Abstract
This paper reviews the various aspects of tissue regeneration during the process of tissue expansion. “Creep” and mechanical and biological “stretch” are responsible for expansion. During expansion, the epidermis thickens, the dermis thins out, vascularity improves, significant angiogenesis occurs, hair telogen phase becomes shorter and the peripheral nerves, vessels and muscle fibres lengthen. Expansion is associated with molecular changes in the tissue. Almost all these biological changes are reversible after the removal of the expander.This study is also aimed at reviewing the difficulty in deciding the volume and dimension of the expander for a defect. Basic mathematical formulae and the computer programmes for calculating the dimension of tissue expanders, although available in the literature, are not popular. A user-friendly computer programme based on the easily available Microsoft Excel spread sheet has been introduced. When we feed the area of defect and base dimension of the donor area or tissue expander, this programme calculates the volume and height of the expander. The shape of the expander is decided clinically based on the availability of the donor area and the designing of the future tissue movement. Today, tissue expansion is better understood biologically and mechanically. Clinical judgement remains indispensable in choosing the size and shape of the tissue expander.
KEY WORDS: Mathematics of tissue expansion, tissue expander, tissue expansion, tissue regeneration
INTRODUCTION
Tissue expansion is one of the greatest innovations of the 20th century in plastic surgery. When Radovan, 1982, presented his experience of breast reconstruction using tissue expansion in 68 patients, the late William Grabb predicted that “tissue expansion will have a major impact in reconstructive surgery”.[1] This prediction has become a reality and, now, the tissue expansion has become an essential part of the surgical armamentarium of most plastic, reconstructive and aesthetic surgeons.
Historically, surgical tissue expansion was first reported by Neumann.[2] He expanded the skin above the ear for coverage of the cartilage graft for reconstruction of a subtotally avulsed ear. This was based on the simple argument that abdominal wall and breast enlarge many-fold in pregnant women but still maintain their normal thickness and skin appendages.
Innovation by Radovan opened a new vista.[1] Tissue expansion was initially used for head and neck reconstruction, but now is being used in almost every part of the body. Tissue expansion enlarges the existing tissue, maintaining its colour, texture, hair-bearing qualities and innervations; hence, this technique is suited the most for head neck region and breast.
Choosing an expander for a specific defect still remains a grey area. There are many mathematical, geometrical and computer-based models for calculation of the required expansion for choosing the correct expander size. They will be reviewed and discussed and a new user-friendly computer programme to help choose the optimum size of the expander will be presented.
BIOLOGICAL PROPERTY OF THE SKIN LEADING TO TISSUE EXPANSION
It was the curiosity of the surgeons as well as the scientists, which led to investigations into the changes that tissue expansion causes in various tissues. Extensive studies have been carried out on the effect of tissue expansion on the skin, nerve, muscle and bones.
Viscoelastic property
Tissue expansion involves a combination of creep and biological stretch. In “Creep”, when a constant force is applied to stretch the skin, it continues to extend. In “Biological stretch”, the skin or any other tissue enlarges whenever a force is applied. In tissue expansion, the tissue is stretched without affecting the quality of the original tissue. If the force is too much or applied too fast then the dermis will rupture, resulting in striae formation. Argenta (1984) observed the softening of the overlying skin within 24–48 h of injection into a tissue expander. This indicates the role of “Creep” in expansion.[3]
BIOLOGICAL EFFECT OF TISSUE EXPANSION
When the skin is expanded using a tissue expander, the epidermal thickness increases. However, after removal of the expander, the epidermal thickness gradually returns back to normal after 4–6 weeks. The pilosebaceous elements are well preserved, although they may be compressed on histological examination. Hyperpigmentation is noticeable because of hyperactivity of melanocytes during expansion’ however, it returns back to normal slowly after removal of the expander.[4]
Histologically, the maximum pressure effect of tissue expansion is seen in the dermis. During expansion, the dermis becomes thinner. A dense fibrous capsule is formed around the expander that progressively increases till the expander remains in vivo. This is in response to the presence of the foreign body (tissue expander) under the skin envelope. Contractile myofibroblasts have been demonstrated in deep dermis adjacent to the capsule and within the capsule. It is hypothesized that the contraction of the expander capsule is akin to wound contraction. The magnitude of capsular contracture decreases over a period of time because of the intraluminal pressure in the expander. The risk of contraction increases in case of infection and/or exposure of the implant.[5] There is increased metabolic activity, increased collagen synthesis and the elastin fibres become altered and fragmented.[6] Mitotic activity of the fibroblast is maximum during the initial period and, later on, it progressively decreases. After removal of the expander, the dermis thickness returns back to normal and the capsule slowly disappears. Tissue expansion of hair bearing scalp shortens the telogen phase, probably because of active epidermal mitosis.[7]
When the expander is placed over a muscle, a depression forms after removal of the implant, which Radovan labeled as “bath-tub” depression. This deformity is due to temporary compression of the muscle and is not the effect of muscle atrophy,[8] and later Gur et al. in 1998 demonstrated that the muscle undergoes significant atrophic changes because of long-standing pressure of the expander. Focal muscle fibre degeneration with glycogen deposits has been histologically demonstrated.[9] When the expander is removed, the histological architecture of the muscle, vasculature and its functions return to normal.
