Abstract
Background:
A characteristic feature of early active psoriatic lesions is the intraepidermal penetration of neutrophils, with attendant formation of Munro-Saboureau microabscesses. Previous immunofluorescence studies have shown reactivity of in vivo binding of stratum corneum antibodies (SCAs) within the Munro-Saboreau microabscesses in cases of psoriasis.
Aims:
In our study, we aimed to investigate any correlation between the SCAs and the Munro-Saboureau microabscesses.
Materials and Methods:
We investigated 50 archival biopsies of psoriasis with Munro-Saboureau microabscesses, and attempted to confirm antibody colocalization within these microabcesses via immunohistochemistry staining. As controls, we utilized 50 skin biopsies from healthy patients undergoing esthetic plastic surgery procedures.
Results:
Within the Munro-Saboureau microabscesses, the following markers were statistically significantly positive relative to controls: CD1a, CD8, CD23, cyclooxygenase-2, myeloid histoid antigen, albumin, fibrinogen, kappa, lambda, von Willebrand factor, IgG, IgM, IgD, complement/C3c, C3d, myeloperoxidase, and carcinoembryonic antigen (P < 0.05). Autoreactivity to blood vessels was also detected, with multiple immunoglobulins and complement factors.
Conclusions:
We document important correlations between the Munro-Saboureau microabscesses, SCAs, and other immunoreactants.
Keywords: Autoimmunity, Blood vessels, Munro-Saboureau microabscesses carcinoembryonic antigen, Psoriasis, von Willebrand factor
Introduction
Psoriasis is a benign but hyperproliferative skin disease.[1–3] The classic histologic features of psoriasis are well documented.[1–3] A characteristic feature of early and active psoriatic lesions is the intraepidermal penetration of neutrophils, with the formation of Munro-Saboureau microabscesses within the stratum corneum. In pustular psoriasis, the accumulation of neutrophils dominates the clinical picture. Classic histologic features include (1) confluent parakeratosis, (2) Munro-Saboureau microabscesses, (3) attenuation of the granular layer (except on nail tissue, where hypergranulosis may exist), (4) overall epidermal psoriasiform hyperplasia, (5) elongation and edema of the dermal papillae, (6) thinning of the suprapapillary epidermal plates, (7) an increase in epidermal keratinocytic mitoses, and (8) proliferation of dermal papillary blood vessel endothelial cells, with hypertrophy and vasodilatation of the papillary capillaries.[1–3] Kogoj microabscesses (neutrophilic aggregates in the epidermal stratum spinosum) represent a specific diagnostic clue for psoriasis, but are often not present.[1–3]
Clinically, psoriasis is a common, chronic, relapsing, and papulosquamous dermatitis, with overlying silvery scales. The scalp, sacral region, and extensor surfaces of extremities are commonly involved, and flexural and intertriginous areas may be affected in “inverse psoriasis”.[1–3] Clinical involvement of the nails is frequent. Oral lesions (i.e., geographic stomatitis and/or glossitis) are commonly described. Five to eight percent of psoriatic patients develop arthritis. Interphalangeal joints are characteristically involved, but large joints may also be affected. Histologically, psoriasis is a dynamic dermatosis with changing features over the life of an individual lesion; thus, we can histologically classify individual lesions in early, intermediate or advanced stages.[1–3]
Previous direct and indirect immunofluorescence (DIF, IIF) studies by Beutner have shown reactivity of stratum corneum antibodies (SCAs), and their in vivo binding in cases of psoriasis.[4–10] One of these studies showed (in 193 cases of psoriasis and 89 cases of other dermatoses) that all fully developed, active psoriatic lesions had IgG deposits in the stratum corneum at the sites of SCAs, presumably due to in vivo binding of SCAs.[4–10] Evidence of autoimmunity has also been described in psoriasis.[6,7] Partial or complete saturation of the SCAs could be observed by the performance of IIF tests for SCAs in such specimens. In our study we investigated whether any correlation existed between the SCAs and other immunoreactants, especially within Munro-Saboureau microabscesses.
