nephropathy complicates approximately 5% of pregnancies in women with pre-existing diabetes. Most affected pregnancies are in women with type 1 diabetes. Disease progression is characterized by hypertension and deteriorating glomerular filtrations rate. Progression of diabetic nephropathy can be attenuated by aggressive treatment of hypertension and intensive glycemic control.[33] Some women with diabetic nephropathy display the expected increase in glomerular filtration noted in normal pregnancy; others do not experience a significant increase. Women with overt diabetic nephropathy experience increased proteinuria in pregnancy. The greater the proteinuria at onset of pregnancy, the greater is its increase during the pregnancy. Protein excretion can double or triple in the third trimester compared with the first, and can confuse the diagnosis of pre-eclampsia. Approximately 50% of women deliver preterm iatrogenically because of maternal or fetal indications, 15% have fetuses with intrauterine growth restriction (IUGR), and pre-eclampsia occurs in approximately 50%. Women with a prepregnancy creatinine of greater than 1.5 mg/ dL have the highest perinatal complication rate.[34] Antihypertensive therapy delays progression of diabetic nephropathy. Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers have clearly been shown to be superior in slowing progression of microalbuminuria in women with diabetes with and without hypertension.[35] Unfortunately, these medications are teratogenic throughout pregnancy and cannot be used during pregnancy.[36]
Diabetic retinopathy, still one of the leading causes of blindness and visual disability in the world, is most often associated with long-standing type 1 diabetes. Evidence shows that diabetic retinopathy advances with pregnancy, at least for the short term.[37] However, pregnancy does not seem to have long-term consequences on diabetic retinopathy. Controversy exists over whether the microvascular changes in the eye are from pregnancy itself or the rapid improvement of glycemic control that occurs in some women when pregnancy is discovered. Factors associated with progression of diabetic retinopathy in pregnancy are the duration of type 1 diabetes, the presence of chronic hypertension or pre-eclampsia, the degree of hyperglycemia, poor glycemic control at conception, and the stage of disease at onset of pregnancy.[38] Fluid retention, vasodilation, and increased blood flow in pregnancy are believed to accelerate the loss of autoregulatory function of the retinal capillary bed. Diabetic retinopathy can be classified as background, preproliferative, or proliferative, depending on progression. Progression from nonproliferative to proliferative retinopathy ranges from 6% to 30% depending on severity.[39] The treatment of diabetic retinopathy using laser photocoagulation is as effective in pregnancy as outside of pregnancy and should not be delayed.
Diabetic neuropathy in pregnancy has not been well studied. A short-term increase in distal symmetric polyneuropathy may occur in association with pregnancy, but at least in one study the increase appeared to be transient.[40] Women with diabetic gastroparesis may experience more protracted nausea and vomiting of pregnancy. This complication should be considered and treated. Coronary artery disease is not commonly seen in pregnant women with diabetes. Information related to the incidence of coronary artery disease in pregnant women with diabetes is sparse and only case reports exist in the literature. Data are insufficient to extrapolate recommendations. However, women with pre-existing angina or myocardial infarction should generally not be encouraged to become pregnant, particularly if they have diminished cardiac function.
Diabetic ketoacidosis (DKA) is an uncommon occurrence in treatment-compliant women with type 1 diabetes, despite the increased risk for this complication associated with the ketogenesis of normal pregnancy. However, DKA is a common complication in undiagnosed diabetes. Any pregnant woman with vomiting or dehydration and blood sugars greater than 200 mg/dL should have electrolytes, plasma bicarbonate, and serum acetone levels measured to confirm DKA diagnosis. Arterial blood gasses should be obtained if the plasma bicarbonate is low and acetone is present. The precipitant of DKA is often infection, which should be diagnosed and treated promptly. Resolution of DKA can be slower in pregnancy. DKA is often associated with a non-reassuring fetal heart rate tracing, which in most cases resolves once the metabolic acidosis improves. However, despite improved management, DKA remains an important cause of fetal loss in diabetic pregnancies.[41]
Principles of Management in Pregnancy
Preconception
Management of the pregnant diabetic woman is a complex task that ideally begins before conception. In the preconception period, insulin regimens can be modified to improve glycemic control, cholesterol-lowering medications should be discontinued, and angiotensin-converting enzyme inhibitors should be discontinued or changed to a calcium channel blocker. Folic acid supplementation is instituted. Baseline renal function must be assessed to evaluate risk in a pregnancy and an ophthalmologic evaluation performed. Other health or genetic risks should also be addressed. Counseling regarding specific risks and expectations in a diabetic pregnancy should be provided.