Surgeons have attempted to expand the Tensor Fascia Lata muscle. The expansion of skeletal muscle preserves the histology of the muscle. The average number of sarcomeres in the muscle fibres increases significantly. The vessels become longer with the development of the arterial network. These findings suggest that increase in the muscle length following tissue expansion is due to the biological growth process.[10] It has been observed that adipose tissue undergoes permanent atrophy to the extent of 30–50% with loss of fat cells due to the pressure caused by the tissue expander.[11]
The effect of expansion has been studied in an experimental model. The expander causes thinning of the cranial bone under the expander due to direct pressure effect. However, the bone density remains unaffected.[12] Usually, a periosteal inflammatory reaction is observed in the margin of the expander. Clinically, the cranial bones are deformed more often than the long bones due to the pressure effect of the expanders. Such bony deformities caused by an expander return back to normal after removal of the expander balloon.
Calvarial resorption directly under the expander was noted in two adults during forehead expansion. This was noticed after removal of the implant, resulting in a conspicuous skeletal deformity.[13] In children, periosteal reaction was the most frequent change. Bone resorption and thinning occurred in a large number of patients, and downward displacement was observed in a few patients.[14,15]
Effect on vascularity
The expanded skin is more vascular than the normal adjacent skin. This has been shown experimentally and has also been observed clinically by most of the surgeons.[16] The flaps raised out of the expanded skin and hence have better chances of survival.[6]
Increased vascularity is partly because of the presence of the capsule. The pattern of vascularity in the expanded skin is comparable to that of the delayed flaps.[16] An expanded flap may also be labeled as a delayed flap.
Temporary hypoxia caused by the pressure of the expander is one of the explanations for the increase in vascularity or angiogenesis. A significantly higher number of the expressing vascular endothelial growth factor has been observed in the expanded skin as compared with the unexpanded adjoining tissue. This has given a new dimension to the biological explanation of tissue-expanded angiogenesis.[17] Overall, tissue expansion causes increase in the thickness of the epidermis, the dermis thins out, a capsule is formed around the expander, angiogenesis occurs, the vascularity of the skin improves and the underlying bone may get deformed. To summarise, mechanical stretching as well as biological growth occurs during tissue expansion. The histological normalcy is achieved in a few weeks time after the removal of the expander. Overall enlargement of the tissue is used for resurfacing the defect. The biological effects of tissue expansion have been enlisted in Table 1.
Table 1.
Biological effects of tissue expansion

IS IT A “DIVIDEND” OR A “LOAN”?
The following factors contribute to the enlargement of the skin during tissue expansion:
Initially, there is a significant movement of the adjacent skin over the expander.[19]
Increased mitotic activity in the skin over the expander has been demonstrated using video-radiographic studies.[19]
Biological growth of the tissue by cell proliferation is responsible for reducing the tension applied by the expander. Gradually, the tension reduces to the resting level, indicating a process of biological creep.[20]
Based on these observations, Austad et al., 1986, concluded that tissue expansion is a net “dividend” of tissue gained.[21]
EXPANSION OF TISSUES OTHER THAN THE SKIN
Success of the expansion of the skin encouraged some innovative surgeons to use the same principle for the enlargement of other tissues experimentally and clinically. There are very interesting observations and findings reported in the literature. In an experimental attempt to expand the saphenous neurovascular bundle, it was observed that the artery and the vein could be expanded rapidly by 140% within 4 days without causing thrombosis, disruption of blood vessels or any other complication. In spite of increased length of the vessels, the histological architecture remained normal, indicating smooth muscle cell proliferation in the subendothelial region and regeneration of endothelial cells.[22] In the same study, the expanded vessels were used for microvascular anastomosis and the patency rate was found to be equal to that in the un-expanded vessels.[22] This knowledge was expected to increase the application of tissue expansion on blood vessels and other tissues; however, this has not happened over the past 25 years.