Materials and Methods
Subjects of study
Fifty biopsies reported as psoriasis were reviewed, obtained from two private dermatopathology** laboratories; each biopsy was evaluated independently by two board certified dermatopathologists. Each biopsy received a research histologic diagnosis of active psoriasis, and also histologically demonstrated the presence of Munro-Saboreau microabscesses (MSMs). As controls, 50 skin biopsies from healthy, non-psoriatic patients undergoing plastic surgery were utilized. All samples were tested anonymously to comply with Institutional Review Board (IRB) requirements. The medication history, current medications, and clinical site of the biopsies were obtained from the original requisition form for each biopsy. All biopsies were fixed in 10% buffered formalin, and processed for hematoxylin and eosin (H and E) staining. The samples were then cut at 4 μm thickness, and submitted for H and E staining and immunohistochemistry (IHC) analysis as previously described.[11–16]
Immunohistochemistry
We tested for the following antibodies by IHC: polyclonal rabbit anti-human IgG, polyclonal rabbit anti-human IgA, IgM, IgD, IgE, Complement/C3c, C3d, C1q, fibrinogen, albumin, kappa light chains, lambda light chains, CD117/c-kit, myeloperoxidase, S-100, chromogranin A, PGP 9.5, and calcitonin. We also utilized monoclonal mouse anti-human CD3, CD4, CD5, CD8, CD19, CD20cy, CD45, CD68, myeloid/histoid antigen, HLA-DPDQDR antigen, cyclooxygenase-2 (COX-2), linker for activation of T cells (LAT), T-cell antigen receptor zeta chain (ZAP-70), ribosomal protein S6-p240 (phosphorylation site specific), topoisomerase IIα, mast cell tryptase (MCT), Bcl-2, calretinin, carcinoembryonic antigen (CEA), CD1a, CD7, CD10, CD23, CD31, CD34, CD43, CD44, CD57, CD34 class II, Ki-67, neurofilament, p53, anti-proliferating cell nuclear antigen (PCNA), synaptophysin, survivin, von Willebrand factor (VWF), serotonin, epithelial membrane antigen (EMA), cyclin D1, Mart-1/Melan-A/CD63, tyrosinase, pancytokeratin (Clone AE1/AE3), D2-40, and vimentin. All of our antibodies were obtained from Dako, (Carpinteria, California, USA). From all patients we obtained written consents, as well as IRB permission. Archival samples were used without any identifiers. The studies were performed as previously described.[11–16]
Staining intensity
The staining intensity of these antibodies was evaluated qualitatively by two independent observers, as well as in a semiquantitative mode by automated computer image analysis designed to quantify IHC staining in hematoxylin counterstained histologic sections. Slides were then scanned with a ScanScope CS scanner (Aperio Technologies, Vista, California, USA), utilizing brightfield imaging at 20× and 40× magnifications. The strength of the staining was evaluated on a scale from 0 to 4, where 0 represented negative staining and 4+ indicated the strongest staining. We then calculated the area of positive signal, divided by the studied area.
Controlling for non-specific binding by IHC and DIF
Controlling for non-specific antibody binding to the stratum corneum was performed, because antibodies can bind non-specifically to other targets or tissue components. To address these issues, we used specific primary and secondary antibodies for each marker. To minimize cross-reactivity, we utilized highly cross-adsorbed secondary antibodies. In addition, we used specific blocking reagents and specific antigen retrievals to ensure high-fidelity binding of antibodies. To test for specificity, secondary antibodies were applied in the absence of primary antibodies; all residual staining was then considered nonspecific. In immunofluorescence experiments, we also controlled for possible autofluorescent molecules that could be contained in tissues, their presence was to be detected best in the absence of secondary antibodies.
Statistical methods
Differences between the control and psoriasis subject groups were evaluated using GraphPad Software statistical analysis, and using Student's t-test. We considered a correlation present with a P-value of 0.05 or less, assuming a normal distribution of the samples.
Results
Within the Munro-Saboureau microabscesses, the following IHC markers were statistically significant relative to controls: CD1a, CD8, CD23, cyclooxygenase-2, myeloid histoid antigen, albumin, fibrinogen, kappa, lambda, VWF, IgG, IgM, IgD, C3c, C3d, myeloperoxidase, and CEA (P<0.05). Autoreactivity to blood vessels was also detected with multiple immunoglobulins and complement factors.