First trimester
Women who present in the first trimester with poorly controlled diabetes require rapid normalization of blood sugar to try to prevent congenital malformations and hypoglycemia. Hospitalization may be required to reevaluate diet, modify insulins, and adjust blood sugars expeditiously. Education regarding the importance of dietary intake and glycemic control to the health of the fetus can be helpful to motivate women who do not have their diabetes under control. Women with type 2 diabetes with good glycemic control may not need a further increase in insulin until the second trimester. However, on average, women with type 1 diabetes will require an additional 0.9 units of insulin per kilogram of body weight.[42] The need for increased insulin in women with type 1 diabetes in the first trimester should be individualized depending on glycemic control, food intake, and consideration of the transient drop of insulin requirement that may occur in some women the late first trimester. Anorexia, nausea, and vomiting during the first trimester can decrease oral intake and predispose to hypoglycemia. Severe hypoglycemia in pregnancy is most common in the first trimester. Changes in timing or dose of insulin may be required. Glycemic disturbance is usually less severe in pregnant women with type 2 diabetes than in those with type 1. If not done in the preconception period, medications should be modified as noted earlier. Initial evaluation of women with diabetes includes the usual prenatal laboratory studies performed for non-pregnant women. In addition, laboratory studies should be obtained to assess organ damage and determine a baseline for the risk for pre-eclampsia later in pregnancy. These tests include liver enzymes, renal function, HbA1c, and a 24-h urine for protein and creatinine clearance. Asymptomatic bacteriuria should also be assessed similar to other pregnant women. Clinical judgment dictates whether a chest radiograph, electrocardiogram, or maternal echocardiogram should also be obtained. Certainly further assessment of the heart is warranted in women who have hypertension, history of pulmonary edema, angina, or myocardial infarction. Ophthalmologic examination with assessment of the retina should be performed at least in each trimester. Obstetric ultrasound to document viability early in the evaluation should be obtained. First trimester screening is particularly useful in women with pre-existing diabetes. Nuchal translucency can be used for early screening for not only chromosomal abnormalities but also complex congenital heart disease.
Second trimester
Insulin requirements increase notably in the second trimester, and frequent adjustments may be needed. Targeted ultrasound for congenital anomalies at 16–18 weeks and fetal echocardiogram at 20–22 weeks should be performed with subsequent ultrasound for fetal growth every 3 to 4 weeks. Maternal serum screening can be helpful in screening for open fetal defects.
Third trimester
Insulin requirements to maintain good glycemic control continue to increase and may reach 140% of pre-pregnancy doses. Hospitalization for glucose control may be required, particularly for noncompliant women at highest risk for stillborn. Twice-weekly NST should be initiated by 32 weeks’ gestation.[43] In women with hypertension and IUGR, testing can begin at 28 gestational weeks. The contraction stress test and biophysical profile are generally used when the NST is nonreactive. Doppler assessment of umbilical artery waveforms should be reserved for further assessment of suspected IUGR fetuses. Women with well-controlled diabetes, normal antenatal testing, and normally grown fetuses can go into spontaneous labor, with induction reserved until approximately 40 weeks’ gestation. Early delivery without maternal or fetal indication in women with diabetes is no longer the norm unless fetal lung maturity is documented. Cesarean delivery should be reserved for other obstetric indications, fetal compromise, or estimated fetal weight greater than 4500 g.
Intrapartum
Tight glycemic control in labor helps decrease neonatal hypoglycemia in women with pre-existing diabetes.[44] This degree of control is best accomplished with an intravenous insulin infusion during labor. Women should be instructed to not take their basal or long-acting insulin when in labor or the day of labor induction, and to begin an insulin infusion. After delivery, the infusion can be discontinued or, if a cesarean delivery was needed and full diet not instituted, it can be continued but insulin decreased. One algorithm increases the infusion rate by 0.5 U/h based on blood glucose values, so that normal saline is used if the blood sugar is 81 to 100 mg/dL, 0.5 U/h if the blood sugar is 101 to 140 mg/dL, 1.0 U/h if the blood sugar is 141 to 180 mg/dL, and so forth.
Postpartum
Insulin requirements decrease quickly after delivery of the placenta. Insulin dosing can either be decreased by 40% to 50% or can be changed to pre-pregnancy doses. As soon as the cord is cut, the rate of insulin infusion should be approximately halved as insulin sensitivity returns to normal within minutes.
