Abstract
Background:
The frequency of encountering radiodermatitis caused by X-ray fluoroscopic procedures for ischaemic heart disease is increasing. In severe cases, devastating ulcers with pain, for which conservative therapy is ineffective, emerge. Radiation-induced ulcers are notorious for being difficult to treat. Simple skin grafting often fails because of the poor state of the wound bed. A vascularized flap is a very good option. However, the non-adherence of the well-vascularized flap with the irradiated wound bed is frequently experienced.
Aim:
To ameliorate the irradiated wound bed, bone marrow-derived platelet-rich plasma (bm-PRP) was delivered during the surgery.
Materials and Methods:
Four patients with severe cutaneous radiation injury accompanied by unbearable pain after multiple fluoroscopic procedures for ischaemic heart disease were treated. Wide excision of the lesion and coverage with a skin flap supplemented with bm-PRP injection was performed.
Results:
All patients obtained wound closure and were relieved from pain. No complication concerning the bone marrow aspiration and delivery of bm-PRP was observed.
Conclusions:
Supplementation of bm-PRP can be an option without major complications, time, and cost to improve the surgical outcome for irradiated wounds.
KEY WORDS: Bone marrow, cardiac fluoroscopy, platelet-rich plasma, radiation ulcer, skin flap
INTRODUCTION
Fluoroscopy of the heart, including coronary angiography, is a frequently performed procedure to diagnose heart diseases. The number of fluoroscopically guided interventional procedures is sharply rising. They occur in some open-chest surgical procedures. For some patients with ischaemic heart diseases, multiple coronary angiography and angioplasty procedures are repeatedly performed. The same region of a patient's skin may be irradiated during years of subsequent procedures. Frequency to encounter radiodermatitis caused by fluoroscopic procedures is increasing. Symptoms of the lesion range from mild to devastating. In severe cases with necrotic tissue or ulceration, which is frequently accompanied with intolerable pain, conservative therapy is ineffective. Surgical replacement of the affected skin is required. Simple skin grafting often fails because of the inadequate wound bed. There always rises a question, to what extent debridement should be done. A vascularized flap is a very good option. However, the non-adherence of the well-vascularized flap with the irradiated wound bed is frequently experienced. Lack of wound healing ability of the wound bed is speculated to be the cause.
The delivery of bone marrow cells to non-healing wounds have been reported to be effective in wound bed preparation.[1] There are also reports about the effectiveness of platelet-rich plasma (PRP) for treating chronic wounds.[2–4] The authors have reported a simple, low-cost technique to concentrate bone marrow cells and platelets.[5] The product is called bone marrow-derived platelet-rich plasma (bm-PRP).
In this report, patients with devastating cutaneous radiation injury after multiple fluoroscopic procedures are described. Wide excision of the lesion and coverage with a large skin flap supplemented with bm-PRP were done. Favorable results with relief from the pain and coverage of the ulcers were obtained.
MATERIALS AND METHODS
This study protocol was approved by the Institutional Review Board. The study was conducted in full accordance with the Declaration of Helsinki. The patients were informed of the operations and procedures and signed the written consent form beforehand. Four patients presenting with radiation-induced ulcer after cardiac fluoroscopic procedures were treated [Table 1]. Wide excision and coverage with local flaps were performed in conjunction with bm-PRP injections.
Table 1.
Profiles of patients

Processing bm-PRP
The processing parameters were defined and modified with our previous experience in processing PRP from peripheral blood.[5] The anterior or posterior iliac crest was stabbed with a bone marrow needle. Bone marrow was aspirated into a 20 ml syringe containing 3 ml of ACD-A solution (2.2% sodium citrate, 0.8% citrate, 2.2% glucose) (Terumo, Tokyo, Japan) as an anticoagulant. For the patients in this report, 80 ml in total of the bone marrow was aspirated. The aspirate was poured into centrifuge tubes and spun at 100g for 20min with a portable centrifuge placed beside the operating table. The supernatant, including the buffy coat and slightly red layer, was aspirated and transferred to the other tubes. Secondary centrifugation was done at 1000g for 10 min. Clear supernatant was aspirated out, and the precipitate was resuspended. Approximately 10 ml of bm-PRP was obtained. The concentration procedure was done simultaneously while the surgery was performed.