Manders et al., 1986, attempted to elongate the median nerve in a case of neuroma. They also tried to use the principle of tissue expansion to enlarge the bowel, bladder, etc.[23]
Martini et al., 1998, used rapid intraoperative expansion of facial nerves for repair of a defect in the nerve. The functional results were comparable with that of the grafted nerve.[24] Ikeguch et al., 1998, expanded the ureter to use the tissue for augmentation of the urinary bladder in pigs.[25]
Tissue expanders have been used to expand the bony orbit in cases of congenital anophthalmos. It has been found to be safe and effective in increasing the bone growth and enlargement of the socket.[26]
Chudacoff et al., 1996, expanded bilateral labia majora to use the expanded tissue as flaps to line the neovagina in cases of vaginal agenesis.[27]
ESTIMATION OF TISSUE REGENERATION WITH TISSUE EXPANSION
Most often, the choice of the tissue expander is arbitrary, and it is usually based on clinical judgement [Figure 1]. Attempts have been made to calculate the desired dimensions of the expander using computer programmes and mathematical calculations.
Figure 1.
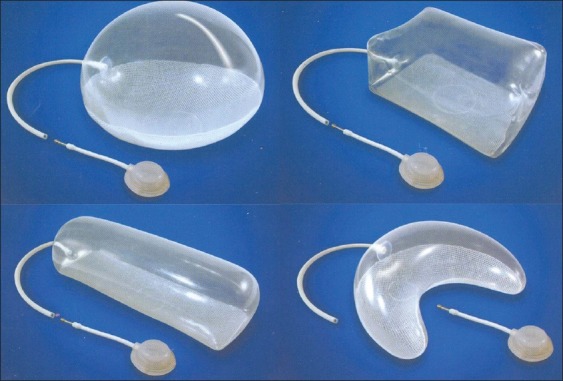
Common types of tissue expanders used in clinical practice: spherical, rectangular, cylindrical and crescent shaped
A computer programme was written in BASIC language for an IBM personal computer. This programme requires the diameter of the implant base and the desired surface area gain. The programme estimates the capacity of a spherical tissue expander.[28] Another computer-aided programme based on C++ and linked by C++ compiler calculates the volume of a rectangular tissue expander after feeding the length and breadth of the lesion, considering them as the size of the base of the expander. Raposio and Santi, 1997, considered the base dimensions of a rectangular tissue expander as equal to the dimensions of the defect. They multiplied the surface requirement by 3/2 as a “correcting factor” to take care of the tissue loss during tissue transfer.[29] Patel in 1986 contradicted Shively and presented a mathematical calculation to predict the desired volume and height of a spherical expander without the aid of a computer [Figure 2]. He opined that when an analytical calculation is possible then there is no need of a computer for these simple calculations.[30] The equations are as follows:
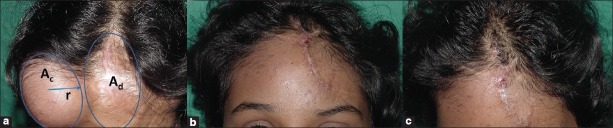
Figure 2.