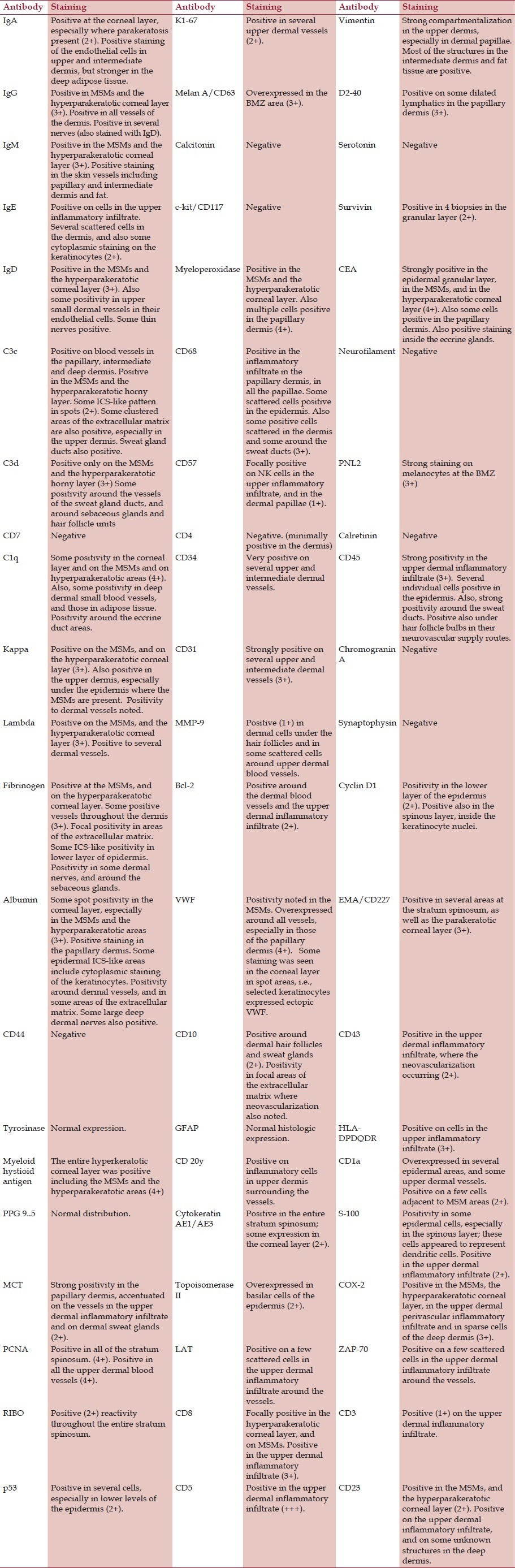
In Table 1, we summarize our results. The areas in gray show which antibodies were simultaneously positive within the Munro-Saboureau microabscesses.
Table 1.
Antibody staining patterns in patients with active psoriasis, with attention to Munro-Saboureau microabscesses (MSMs)
Notably, ectopic expression of VWF in the Munro-Saboureau microabscesses and the hyperparakeratotic epidermal stratum corneum was seen in greater than 80% of the psoriasis cases, as well as in the papillary dermis (P<0.05, [Figure 1]).
Figure 1.
