Essentially all animal cells have the ability to kill themselves by activating an intrinsic cell suicide program (1, 2). The execution of this program leads to a morphologically distinct form of cell death termed apoptosis (3). During the last few years, significant progress has been made in identifying important components of the cell death program, and there is considerable hope that this knowledge can be exploited to manipulate apoptosis for therapeutic benefits. The paper by MacCorkle et al. (4) illustrates how chemically induced dimerization (CID) of cysteine proteases can be used for targeted cell ablation.
Apoptosis involves the activation of an unusual class of cysteine proteases, termed caspases (for cysteine aspartic acid-specific protease) (5, 6). The original evidence for an involvement of caspases in apoptosis came from genetic analyses of programmed cell death in the nematode C. elegans: loss-of-function mutations in ced-3, which encodes a caspase, lack programmed cell death during development (7, 8). A large number of mammalian caspases have been isolated in recent years, and evidence for their involvement in apoptosis has come from a variety of inhibition and overexpression studies (5). Caspases are synthesized as inactive zymogens that need to be proteolytically processed to generate the active enzyme (6). Based on the known three-dimensional structure of two caspases (caspase-1 and -3), it is thought that the active protease consists of two heterodimers that are produced by internal proteolytic cleavages of the pro-enzyme. This leads to the removal of an N-terminal prodomain and the generation of a large (p20) and small (p10) subunit. Significantly, these cleavages occur after aspartate residues at substrate recognition consensus sites for caspases, and mature caspases can often cleave and activate their own as well as other caspase zymogens in vitro. This has led to the idea of a caspase cascade in which “initiator” caspases transmit signals to “executioners” to activate cell death (5, 6, 9). The central question that has remained is how such a caspase cascade is initially activated in response to death-inducing stimuli. Considerable progress toward answering this question has come from several recent reports that the oligomerization of pro-caspases can be sufficient for autoprocessing and cell killing (4, 10, 11).
The initial clues suggesting that caspases might be activated by oligomerization came from studies of the CD-95 (Fas/Apo-1) (12). In this well characterized system, the binding of a trimeric ligand (FasL) to CD-95 induces receptor trimerization. This trimerization leads to the recruitment of a caspase zymogen (pro-caspase-8) into a complex with the CD-95 receptor and the adaptor molecule FADD/MORT1. Upon recruitment, the single polypeptide chain of pro-caspase-8 is rapidly cleaved into the catalytically active subunits. The active caspase-8 is thought to cleave and activate downstream caspases, thereby initiating a proteolytic cascade that eventually leads to apoptosis. These observations suggested the possibility that bringing the zymogen molecules into close proximity might be sufficient for autoprocessing via transproteolysis. To test this idea, several groups constructed pro-caspase molecules that can be aggregated in vivo by the addition of a nontoxic, lipid-permeable chemical (4, 10, 11). This strategy, termed chemically induced dimerization, uses the FK506 binding protein FKBP12 (13). Proteins containing a FKBP12 domain can be efficiently dimerized by adding nontoxic dimeric derivatives of FK506, such as FK1012 or its synthetic analogs. Expression constructs with FKBP12 fused to the N terminus of the inactive pro-caspase were generated and expressed in different cells. Significantly, chemically induced dimerization of these chimeric proteins led to efficient induction of apoptosis. All cell types tested were susceptible to killing by this approach and cell killing dependent on caspase activity. Furthermore, oligomerization of caspase-8 was sufficient to induce pro-caspase processing in vitro (10). Finally, by mutating the internal cleavage sites of caspase-8, Muzio et al. (11) were able to demonstrate a weak proteolytic activity (approximately two orders of magnitude lower than the active caspase) of the pro-caspase. Taken together, these results suggest a simple “proximity” model for the activation of caspases: if brought into close contact, the weak proteolytic activity of two zymogen molecules would be sufficient to cleave one another to form the active enzyme and thereby start a proteolytic cascade. At least in general terms, this model of transproteolysis resembles activation of the complement protease cascade and tyrosine kinase activation by intermolecular cross-phosphorylation (14). (Fig. 1)
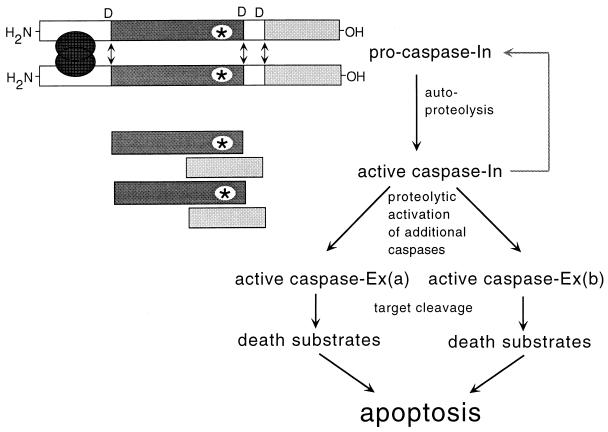
Figure 1.