RESULTS
The cell counting of the bone marrow aspirate and bm-PRP after the concentration process is presented in Table 2.
Table 2.
Cell counting before and after the concentration procedure
All four patients obtained wound closure and relief from pain. All flaps adhered well to the wound bed and no fluid pooling was observed. So far, no recurrence of erosion or ulceration has been observed in our treated patients, with the longest follow-up of 4 years.
Case 1
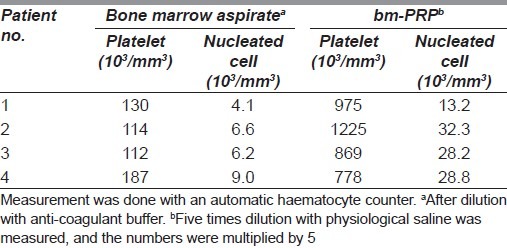
A 42-year-old man, 171 cm height, 88kg weight (body mass index: 30.0), presented with a skin ulcer on the right subscapular area. He complained of insomnia with excruciating pain. His medical record revealed that he had been suffering from diabetes mellitus, hypertension, and old myocardial infarction. He had undergone 1 h of coronary angiography and 5 h of subsequent percutaneous transluminal coronary angioplasty. Aspirin was prescribed as an antiplatelet agent. Two months later, erythema with itch emerged on his back. It developed into a non-healing ulcer with pain, 11 months after the procedures. He could not quit pack-a-day smoking, even after strong suggestions by cardiologists and dermatologists. Large amounts of methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-sensitive Staphylococcus aureus (MSSA) and a small amount of Enterococcus faecalis were detected in the ulcer. Surgical debridement of necrotic tissue under local anaesthesia was done to the extent that wound edge bleeding could be seen, intending wound bed preparation and secondary skin grafting. However, necrosis of wound edge and floor progressed, and severe pain persisted. The secondary operation plan was altered to flap coverage. The prescription of aspirin was stopped for the presurgical period. Under general anaesthesia, the posterior iliac crest was stabbed with a needle, and 80 ml of the bone marrow was aspirated. With a centrifuge placed beside the operation table, approximately 10 ml of the bm-PRP was produced simultaneously when the surgical debridement of the patient was done. Into the wound floor and edges, the bm-PRP was injected. The local flaps were elevated to cover the defect. Soon after the secondary operation, the patient was relieved from the persistent pain. Delayed necrosis of the flap edges was observed, but it healed spontaneously. Recurrence of the ulcer has not been seen for 4 years [Figure 1].
Figure 1.
(a) A 42-year-old man. Eleven months after percutaneous transluminal coronal angioplasty. Painful ulcer, causing insomnia, was observed on his right subscapular area. (b) Surgical debridement with the index of bleeding from the edge was performed. (c) Twenty days after initial debridement, granulation was poor, and extension of tissue necrosis was observed. Insomnia with pain continued. (d) Further surgical debridement was done, and the adjacent skin flap was raised. (e) Into the wound floor and edges, bm-PRP was injected. (f) Eight months after the reconstructive surgery, there was no pain complaint
Case 2
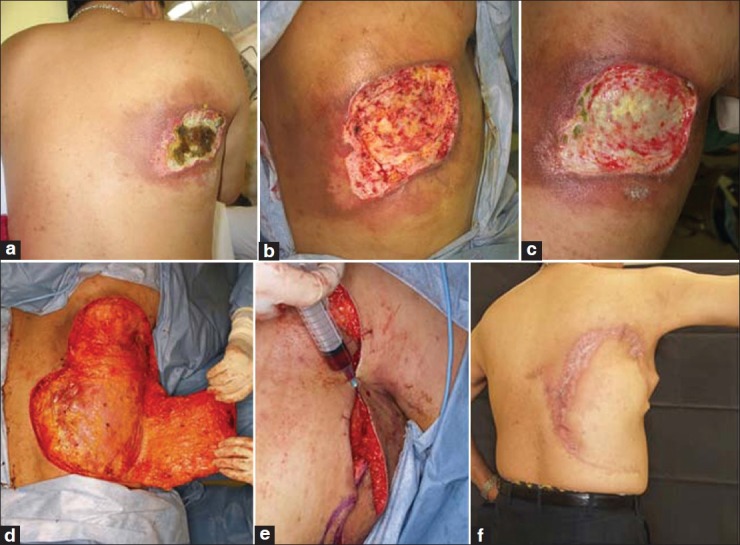