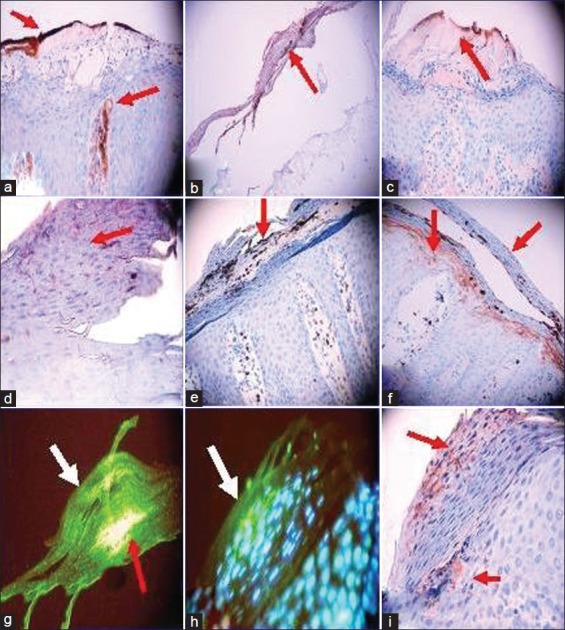
Relationship between Munro-Saboureau microabscesses and autoantibodies in psoriasis. (a) A representative IHC photomicrograph demonstrating ectopic expression of VWF in the Munro-Saboureau microabscesses (MSMs) and the hyperparakeratotic epidermal stratum corneum as well as in the papillary dermis (brown staining; red arrows). (b) Positive IHC staining in the Munro-Saboureau microabscesses and the hyperparakeratotic epidermal stratum corneum utilizing anti-myeloid histoid antigen antibody (brown staining; red arrow). (c) Positive IHC staining in the Munro-Saboureau microabscesses and the hyperparakeratotic epidermal stratum corneum using anti-human kappa light chains antibody (brown staining; red arrow). (d) Positive IHC staining in the MSMs and the hyperparakeratotic epidermal stratum corneum utilizing anti-human IgD (violet/brown staining; red arrow). (e) Positive IHC staining at the MSMs and the hyperparakeratotic epidermal stratum corneum via anti-human myeloperoxidase (dark staining; red arrow). (f) Positive IHC staining in the MSMs and the hyperparakeratotic epidermal stratum corneum utilizing anti-human CEA antibody (dark staining; red arrows). (g) A representative DIF with double staining, highlighting the SCAs. The green staining represents FITC conjugated anti-human IgG (white arrow). The red arrow indicates simultaneous reactivity as yellow/pink staining of FITC conjugated anti-human IgM. (h) A representative DIF with FITC conjugated anti-human IgG showing the SCAs (green staining, white arrow). The light blue staining represents epidermal stratum spinosum keratinocyte nuclear counterstaining with 4’-6-Diamidino-2-phenylindole (DAPI). The nuclear counterstaining indicates (1) that the primary IgG reactivity is in the upper left, non-nuclear epidermal stratum corneum area where parakeratosis is present; and (2) that the subjacent stratum spinosum keratinocytes have not yet lost their nuclei. (i). IHC staining with anti-human IgG showing positivity at the SCAs (violet/brown staining; red arrows)
In addition, CEA was positive in the Munro-Saboureau microabscess areas, and on many of the upper dermal inflammatory cells in 90% of the psoriasis biopsies. These results were statistically significant in comparison with the controls (P<0.05). Mart-1/Melan A/CD63 and PNL2 were overexpressed in the BMZ area in 75% of the psoriasis cases. We observed melanin and/or melanocyte cell debris within the Munro-Saboureau microabscesses. We further noted increased melanin pigment between epidermal keratinocytes, relative to the controls.
Endothelial proliferation was confirmed by Ki-67, CD31, CD34, and VWF overexpression in the papillary tip and upper dermal blood vessels, and VWF was positive in 95% of the Munro-Saboureau microabscesses (P < 0.05). In addition, we detected significant autoreactivity against the upper dermal blood vessels using anti-IgG, IgM, IgA, IgD, Complement/C3, fibrinogen, and albumin. The following immunoreactants were positive at the level of the Munro-Saboureau microabscesses in 95% of the psoriatic samples: CD8, CD23, COX-2, myeloid histoid antigen, CD1a, albumin, fibrinogen, kappa, and lambda light chains, VWF, IgG, IgM, IgD, Complement/C3d, C3c, myeloperoxidase, and CEA antibodies (P < 0.05). No control biopsies were positive. D2-40 staining demonstrated that the lymphatics in 93% of the psoriasis cases were dilated, especially in the dermal papillary tip regions. The lymphatic dilatation was not observed in the controls (P < 0.05, [Figure 1]).