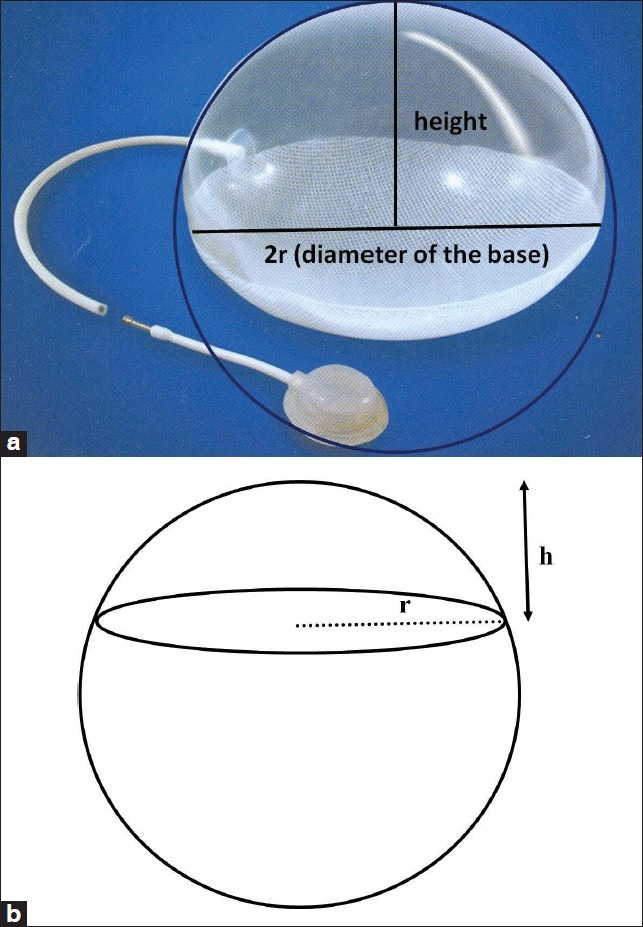
(a) Spherical expander is usually a part of the sphere: Its height and diameter has been shown. (b) A diagrammatic representation of a spherical tissue expander in which the expander is part of the sphere. It may not always be a half or full sphere. ‘r’ is the radius of the base of the expander and ‘h’ is the height of the expander
Ac = Ad +πr2 + area of rotation
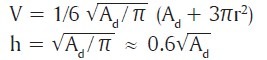
Where “Ac” is the surface area of a convex surface of a sphere, Ad is the surface area of the recipient defect, “V” is the volume, “r” is the radius of the base and “h” is the height of the spherical tissue expander. The area of rotation is not taken into consideration as overestimation can be done while performing the final calculation.[30]
While presenting a mathematical modeling of tissue expanders, Duits et al., 1989, warned that these mathematical calculations estimate the surface area of the expander,[31] which may not exactly be the same as the area of skin gained because of the “stretch back” phenomenon presented by Nordstrom in 1984.[32] Duits et al. emphasized a very important factor that the area gained in a spherical tissue expander is related to its height and has no correlation with its radius/diameter.
Area gained in a spherical expander = πh2
Volume of a spherical expander = 1/8 πd2h + 1/6 πh3
Duits et al., 1989, further presented mathematical calculations for rectangular and crescent-shaped expanders.[31] A general scheme to calculate the volume and surface area of spherical and rectangular tissue expanders by simple arithmetic calculations has been proposed by many authors on almost the same principle.[31,33,34]
Bhandari presented a simplified version of the mathematical calculation to calculate the volume of a spherical expander by giving many examples of different types of defects.[34] He has considered the need to add 20–30% extra tissue for rotation, dog ear and mechanical creep resulting into “stretch back”. He has also pointed out that calculations should also take into consideration the inward displacement of the underlying tissue due to the pressure effect of the tissue expander.[34] According to Bhandari, 2009, the total surface area required for resurfacing the defect = surface area of the defect + surface area of the donor site + 20% of the defect and donor site surface areas.
The surface area gain using tissue expanders is debatable. In an in vivo model, it was found to be entirely different. The surface area gained in vivo was significantly less as compared with the in vitro model and that determined by the mathematical calculation.[33] van Rappard et al. found that with spherical or semispherical expanders, the surface area gain was only 25% of the mathematical calculation, and it was only 38% and 32% with rectangular and crescent shape expanders, respectively. This was true for expanders of any size. They concluded that mathematical calculations can only guide the choice of an expander. The dimensions calculated need to be multiplied by an appropriate factor for round (6), rectangular (3.75) and crescent-shaped (4.5) expanders. Further, it requires a sound clinical judgement to finally decide the shape and size of the expander.[33]
Recently, 3-D photogrammetry has been used for presurgical estimation of volume deficiency in a series of craniofacial microsomia patients. The volume deficiency was calculated by superimposing the 3-D mirror image of the normal side in these patients. 3-D photogrammetry estimated the requirement of tissue expansion. This was further used for volumetric changes during the postoperative period.[35]
With advancement in computer programmes, the mathematical calculations have become easy. In clinical practice, while selecting a tissue expander, it is difficult to decide the volume of the expander for a given defect. Mathematical calculations given in the literature are feasible but awkward for medical personnel, as we are not in the habit of using these formulae. This difficulty can be overcome by using a computer programme. We have used the Microsoft excel sheet to calculate the volume of tissue expander for a given defect. This excel programme automatically calculates the volume on providing the area of the defect and length and the width of the base of the tissue expander to be used.