A 59-year-old man, 165 cm height, 70 kg weight (body mass index: 25.7), with hypertension and hyperlipidaemia consulted us for the treatment of a painful ulcer on his right back. He had experienced six times of coronary angiography. Stents had been placed to expand the stenoses. Aspirin and clopidogrel sulfate had been administered as antiplatelet agents. He quit smoking on advice. Two months after the last procedure, erythema with itch appeared on his back and worsened into a painful ulcer in 8 months. His primary anxiety was the liberty from the pain. A large amount of MSSA was detected in the ulcer. After the presurgical suspension of the antiplatelet agents, the operation was performed. Under general anaesthesia, the posterior iliac crest was stabbed, and 80 ml of the bone marrow was aspirated, and the bm-PRP was processed during the debridement was performed. The bm-PRP was injected into the wound bed and edges through a 23 G needle. With local skin flaps, the defect was covered. Soon after the operation, the patient realized that the burden of the pain was gone. Delayed necrosis of the flap edges was seen, but it healed spontaneously. No recurrence of the ulcer has been seen for 2 years so far [Figure 2].
Figure 2.
(a) A 59-year-old man. An ulcer with severe spontaneous pain was observed on his right dorsum, 8 months after the last fluoroscopic angioplasty procedure. (b) Excisional design. (c) Surgical debridement was done, and the skin flaps were raised. (d) Injection of bm-PRP into the wound bed and edges. (e) Seven months after the surgery, the Pain-free condition was obtained. (f) Cross-sections of the debrided tissue. Marked fibrosis throughout the sections can be observed. (g) A high magnification micrograph of the deep portion of the specimen. Significant fibrotic tissue replaced the muscular structure. A decrease in the viable cells is obvious (haematoxylin-eosin staining)
DISCUSSION
Fluoroscopy results in radiation exposures of 0.02–0.05 Gy every minute, and in extreme circumstances, reaches up to 0.5 Gy per minute. Image recording requires an even higher dose.[6] The average radiation dose in routine cardiac catheterization is 2.5 Gy, whereas in percutaneous interventions, the dose rises to 6.4 Gy.[7]
Skin damage can be caused by the cumulative dose from multiple procedures, each of which is individually insufficient to cause injury. For some patients with ischaemic heart diseases, multiple coronary angiography and angioplasty procedures are repeatedly performed. The same region of a patient's skin may be irradiated during years of subsequent procedures. Radiation dose evaluation mapping of the site is not easy and is often impossible, especially in repetitive fluoroscopy cases. This makes it more difficult to deal with these ulcers than the oncological radiation therapy-induced damage that can be evaluated with medical records. Scattering of X-rays also has to be accounted for.
When the tissue damage is shallow, adequate debridement and a simple free skin graft are good enough. However, when the damage extends deep into the underlining muscles or bones, which is often the case with γ-ray or X-ray irradiation, or when the total debridement of the affected tissue cannot be done, flap coverage should be performed. It is always a question, to what extent debridement should be done. Empirically, bleeding from the edge cannot be the index. Coverage with a contralateral side latissimus dorsi musculocutaneous flap may be an option. In these cases, the flap is within the site of X-ray exposure, because it is rare that fluoroscopy is performed only with one-sided view. Even though it seems intact on the surface, hidden radiation damage of the flap should not be underestimated. Furthermore, it is not rare to encounter vividly surviving flaps floating, not adhering to the irradiated wound bed. The lack of the wound-healing ability of the wound bed is speculated to be the cause.