EMA/CD227 was positive (+++) in the majority of the proliferating epidermal stratum spinosum areas of the psoriasis biopsies, and was statistically significant in comparison with the controls (P<0.05). In addition, PCNA was positive in the psoriatic biopsies, in comparison to the controls (P<0.05%); 80% of the psoriatic biopsies showed strongly positive staining. The controls demonstrated no specific positivity to these antibodies (P<0.05%, [Figure 1]).
Discussion
DIF, IIF, and IHC studies have previously confirmed the reactivity of SCAs and their in vivo binding in cases of psoriasis.[5,8] It has been shown via the mixed agglutination technique on tissue sections that 25 patient's psoriatic lesions contained immunoglobulins of the IgG and IgM classes in the epidermis. The authors estimated the maximum immunoglobulin concentrations were around the dermal-epidermal junction, and above the dermal papillae.[17–20] Anti-Ig activity consistent with rheumatoid factor of multiple immunoglobulin classes was also present at these sites. Further, the anti-IgG factors were eluted from the sections by acid buffer. Next, the sections would again absorb anti-IgG factors if the reaction with sensitized cells was repeated. Fixatives utilized in tissue section preparation assisted in modulating the reactions.[17–20]
Interestingly, SCAs as detected by DIF and IIF can also be absorbed from human sera with homogenates of normal human callus which contain stratum corneum antigens.[9,10] Titers of concurrently present antibodies remain unchanged in sera absorbed via callus, demonstrating that the absorption is specific.[17–20] SCAs reacting in a passive hemagglutination assay with an antigen derived from human callus can be specifically absorbed by means of a trypsin-phenol-water extraction with the homologous antigen. However, absorption with callus fails to remove the hemagglutinatin antibodies and absorption with the trypsin-phenol-water antigen preparation fails to remove SCAs as detected by IIF. The authors suggest that these data indicate a non-identity relationship between these antigen systems, despite their similar histological features and anatomical distribution.[9]
One psoriasis pathophysiologic hypothesis favors that the formation of immune complexes may be responsible for the “squirting papilla” phenomenon. In addition, this hypothesis proposes that conversion of the stratum corneum - which normally contains inaccessible antigens - into its reactive form seems to be induced by trauma, which would in turn induce proteases of neutrophils or other cells, and/or inflammatory and chemotactic agents. Stimulation of protease production by neutrophils appears to be an important factor in the pathogenesis of psoriasis. The stratum corneum of the epidermis is probably the target, and becomes an antigen for SCAs present in the circulation.[17–20] Of note, a passive flux of molecules through the epidermis beneath the pustules cannot solely account for our findings, because multiple other antibodies were negative at the stratum corneum.
Normal titer stratum corneum autoantibodies, including the apparent primary “ending” antibodies to N-acethylglucosamine have been found to not have disease-specific associations; however, in vivo reactions of large amounts of these antibodies do indeed produce pathological changes. The question thus to be addressed is what factors induce their pathologic reactions.
In our findings, we corroborated previously documented findings regarding SCAs. Due to technological advances and accessibility to more antibodies, we were able to determine that there is a 100% correlation between (1) the presence of Munro-Saboureau microabscesses in psoriatic cases versus controls, and (2) presence in psoriatic patients versus controls of the following antibodies: CD1a, CD8, CD23, COX-2, myeloid histoid antigen, albumin, fibrinogen, kappa light chains, lambda light chains, VWF, IgG, IgM, IgD, complement/C3c, complement/C3d, myeloperoxidase, and CEA. In addition, all the psoriatic cases demonstrated positive staining for melanin in the Munro-Saboureau microabscesses (P<0.05).