USE OF MICROSOFT EXCEL SPREAD SHEET FOR CALCULATING THE VOLUME OF AN EXPANDER
We have designed a method of mathematical calculation in the Microsoft Excel sheet for the ease of calculation of volume and height of a rectangular/cuboid tissue expander, when the area of defect to be resurfaced and the base of the expander or donor area are known [Table 2]. This spread sheet has been designed based on the mathematical calculations already discussed.[30,31,33,34] Hence, the steps of designing the excel sheet are not elaborated.
Table 2.
Excel sheet to calculate volume and height of rectangular/cuboid expander

The Excel sheet has been reproduced. The formula for calculation of the volume is represented in the B5 cell of the table and the formula for calculation of height of a cuboid/rectangular expander is represented in the B6 cell of the table. This formula in the Excel spread sheet automatically calculates the volume (as in B5 cell) and height (as in B6 cell). In case of cuboid expander, the length and breadth will be the same.
In case of spherical expander, the Excel spread sheet has been written to calculate the volume and height of the balloon when the defect area and the diameter of the base of the balloon are known [Table 3]. One should keep in mind that the diameter of the base of the spherical cap is used, not the diameter of the balloon itself, which will be different depending on the type of the expander [Figures 2a and b].
Table 3.
Excel spread sheet to calculate volume and height of a spherical expander

HOW TO USE THIS EXCEL SHEET
After opening a new spread sheet, the above table is copied. The text of column “A” and formula of column “B” should be pasted in the respective columns. B1, B2 and B3 should be replaced with respective data for a spherical expander and B1, B2, B3 and B4 for a rectangular/cuboid expander. Volume and height of the expander will be presented automatically in the respective cells [Figures 4a and 5b]. The additional allowance percent is flexible and is to be decided by the surgeon.
Figure 4.
(a) Scar over the forehead and scalp planned for resurfacing using an expander. Area of the defect (Ad) was 55 sq cm and radius of a spherical expander (r) was 4 cm. Additional tissue estimated was 20%. When these data were fed to the excel sheet, It calculated for 186 cc expander with height of 4.92 cm. A 200 cc hemispherical tissue expander has been used. (b) Front view of the postoperative result after removal of the expander and resurfacing of the defect. (c) Looking down view of the postoperative result after removal of the expander and resurfacing of the defect
Figure 5.
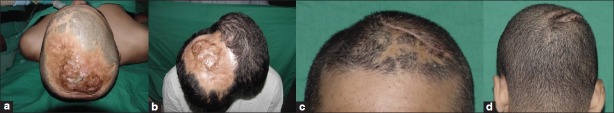
(a) A non-hair bearing 10 cm × 6 cm scar over the occipital region. (b) Occipital scar size was 60 sq cm. The base size of the expander was 7.8 × 5.9 cm. Additional tissue requirement was estimated as 40%. The volume of a rectangular tissue expander required to cover the defect was 172 cc and height is 3.74 cm. A 240 cc expander was used. (c) Postoperative result after removal of the expander and resurfacing of the scar with hair bearing expanded scalp
ADDITIONAL ALLOWANCE
There are certain areas in the expanded tissue that do not participate in the resurfacing of the defect after removal of the expanders. And, there are factors that require extra tissue for the movement of the tissue. While estimating the dimension of the required tissue, one should calculate additional expansion for the reasons given in Table 4. For example, in Figure 3a, 20% additional tissue has been calculated, while in Figure 4b, 40% additional tissue has been calculated. This additional allowance has been given a place in the computer Microsoft excel sheet in the form of B4% for rectangular and B3% for spherical expanders.
Table 4.
Factors requiring additional tissue than that mathematically calculated

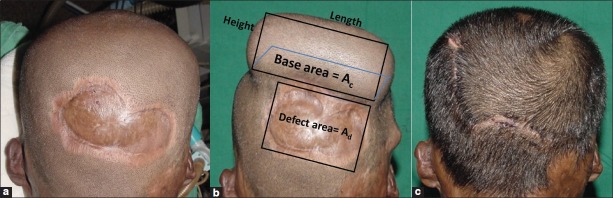
Figure 3.