Cell killing by pro-caspase aggregation. Caspases are synthesized as inactive pro-enzymes. When pro-caspases are brought into close proximity, they can self-activate through internal proteolysis that occurs after specific aspartic acid (D) residues. These cleavages result in the removal of an inhibitory N-terminal domain and the formation of a small and large subunit. Two small and two large subunits associate with each other to form the active heterotetrameric enzyme. The activated “initiator caspase” (caspase-In) can proteolytically process and activate additional pro-caspase molecules, including downstream “executional caspases” (caspase-Ex), thereby initiating a caspase cascade. The activity of this cascade leads to the proteolysis of a variety of specific target proteins and mediates the coordinate destruction of organelles and macromolecules during apoptosis. The aggregation of pro-caspases is normally regulated by adapter molecules that interact with the prodomain, but recombinant molecules have been constructed that can be aggregated by small, lipid-permeable chemicals.
MacCorkle et al. (4) emphasize the utility of cell ablation via chemically induced caspase aggregation for developing safe vectors for gene therapy. Because genetically modified cells may become deleterious to the host at some point, a method for their safe and efficient elimination is needed. As the authors point out, caspase-based “suicide switches” offer several advantages for this purpose. First, cell ablation by induction of apoptosis is an efficient and clean process: evolution has designed apoptosis as a mechanism for the removal of cells that are no longer needed, including damaged and virus-infected cells (1, 2). During the execution of apoptosis, nothing escapes the dying cell, and cell corpses are rapidly cleared and degraded by phagocytosis. As a result, death occurs almost unnoted and without pain. In contrast, necrotic deaths involve cytoplasmic leakage and are typically associated with inflammation and pain.
To be clinically suitable, an “artificial death switch” (ADS) should satisfy several criteria. First, cytotoxicity must be very low in the uninduced state. Second, the dimerizing chemical must be nontoxic for the (unaltered) host cells. Third, the method should be effective in many different cell types, and ablation of the targeted cells should be rapid and highly efficient to avoid the emergence of resistant clones. Finally, the ADS components should not trigger an immune response. Although the satisfaction of these criteria remains to be tested in animal models, the results obtained with caspase-based ADSs in cell culture experiments are encouraging. At least for caspase-3, basal toxicity was very low, and it is likely that the slight autotoxicity that was observed for caspase-1 constructs can be further reduced or eliminated in the future. Likewise, the FK506-derivatives used for dimerization are well characterized and nontoxic. Also, because syngenic proteins can be used, an immune response against the ADS is very unlikely.
In theory, the use of caspases for the activation of apoptosis should leave very little room for a cell to avoid death. The machinery to execute apoptosis appears to be present in all of our cells at all times, and the activation of “executioner caspases”, such as caspase-3, is thought to cause irreversible damage to a cell. Therefore, the finding by MacCrokle et al. (4) that high levels of the anti-apoptotic protein Bcl-XL protected against caspase-3-mediated death comes as a bit of a surprise. On the other hand, it is not very likely that normal cells would express the very high levels of Bcl-XL protein required for protection under these conditions. However, it is possible that cells can induce protective functions in response to genetic modifications during gene therapy. In particular, inhibitors of active caspases, such as the virally encoded p35 and crmA proteins (15), are expected to inactivate caspase-based ADSs, and certain viral infections may pose an extra risk during gene therapy. In addition, cellular homologs of these viral inhibitors may exist and complicate matters further could be induced. Transgenic mouse studies with caspase-based ASDs should provide a better assessment of these potential risks.