Women with diabetes who breastfeed have lower daily blood glucose levels and generally require less insulin. Breastfeeding may also have a protective effect against the development of type 1 diabetes in childhood.[45]
Conclusion
Diabetes, especially type 1 diabetes, can be a challenge in pregnancy, but with education, close monitoring, and latest therapeutic modalities, these women can have healthy newborns. Close attention to diet, glycemic control, metabolic stresses, and early diagnosis and monitoring of complications can make pregnancy a successful experience for women with diabetes. It is our responsibility as women's health care providers to make it happen.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Magon N. Gestational diabetes mellitus: Get, set, go from diabetes capital of the world to diabetes care capital of the world. Indian J Endocrinol Metab. 2011;15:161–9. doi: 10.4103/2230-8210.83398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sicree R, Shaw J, Zimmet P. Diabetes Atlas International Diabetes Federation. 4th ed. Belgium: International Diabetes Federation; 2009. The Global Burden: Diabetes and impaired glucose tolerance; pp. 1–105. [Google Scholar]
- 3.The DIAMOND Project Group. Incidence and trends of childhood type 1 diabetes worldwide 1990-1999. Diabet Med. 2006;23:857–66. doi: 10.1111/j.1464-5491.2006.01925.x. [DOI] [PubMed] [Google Scholar]
- 4.Dabelea D. The accelerating epidemic of childhood diabetes. Lancet. 2009;373:2027–33. doi: 10.1016/S0140-6736(09)60874-6. [DOI] [PubMed] [Google Scholar]
- 5.White P. Pregnancy complicating diabetes. Am J Med. 1949;7:609–16. doi: 10.1016/0002-9343(49)90382-4. [DOI] [PubMed] [Google Scholar]
- 6.Hare JW, White P. Gestational diabetes and the White classification. Diabetes Care. 1980;3:394–6. [Google Scholar]
- 7.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33:S62–9. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freinkel N. Gestational diabetes 1979: Philosophical and practical aspects of a major health problem. Diabetes Care. 1980;3:399–401. doi: 10.2337/diacare.3.3.399. [DOI] [PubMed] [Google Scholar]
- 9.Singal DP, Blajchman MA. Histocompatibility (L-A) antigens, lymphocytotoxic antibodies and tissue antibodies in patients with diabetes mellitus. Diabetes. 1973;22:429–32. doi: 10.2337/diab.22.6.429. [DOI] [PubMed] [Google Scholar]
- 10.Nerup J, Platz P, Anderson OO, Christy M, Lyngsoe J, Poulsen JE, et al. HL-A antigens and diabetes mellitus. Lancet. 1974;2:864–6. doi: 10.1016/s0140-6736(74)91201-x. [DOI] [PubMed] [Google Scholar]
- 11.Magon N, Seshiah V. Gestational diabetes mellitus: Noninsulin management. Indian J Endocrinol Metab. 2011;15:284–93. doi: 10.4103/2230-8210.85580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisenbarth BS. Type I diabetes mellitus. A chronic autoimmune disease. N Engl J Med. 1986;314:1360–8. doi: 10.1056/NEJM198605223142106. [DOI] [PubMed] [Google Scholar]
- 13.Todd IA, Bell N, McDevitt HO. A molecular basis for genetic susceptibility to type I (insulin dependent) diabetes: Analysis of the HLA-DR association. Diabetologia. 1984;24:224–30. doi: 10.1007/BF00282704. [DOI] [PubMed] [Google Scholar]
- 14.Kobberling I, Bruggeboes B. Prevalence of diabetes among children of insulin-dependent diabetic mothers. Diabetologia. 1980;18:459–62. doi: 10.1007/BF00261701. [DOI] [PubMed] [Google Scholar]
- 15.Warram IH, Krolewski AS, Gottlieb MS, Kahn CR. Differences in risk of insulin-dependent diabetes in offspring of diabetic mothers and diabetic fathers. N Engl J Med. 1984;311:149–52. doi: 10.1056/NEJM198407193110304. [DOI] [PubMed] [Google Scholar]