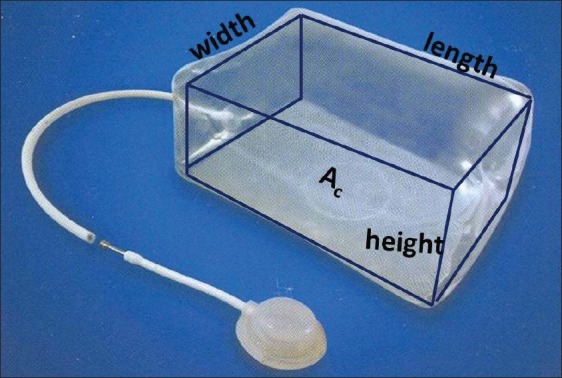
A diagram depicting the dimensions of a rectangular expander. Ac is the base area of the expander. When tissue expander is placed, five sides of the expander participate in tissue enlargement except its base (Ac)
Lastly, even if one has chosen a smaller expander or after filling the expander to its capacity, if one feels that the expanded tissue may fall short, then one can overinflate the expander without the risk of rupture.[36] This is in regular clinical practice. Hence, one has to generate extra tissue to resurface the defect as well as the donor site, which may differ from case to case.
CLINICAL APPLICATION
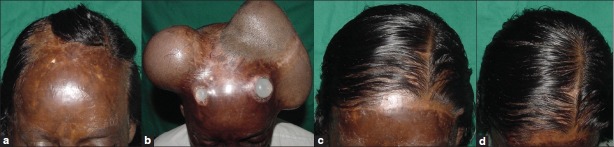
This Excel spread sheet has been used by the first author for selection of tissue expander in 20 patients over a period of 2 years. It has been observed that the volume of the available expander with specific base size is fixed. There are not too many choices of expanders of different volumes with the same base size in the market. Hence, this software is a guide for choosing the volume of an expander. This has helped us in choosing expanders for patients. We selected a 200 cc hemispherical expander for a patient with scar over the scalp and forehead [Figure 4], a 250 cc rectangular expander for an occipital scalp scar [Figure 5], a large tissue expander of 450 cc for a hairless and adherent scar over the scalp [Figure 6] and two spherical expanders for postburn alopecia and scalp scar [Figure 7].
Figure 6.
(a) A 20 cm × 18 cm hairless scar over the vertex. (b) Single 450 cc spherical tissue expander has been used. (c) Front view of the postoperative result after removal of the expander and resurfacing of the scar with hair bearing scalp. (d) Occipital view of the postoperative result after removal of the expander and resurfacing of the scar with hair bearing scalp
Figure 7.
(a) Post thermal burn hairless scar over the scalp and forehead. (b) Two spherical expanders have been used with guidance with the excel spread sheet. The ports of the expanders are exposed through the scar. (c) Front view of the patient after removal of the expander and replacing the scar- 4 months follow up. Front hairline has been reconstructed. (d) View of the patient's scalp while looking down, after removal of the expander and replacing the scar-4 months follow up. Hairless scar has been replaced with hair bearing scalp
CONCLUSIONS
Knowledge of tissue regeneration in tissue expansion is important for its clinical application. The beginner is concerned about the biological changes caused by this procedure. Most of these changes are temporary and revert back to normal after removal of the expander. Ultimately, one is able to achieve increased surface area of the tissue by mechanical creep and biological stretch, which is used for resurfacing of the defect.
The main difficulty of this innovative surgical technique is the inadequacy of the tissue at the time of final reconstruction. Tissue expansion causes enlargement of the tissue; however, there are many factors that increase the requirement of tissue. The surgeon has to adequately plan for these additional requirements. Till today, choosing an appropriate expander is the major shortcoming of this surgical and biological marvel. As the mathematical calculations and computer programmes available in the literature are not popular, a new user-friendly programme is being introduced. This programme requires further evaluation to evaluate the inter-rater reliability. With a large experience of using tissue expanders in different parts of the body, the shape of the expander is decided based on the shape of the defect to be resurfaced, the size and shape of the donor site and reverse planning of the flap movement after tissue expansion. Clinical judgement remains the key to success.