Bone marrow cells, including the so-called stem cells, are gaining a good reputation for accelerating wound healing. They are also positively estimated to improve the ischaemic state of limbs.[8–10] Delivery of bone marrow cells to the ischaemic heart is expected to encourage neovascularisation and cardiac muscle preservation.[11] A successful investigation about the local administration of culture-expanded bone marrow cells to the radiation burns, in which conventional skin grafting failed, has been done.[12] Bey et al. observed poor healing, despite coverage with a latissimus dorsi muscle flap after failure with conventional excision, dermal substitute coverage, and skin grafting. They were able to cover the defect only after the local administration of culture-expanded bone marrow cells in combination with a radial forearm flap and skin grafting.[13]
Platelet-rich plasma is also attracting attention as a good source of several growth factors that accelerate wound healing. There have been clinical reports about the effectiveness of PRP in treating chronic wounds.[2–4]
The authors previously reported a method for concentrating bone marrow aspirate, which is a simple and low-cost procedure to enrich bone marrow cells (working cells) and platelets (reservoir of growth factors) simultaneously.[5] Among the enriched cells, the relatively larger cells (e.g., multinucleated cells), which potentially cause inflammation, were confirmed to be reduced; in contrast to this, the smaller cells were concentrated. The growth factors contained in platelets derived from the bone marrow aspirate were at the same level as those in platelets derived from the peripheral blood.[5] The local injection of bm-PRP into the wounds on rabbits’ persistent ischaemic limbs accelerated wound healing. Injected cells were confirmed to survive at least 4 weeks after transplantation (submitting to a journal).
Because the goal of this study was initially the wound closure, objective evaluation of the pain level was not performed. The mechanism of the devastating pain of the radiation-induced ulcer is still unclear. Local ischaemia and burden of bacteria, which cause inflammation, can be speculated as the causes. In a previous report of treating late radiation necrosis of the soft tissues with pentoxifylline, a haemorrheologic agent, all patients had pain relief.[14] At least partly, improving the ischaemic state seems to reduce the pain. In our Case 1, severe pain remained even after wide debridement with the index of bleeding from the wound edge. The pain went away after flap coverage with bm-PRP. The pain-relieving effect of the operation, including bm-PRP injection, was remarkable also in the other cases. The anti-inflammatory activity of expanded bone marrow cells has been reported.[15,16] The delivery of bone marrow cells might have some good effects on pain relief, coupled with the coverage with well-vascularized flap.
Small delayed necrosis of the flap edges was seen. The edges appeared to be vital for 5–7 days after the surgery, though they changed colors after that and finally necrosed. This phenomenon is not often seen in normal skin flaps. The flaps used for the coverage were located adjacent to the necrotic lesions. In addition, the necrosed edges were at the margins of the debridement where the radiation exposure and scattering ray might have been extended. It is not easy to define the extent of irradiation and damage to the tissues at the time of treatment. However, it is too early to attribute the edge necrosis only to the irradiation, considering patients’ back grounds and the tension. Closure of the necrosed area was seen within a certain period of time.
The injection of bm-PRP into the tissue without platelet activation, rather than spreading on, was selected because of the certainty for cell delivering. The calcium level will be gradually normalized, supplied from the surrounding tissue, and spontaneous activation of the platelets will take place, with the exposure to collagen and other activating factors.
No major complication with the aspiration of bone marrow and delivery of bm-PRP was observed. Harvesting bone marrow aspirate required extra 5min for the surgery. No additional time was needed for processing bm-PRP, because it was done while the surgical debridement was performed.