The utility of caspase-based ADSs is almost certainly not restricted to the termination of gene therapy. By targeting specific cell types and tissues for ablation, this method might be useful to generate animal models for injuries and degenerative diseases. In the long term, it may be possible to use this technology for the treatment of cancer, or to fight viral infections. Such a use would obviously require the selective activation of ADSs in the targeted cells. Whereas a solution to this problem is far from obvious right now, a two-component ADS system may ultimately provide the necessary specificity by combining selective drug targeting/delivery with molecular differences between normal and tumor cells.
Another interesting question that remains to be answered is whether aggregation-induced activation is a general mechanism of caspase regulation or whether it is a property of initiator caspases. Because apoptosis is an irreversible phenomenon, the activation of a caspase cascade has to be tightly controlled. If aggregation of pro-caspases is indeed a key step in apoptosis induction, much of the regulatory input will be devoted to control this step. The identification of proteins that promote and inhibit aggregation and understanding their precise molecular mechanism of action will almost certainly provide many interesting new opportunities to control cell death in therapeutically relevant ways.
Footnotes
The companion to this commentary is published on pages 3655–3660 in issue 7.
Abbreviation: ADS, artificial death switch.
References
- 1.Steller H. Science. 1995;267:1445–1449. doi: 10.1126/science.7878463. [DOI] [PubMed] [Google Scholar]
- 2.Jacobsen M D, Weil M, Raff M C. Cell. 1997;88:347–354. doi: 10.1016/s0092-8674(00)81873-5. [DOI] [PubMed] [Google Scholar]
- 3.Kerr J F R, Wyllie A H, Currie A R. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacCorkle R A, Freeman K W, Spencer D M. Proc Natl Acad Sci USA. 1998;95:3655–3660. doi: 10.1073/pnas.95.7.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takahashi A, Earnshaw W C. Curr Opin Genet Dev. 1996;6:50–55. doi: 10.1016/s0959-437x(96)90010-6. [DOI] [PubMed] [Google Scholar]
- 6.Salvesen G S, Dixit V M. Cell. 1977;91:443–446. doi: 10.1016/s0092-8674(00)80430-4. [DOI] [PubMed] [Google Scholar]
- 7.Ellis R E, Yuan J, Horvitz H R. Annu Rev Cell Biol. 1991;7:663–698. doi: 10.1146/annurev.cb.07.110191.003311. [DOI] [PubMed] [Google Scholar]
- 8.Yuan J, Shaham S, Ledoux S, Ellis H M, Horvitz H R. Cell. 1993;75:641–652. doi: 10.1016/0092-8674(93)90485-9. [DOI] [PubMed] [Google Scholar]
- 9.Thornberry N A, Rano T A, Peterson E P, Rasper D M, Timkey T, Garcia-Calvo M, Houtzager V M, Nordstrom P A, Roy S, Vaillancourt J P, et al. J Biol Chem. 1997;272:17907–17911. doi: 10.1074/jbc.272.29.17907. [DOI] [PubMed] [Google Scholar]
- 10.Yang X, Chang H Y, Baltimore D. Mol Cell. 1998;1:319–325. doi: 10.1016/s1097-2765(00)80032-5. [DOI] [PubMed] [Google Scholar]
- 11.Muzio M, Stockwell B R, Stennicke H R, Salvesen G S, Dixit V M. J Biol Chem. 1998;273:2926–2930. doi: 10.1074/jbc.273.5.2926. [DOI] [PubMed] [Google Scholar]
- 12.Nataga S. Cell. 1977;88:355–365. [Google Scholar]
- 13.Crabtree G R, Schreiber S L. Trends Biochem Sci. 1996;21:418–422. doi: 10.1016/s0968-0004(96)20027-1. [DOI] [PubMed] [Google Scholar]
- 14.Ullrich A, Schlessinger J. Cell. 1990;61:203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- 15.Clem R J, Hardwick J M, Miller L M. Cell Death Differ. 1996;3:9–16. [PubMed] [Google Scholar]