Our study demonstrated that the Munro-Saboreau microabcesses seem to have and/or create ectopic expression of selected molecules, including vascular antigens and CEA. CEA is primarily produced during fetal development, and acts as a glycoprotein involved in cell adhesion. CEA and related genes make up the CEA family, belonging to the immunoglobulin superfamily. The normal production of CEA stops before birth. Therefore, it is not classically present in the serum of healthy adults, although levels are raised in heavy smokers. Anti-CEA is expressed in the sweat glands of normal human skin and by sweat gland tumors.[21]
Carcinoembryonic antigen-related cellular adhesion molecule 1 (CEACAM1/CD66a) is a cell-surface glycoprotein, belonging to the CEA family and expressed by human neutrophils, epithelial cells, and activated T and NK cells.[22] CEACAM1/CD66a is expressed as a cell-surface molecule with different isoforms, and can also be secreted as a soluble protein. Previous studies have also demonstrated that keratinocytes in the outer epidermal layers of psoriatic skin express CEACAM1/CD66a, unlike these layers (1) in healthy skin or (2) in cutaneous lesions of patients with atopic or nummular dermatitis.[21–24] The authors further studied the stimulation of primary human keratinocytes and in vitro reconstituted epidermis with culture supernatants of activated psoriatic lesion infiltrating T cells, IFN-gamma, or oncostatin M (but not IL-17). The supernatant stimulation induced keratinocyte expression of transcripts for the CEACAM1/CD66a long and short isoforms, as well as cell-surface CEACAM1/CD66a.[21–24] Moreover, the uppermost layers of the epidermis in psoriatic lesions often contain neutrophils, a cell with inflammatory and antimicrobial properties.[21–24] The authors demonstrated that cytokine-induced cell-surface expression of CEACAM1/CD66a by keratinocytes in the context of a psoriatic environment might contribute to the persistence of neutrophils. The neutrophils might in turn contribute to chronic inflammation and decreased propensity for skin infections, typical clinical findings in psoriasis patients.[21–24]
The expression of EMA in the spinous layer of lesional psoriatic skin was also unexpected, as this antigen in the epidermis is typically restricted to the acrosyringia (ducts) of eccrine sweat glands. We speculate that this molecule could also be ectopically expressed in the epidermis of psoriatic skin.
Within the Munro-Saboureau microabscesses we found not only strong expression of CEA, but also expression of ectopic VWF and acute inflammatory markers such as COX-2. We found several antigen presenting cell markers (e.g. CD1a, CD68, and myeloid/histoid antigen) surrounding the Munro-Saboureau microabscesses. We also found other immune cell markers such CD23 and myeloperoxidase around the Munro-Saboureau microabscesses, which could indicate an active immune response to internal components of these microabscesses.
Our findings of autoantibody markers such as kappa light chains, lambda light chains, IgG, IgM and IgD, and complement markers such as C1q, C3c, and C3d at the dermal blood vessels are also indicative of a possible autoimmune response against these vessels. The concomitant presence of Bcl-2 at this level indicates an active cell cycle struggle between cell cycle regulators, proto-oncogenes, and apoptotic mechanisms.[25]
Our observed immune reactivity to endothelial cells has been previously documented in palmoplantar psoriasis.[26] The possible autoimmune effects on blood vessels on psoriatic skin thus warrants further investigation, especially given additional documented associations of cardiovascular alterations in psoriatic patients versus controls groups.[27–29]
In summary, the possible genetic factors and environmental stimuli that trigger the immune response seem to act not only via (1) autoantibodies, but also via (2) alterations in the cell cycle regulation, (3) neo-vascularization, and (4) ectopic tissue present within Munro-Saboureau microabscesses. The immune response initiation and specific reactions are based on the strong presence of immunoglobulins that correlate directly with the SCAs. Finally, the presence of autoantibodies and complement in psoriatic dermal blood vessels may cause localized inflammation and alterations in blood flow, in turn contributing to recently documented cardiovascular effects in these patients.
Footnotes
Source of Support: Georgia Dermatopathology Associates, Atlanta, Georgia.
Conflict of Interest: None declared.