Through our experience, we propose a synergetic approach of debridement, flap coverage and bm-PRP supplementation for treating severe radiation ulcers. However, at this moment, there are not enough evidences to support the effectiveness of bm-PRP. Further investigation should be continued.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Yoshitane Tsukamoto for pathological advices.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Badiavas EV, Falanga V. Treatment of chronic wounds with bone marrow-derived cells. Arch Dermatol. 2003;139:510–6. doi: 10.1001/archderm.139.4.510. [DOI] [PubMed] [Google Scholar]
- 2.Knox RL, Hunt AR, Collins JC, DeSmet M, Barnes S. Platelet-rich plasma combined with skin substitute for chronic wound healing: A case report. J Extra Corpor Technol. 2006;38:260–4. [PMC free article] [PubMed] [Google Scholar]
- 3.Driver VR, Hanft J, Fylling CP, Beriou JM. A prospective, randomized, controlled trial of autologous platelet-rich plasma gel for the treatment of diabetic foot ulcers. Ostomy Wound Manage. 2006;52:68–70. [PubMed] [Google Scholar]
- 4.Saad Setta H, Elshahat A, Elsherbiny K, Massoud K, Safe I. Platelet-rich plasma versus platelet-poor plasma in the management of chronic diabetic foot ulcers: A comparative study. Int Wound J. 2011;8:307–12. doi: 10.1111/j.1742-481X.2011.00797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishimoto S, Oyama T, Matsuda K. Simultaneous concentration of platelets and marrow cells: A simple and useful technique to obtain source cells and growth factors for regenerative medicine. Wound Repair Regen. 2007;15:156–62. doi: 10.1111/j.1524-475X.2006.00196.x. [DOI] [PubMed] [Google Scholar]
- 6.Malkinson FD. Radiation injury to skin following fluoroscopically guided procedures. Arch Dermatol. 1996;132:695–6. [PubMed] [Google Scholar]
- 7.Lichtenstein DA, Klapholz L, Vardy DA, Leichter I, Mosseri M, Klaus SN, et al. Chronic radiodermatitis following cardiac catheterization. Arch Dermatol. 1996;132:663–7. [PubMed] [Google Scholar]
- 8.Esato K, Hamano K, Li TS, Furutani A, Seyama A, Takenaka H, et al. Neovascularization induced by autologous bone marrow cell implantation in peripheral arterial disease. Cell Transplant. 2002;11:747–52. [PubMed] [Google Scholar]
- 9.Franz RW, Parks A, Shah KJ, Hankins T, Hartman JF, Wright ML. Use of autologous bone marrow mononuclear cell implantation therapy as a limb salvage procedure in patients with severe peripheral arterial disease. J Vasc Surg. 2009;50:1378–90. doi: 10.1016/j.jvs.2009.07.113. [DOI] [PubMed] [Google Scholar]
- 10.Amann B, Luedemann C, Ratei R, Schmidt-Lucke JA. Autologous bone marrow cell transplantation increases leg perfusion and reduces amputations in patients with advanced critical limb ischemia due to peripheral artery disease. Cell Transplant. 2009;18:371–80. doi: 10.3727/096368909788534942. [DOI] [PubMed] [Google Scholar]
- 11.Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: The BOOST randomised controlled clinical trial. Lancet. 2004;364:141–8. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 12.Lataillade JJ, Doucet C, Bey E, Carsin H, Huet C, Clairand I, et al. New approach to radiation burn treatment by dosimetry-guided surgery combined with autologous mesenchymal stem cell therapy. Regen Med. 2007;2:785–94. doi: 10.2217/17460751.2.5.785. [DOI] [PubMed] [Google Scholar]
- 13.Bey E, Prat M, Duhamel P, Benderitter M, Brachet M, Trompier F, et al. Emerging therapy for improving wound repair of severe radiation burns using local bone marrow-derived stem cell administrations. Wound Repair Regen. 2010;18:50–8. doi: 10.1111/j.1524-475X.2009.00562.x. [DOI] [PubMed] [Google Scholar]
- 14.Dion MW, Hussey DH, Doornbos JF, Vigliotti AP, Wen BC, Anderson B. Preliminary results of a pilot study of pentoxifylline in the treatment of late radiation soft tissue necrosis. Int J Radiat Oncol Biol Phys. 1990;19:401–7. doi: 10.1016/0360-3016(90)90549-y. [DOI] [PubMed] [Google Scholar]
- 15.Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol. 2007;179:1855–63. doi: 10.4049/jimmunol.179.3.1855. [DOI] [PubMed] [Google Scholar]
- 16.Aslam M, Baveja R, Liang OD, Fernandez-Gonzalez A, Lee C, Mitsialis SA, et al. Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. Am J Respir Crit Care Med. 2009;180:1122–30. doi: 10.1164/rccm.200902-0242OC. [DOI] [PMC free article] [PubMed] [Google Scholar]