- 16.Vadheim CM, Rotter N, Maclaren NK, Riley WJ, Anderson CE. Preferential transmission of diabetic alleles within the HLA gene complex. N Engl J Med. 1986;315:1314–8. doi: 10.1056/NEJM198611203152103. [DOI] [PubMed] [Google Scholar]
- 17.Gamble DR. An epidemiological study of childhood diabetes affecting two or more siblings. Diabetologia. 1980;19:341–4. doi: 10.1007/BF00280517. [DOI] [PubMed] [Google Scholar]
- 18.Lewis SB, Wallin JD, Kuzuya H, Murray WK, Coustan DR, Daane TA, et al. Circadian variation of serum glucose, C-peptide immunoreactivity and free insulin in normal and insulin treated diabetic pregnant patients. Diabetologia. 1976;12:343–50. doi: 10.1007/BF00420978. [DOI] [PubMed] [Google Scholar]
- 19.Knopp R, Montes A, Childs M, Li JR, Mabuchi H. Metabolic adjustments in normal and diabetic pregnancy. Clin Obstet Gynecol. 1980;24:21–49. doi: 10.1097/00003081-198103000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Skryten A, Johnson G, Samisoe G, Gustafson A. Studies in diabetic pregnancy. I. Serum lipids. Acta Obstet Gynecol Scand. 1976;55:211–5. doi: 10.3109/00016347609156915. [DOI] [PubMed] [Google Scholar]
- 21.Knopp RH, Chapman M, Bergelin R, Wahl PW, Warth MR, Irvine S. Relationships of lipoprotein lipids to mild fasting hyperglycemia and diabetes in pregnancy. Diabetes Care. 1980;3:416–20. doi: 10.2337/diacare.3.3.416. [DOI] [PubMed] [Google Scholar]
- 22.Szabo AJ, Opperman W, Hanover B, Gugliucci C, Szabo O. Fetal adipose tissue development: Relationship to maternal free fatty acid levels. In: Camerini-Davalos RA, Cole HS, editors. Early diabetes in early life. New York: Academic Press; 1975. [Google Scholar]
- 23.Reece EA, Gabrielli S, Abdalla M. The prevention of diabetesassociated birth defects. Semin Perinatol. 1988;12:292–301. [PubMed] [Google Scholar]
- 24.Kucera J. Rate and type of congenital anomalies among offspring of diabetic women. J Reprod Med. 1971;7:73–82. [PubMed] [Google Scholar]
- 25.Pinter E, Reece EA, Leranth C, Garcia-Segura M, Hobbins JC, Mahoney MJ, et al. Arachidonic acid prevents hyperglycemia-associated yolk sac damage and embryopathy. Am J Obstet Gynecol. 1986;155:691–702. doi: 10.1016/s0002-9378(86)80001-1. [DOI] [PubMed] [Google Scholar]
- 26.Pinter E, Reece EA, Leranth C, Sanyal MK, Hobbins JC, Mahoney MJ, et al. Yolk sac failure in embryopathy due to hyperglycemia. Ultrastructural analysis of yolk sac differentiation of rat conceptuses under hyperglycemic culture conditions. Teratology. 1986;33:73–84. doi: 10.1002/tera.1420330110. [DOI] [PubMed] [Google Scholar]
- 27.Eriksson UI, Borg LA. Protection by free oxygen radicals scavengingenzymes against glucose induced embryonic malformations in vitro. Diabetologia. 1991;34:325–31. doi: 10.1007/BF00405004. [DOI] [PubMed] [Google Scholar]
- 28.Hagay Z, Weiss Y, Zusman I, Peled-Kamar M, Reece EA, Eriksson UJ, et al. Prevention of diabetes associated embryopathy by overexpression of the free radical scavenger copper zinc superoxide dismutase in transgenic mouse embryos. Am J Obstet Gynecol. 1995;173:1036–41. doi: 10.1016/0002-9378(95)91323-8. [DOI] [PubMed] [Google Scholar]
- 29.Reece EA, Homko CJ. Why do diabetic women deliver malformed infants? Clin Obstet Gynecol. 2000;43:32–45. doi: 10.1097/00003081-200003000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Mondestin MA, Ananth CV, Sumulian JC, Vintzileos AM. Birth weight and fetal death in the United States: The effect of maternal diabetes during pregnancy. Am J Obstet Gynecol. 2002;187:922–6. doi: 10.1067/mob.2002.127458. [DOI] [PubMed] [Google Scholar]