References
- 1.Steffen C. William John Munro and Munro's abscess, and Franz Kogoj and Kogoj's spongiform pustule. Am J Dermatopathol. 2002;24:364–8. doi: 10.1097/00000372-200208000-00016. [DOI] [PubMed] [Google Scholar]
- 2.Coimbra S, Figueiredo A, Castro E, Rocha-Pereira P, Santos-Silva A. The roles of cells and cytokines in the pathogenesis of psoriasis. Int J Dermatol. 2012;51:389–95. doi: 10.1111/j.1365-4632.2011.05154.x. [DOI] [PubMed] [Google Scholar]
- 3.Neumann E, Hård S. The significance of the epidermal sweat duct unit in the genesis of pustular psoriasis (Zumbusch) and the microabscess of Munro-Sabouraud. Acta Derm Venereol. 1974;54:141–6. [PubMed] [Google Scholar]
- 4.Dabski K, Glinski W, Beutner EH, Jablonska S. In vitro effects of enzymes of polymorphonuclear leukocytes on the antigenicity of stratum corneum. Int Arch Allergy Appl Immunol. 1985;77:287–91. doi: 10.1159/000233833. [DOI] [PubMed] [Google Scholar]
- 5.Kumar V, Jones P, Beutner EH, Jablonska S. Immunofluorescence studies in psoriasis: Detection of antibodies to stratum corneum in psoriatic scales. Ann NY Acad Sci. 1983;420:361–8. doi: 10.1111/j.1749-6632.1983.tb22224.x. [DOI] [PubMed] [Google Scholar]
- 6.Myśliwiec H, Flisiak I, Baran A, Górska M, Chodynicka B. Evaluation of CD40, its ligand CD40L and Bcl-2 in psoriatic patients? Folia Histochem Cytobiol. 2012;50:75–9. doi: 10.2478/18699. [DOI] [PubMed] [Google Scholar]
- 7.Martin G, Guérard S, Fortin MM, Rusu D, Soucy J, Poubelle PE, et al. Pathological crosstalk in vitro between T lymphocytes and lesional keratinocytes in psoriasis: Necessity of direct cell-to-cell contact. Lab Invest. 2012 doi: 10.1038/labinvest.2012.69. [In press] [DOI] [PubMed] [Google Scholar]
- 8.Qutaishat SS, Kumar V, Beutner EH, Jablonska S. A distinct stratum corneum antigen in psoriasis and its reactions with stratum corneum autoantibodies. APMIS. 1992;100:341–6. doi: 10.1111/j.1699-0463.1992.tb00881.x. [DOI] [PubMed] [Google Scholar]
- 9.Jablonska S, Chowaniec O, Beutner EH, Maciejowska E, Jarzabek-Chorzelska M, Rzesa G. Stripping of the stratum corneum in patients with psoriasis: Production of prepinpoint papules and psoriatic lesions. Arch Dermatol. 1982;118:652–7. [PubMed] [Google Scholar]
- 10.Beutner EH, Jablonska S, Kumar V, Binder WL, Chorzelska TP. A further study on the mechanism of deposition of plasma protein in psoriasis scales. Arch Dermatol Res. 1981;270:217–21. doi: 10.1007/BF00408237. [DOI] [PubMed] [Google Scholar]
- 11.Howard MS, Yepes MM, Maldonado-Estrada JG, Villa-Robles E, Jaramillo A, Botero JH, et al. Broad histopathologic patterns of non-glabrous skin and glabrous skin from patients with a new variant of endemic pemphigus foliaceus-part 1. J Cutan Pathol. 2010;37:222–30. doi: 10.1111/j.1600-0560.2009.01315.x. [DOI] [PubMed] [Google Scholar]
- 12.Abreu-Velez AM, Loebl AM, Howard MS. Autoreactivity to sweat and sebaceous glands and skin homing T cells in lupus profundus. Clin Immunol. 2009;132:420–4. doi: 10.1016/j.clim.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Abreu-Velez AM, Girard JG, Howard MS. Antigen presenting cells in the skin of a patient with hair loss and systemic lupus erythematosus. N Am J Med Sci. 2009;1:205–10. [PMC free article] [PubMed] [Google Scholar]
- 14.Abreu-Velez AM, Dejoseph LM, Howard MS. HAM56 and CD68 antigen presenting cells surrounding a sarcoidal granulomatous tattoo. N Am J Med Sci. 2011;3:475–7. doi: 10.4297/najms.2011.3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abreu-Velez AM, Brown VM, Howard MS. An inflamed trichilemmal (pilar) cyst: Not so simple? N Am J Med Sci. 2011;3:431–4. doi: 10.4297/najms.2011.3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abreu-Velez AM, Klein AD, Smoller BR, Howard MS. Bullous allergic drug eruption with presence of myeloperoxidase and reorganization of the dermal vessels observed by using CD34 and collagen IV antibodies. N Am J Med Sci. 2011;3:82–4. doi: 10.4297/najms.2011.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krogh HK, Tonder O. Antibodies in psoriatic scales. Scand J Immunol. 1973;2:45–51. doi: 10.1111/j.1365-3083.1973.tb02015.x. [DOI] [PubMed] [Google Scholar]
- 18.Krogh HK, Tönder O. Immunoglobulins and anti-immunoglobulin factors in psoriatic lesions. Clin Exp Immunol. 1972;10:623–34. [PMC free article] [PubMed] [Google Scholar]
- 19.Jablonska S, Beutner EH, Binder WL, Jarzabek-Chorzelska M, Rzesa G, Chowaniec O. Immunopathology of psoriasis. Arch Dermatol Res. 1979;23:65–71. doi: 10.1007/BF00417280. [DOI] [PubMed] [Google Scholar]
- 20.Kaneko F, Itoh N, Yoshida H, Suzuki M, Ono I. The cell-components and cytokines in the subcorneal microabscess of psoriasis. Fukushima J Med Sci. 1991;37:103–12. [PubMed] [Google Scholar]
- 21.Maxwell P. Carcinoembryonic antigen: Cell adhesion molecule and useful diagnostic marker. Br J Biomed Sci. 1999;56:209–14. [PubMed] [Google Scholar]
- 22.Egawa K, Honda Y, Kuroki M, Inaba Y, Ono T. Carcinoembryonic antigen and related antigens expressed on keratinocytes in inflammatory dermatoses. Br J Dermatol. 1996;134:451–9. [PubMed] [Google Scholar]
- 23.Rahmoun M, Molès JP, Pedretti N, Mathieu M, Fremaux I, Raison-Peyron N, et al. Cytokine-induced CEACAM1 expression on keratinocytes is characteristic for psoriatic skin and contributes to a prolonged lifespan of neutrophils. J Invest Dermatol. 2009;129:671–8. doi: 10.1038/jid.2008.303. [DOI] [PubMed] [Google Scholar]
- 24.Hagemeier HH, Bhardwaj R, Grunert F, Buchegger F, Goerdt S, von Kleist S, et al. Carcinoembryonic antigen and related glycoproteins in psoriasis. Pathobiology. 1993;61:19–24. doi: 10.1159/000163755. [DOI] [PubMed] [Google Scholar]
- 25.Abreu-Velez AM, Howard WR, Howard MS. Upregulation of anti-human ribosomal protein S6-p240, topoisomerase II α, cyclin D1, Bcl-2 and anti-corneal antibodies in acute psoriasis. Dermatol Online. 2011;2:113–7. [Google Scholar]
- 26.Hagforsen E, Hedstrand H, Rönnelid J, Nilsson B, Michaëlsson G. Sera from patients with palmoplantar pustulosis show immunoreactivity against endothelial cells. Acta Derm Venereol. 2007;87:261. doi: 10.2340/00015555-0217. [DOI] [PubMed] [Google Scholar]
- 27.Thomson AW, Nalesnik MA, Rilo HR, Woo J, Carroll PB, van Thiel DH. ICAM-1 and E-selectin expression in lesional biopsies of psoriasis patients responding to systemic FK 506 therapy. Autoimmunity. 1993;15:215–23. doi: 10.3109/08916939309019930. [DOI] [PubMed] [Google Scholar]
- 28.Shapiro J, Cohen AD, Weitzman D, Tal R, David M. Psoriasis and cardiovascular risk factors: A case-control study on inpatients comparing psoriasis to dermatitis. J Am Acad Dermatol. 2012;66:252–8. doi: 10.1016/j.jaad.2010.11.046. [DOI] [PubMed] [Google Scholar]
- 29.Gelfand JM, Mehta NN, Langan SM. Psoriasis and cardiovascular risk: Strength in numbers, part II. J Invest Dermatol. 2011;131:1007–10. doi: 10.1038/jid.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]