- 31.Dudly D. Diabetic-associated stillbirth: Incidence, pathophysiology, and prevention. Clin Obstet Gynecol. 2007;34:293–307. doi: 10.1016/j.ogc.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 32.Balsells M, Garcia-Patterson A, Gich I, Corcoy R. Maternal and fetal outcome in women with type 2 versus type 1 diabetes mellitus: A systematic review and metaanalysis. J Clin Endocrinol Metab. 2009;94:4284–91. doi: 10.1210/jc.2009-1231. [DOI] [PubMed] [Google Scholar]
- 33.Jovanovic R, Jovanovic L. Obstetric management when normoglycemia is maintained in diabetic pregnant women with vascular compromise. Am J Obstet Gynecol. 1984;149:617–23. doi: 10.1016/0002-9378(84)90245-x. [DOI] [PubMed] [Google Scholar]
- 34.Gordon M, Landon MB, Samuels P, Hissrich S, Gabbe SG. Perinatal outcome and long-term followup associated with modern management of diabetic nephropathy (Class F) Obstet Gynecol. 1996;87:401–9. doi: 10.1016/0029-7844(95)00420-3. [DOI] [PubMed] [Google Scholar]
- 35.Kasiske BL, Kalil RS, Ma JZ, Liao M, Keane WF. Effect of antihypertensive therapy on the kidney in patients with diabetes: A meta-regression analysis. Ann Intern Med. 1993;118:129–38. doi: 10.7326/0003-4819-118-2-199301150-00009. [DOI] [PubMed] [Google Scholar]
- 36.Cooper WO, Hernandez-Diaz S, Arbogast PG, Dudley JA, Dyer S, Gideon PS, et al. Major congenital malformations after first trimester exposure to ACE inhibitors. N Engl J Med. 2006;354:2443–51. doi: 10.1056/NEJMoa055202. [DOI] [PubMed] [Google Scholar]
- 37.Rosenn B, Miodovnik M, Kranias G, Khoury J, Combs CA, Mimouni F, et al. Progression of diabetic retinopathy in pregnancy. Am J Obstet Gynecol. 1992;166:1214–8. doi: 10.1016/s0002-9378(11)90608-5. [DOI] [PubMed] [Google Scholar]
- 38.Lovestam-Adrian M, Agardh CD, Aberg A, Agardh E. Pre-eclampsia is a potent risk factor for deterioration of retinopathy during pregnancy in type I diabetic patients. Diabet Med. 1997;14:1059–65. doi: 10.1002/(SICI)1096-9136(199712)14:12<1059::AID-DIA505>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 39.Chang S, Fuhrmann M, Jovanovich L. The diabetes in early pregnancy study group (DIEP): Pregnancy, retinopathy normoglycemia. A preliminary analysis. Diabetes. 1985;35:3A. [Google Scholar]
- 40.Hemachandra A, Ellis D, Lloyd CE, Orchard TJ. The influence of pregnancy on IDDM complications. Diabetes Care. 1995;18:950–4. doi: 10.2337/diacare.18.7.950. [DOI] [PubMed] [Google Scholar]
- 41.Schneider MB, Umpierrez GE, Ramsey RD, Mabie WC, Bennett KA. Pregnancy complicated by diabetic ketoacidosis, maternal and fetal outcomes. Diabetes Care. 2003;26:958–9. doi: 10.2337/diacare.26.3.958. [DOI] [PubMed] [Google Scholar]
- 42.Langer O, Anyaegbunam A, Brustman L, Guidetti D, Levy J, Mazze R. Pregestational diabetes: Insulin requirements throughout pregnancy. Am J Obstet Gynecol. 1988;159:616–21. doi: 10.1016/s0002-9378(88)80020-6. [DOI] [PubMed] [Google Scholar]
- 43.Kjos SL, Leung A, Henry OA, Victor MR, Paul RH, Medearis AL. Antepartum surveillance in diabetic pregnancies: Predictors of fetal distress in labor. Am J Obstet Gynecol. 1995;173:1532–9. doi: 10.1016/0002-9378(95)90645-2. [DOI] [PubMed] [Google Scholar]
- 44.Curet LB, Izquierdo LA, Gilson GJ, Schneider JM, Perelman R, Converse J. Relative effects of antepartum and intrapartum maternal blood glucose levels on incidence of neonatal hypoglycemia. J Perinatol. 1997;17:113–5. [PubMed] [Google Scholar]
- 45.Sadauskaite-Kuehne V, Ludvigsson J, Padaiga Z, Jasinskiene E, Samuelsson U. Longer breastfeeding is an independent protective factor against development of type 1 diabetes mellitus in childhood. Diabetes Metab Res Rev. 2004;20:150–7. doi: 10.1002/dmrr.425. [DOI] [PubMed] [Google Scholar]