ACKNOWLEDGMENT
The authors thank Dr. Aparna Agrawal, MD, Director Professor LHMC and Associated Hospitals, New Delhi for her suggestions, editing and correction of English transcript. Acknowledgements are due to the residents of the department of Burns, Plastic and Reconstructive Surgery for imparting strategic help, whenever requested.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Radovan C. Breast reconstruction after mastectomy using the temporary expander. Plast Reconstr Surg. 1982;69:195–208. doi: 10.1097/00006534-198202000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Neumann CG. The expansion of an area of skin by progressive distention of a subcutaneous balloon, use of the method for securing skin for subtotal reconstruction of the ear. Plast Reconstr Surg. 1957;19:124–30. doi: 10.1097/00006534-195702000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Argenta LC. Controlled tissue expansion in reconstructive surgery. Br J Plast Surg. 1984;37:520–9. doi: 10.1016/0007-1226(84)90143-7. [DOI] [PubMed] [Google Scholar]
- 4.Pasyk KA, Argenta LC, Hassett C. Quantitative analysis of the thickness of human skin and subcutaneous tissue following controlled expansion with a silicone implant. Plast Reconstr Surg. 1988;81:516–23. doi: 10.1097/00006534-198804000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Coleman DJ. The role of contractile fibroblast in the capsules around tissue expanders and implants. Br J Plast Surg. 1993;46:547–56. doi: 10.1016/0007-1226(93)90104-j. [DOI] [PubMed] [Google Scholar]
- 6.Sasaki GH, Pang CY. Pathophysiology of flaps raised on expanded pig skin. Plast Reconstr Surg. 1984;74:59–67. doi: 10.1097/00006534-198407000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Lee Y, Gil MS, Hong JJ. Histomorphologic changes of hair follicles in human expanded scalp. Plast Reconstr Surg. 2000;92:710–6. doi: 10.1097/00006534-200006000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Radovan C. Tissue expansion in soft-tissue reconstruction. Plast Reconstr Surg. 1984;74:482–90. doi: 10.1097/00006534-198410000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Gur E, Hanna W, Andrighetti L, Semple JL. Light and electron microscopic evaluation of the pectoralis major muscle following tissue expansion for breast reconstruction. Plast Reconstr Surg. 1998;102:1046–51. doi: 10.1097/00006534-199809040-00019. [DOI] [PubMed] [Google Scholar]
- 10.Kim KA, Hong C, Futrcll JW. Histomorphologic Changes in expanded skeletal muscle inn rats. Plast Reconstr Surg. 1993;92:710–6. doi: 10.1097/00006534-199309001-00022. [DOI] [PubMed] [Google Scholar]
- 11.Zhou Y, Yan Z, Zhang H, Lu W, Liu S, Huang X, et al. Expansion and delivery of adipose-derived mesenchymal stem cells on three microcarriers for soft tissue regeneration. Tissue Eng Part A. 2011;17:2981–97. doi: 10.1089/ten.tea.2010.0707. [DOI] [PubMed] [Google Scholar]
- 12.Moelleken B, Mathes S, Cann C, Simmons DJ, Ghafoori G. Long term effects of tissue expansion on cranial and skeletal bone development in neonatal miniature swine: clinical finding and histomorphometric correlates. Plast Reconstr Surg. 1990;86:825–34. doi: 10.1097/00006534-199011000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Antonyshyn O, Gruss JS, Mackinnon SE, Zuker R. Complications of soft tissue expansion. Br J Plast Surg. 1988;41:239–50. doi: 10.1016/0007-1226(88)90107-5. [DOI] [PubMed] [Google Scholar]
- 14.Lari AA. Tissue expansion. J R Coll Surg Edinb. 1992;37:149–54. [PubMed] [Google Scholar]
- 15.Awad MMS. The effect of tissue expanders on the growing craniofacial skeleton. Indian J Plast Surg. 2006;39:22–8. [Google Scholar]
- 16.Cherry GW, Austad E, Pasyk K, McClatchey K, Rohrich RJ. Increased survival and vascularity of random pattern skin flaps elevated in controlled, expanded skin. Plast Reconstr Surg. 1983;72:680–7. doi: 10.1097/00006534-198311000-00018. [DOI] [PubMed] [Google Scholar]
- 17.Lantieri LA, Martin-Garcia N, Wechsler J, Mitrofanoff M, Raulo Y, Baruch JP. Endothelial Growth Factor Expression in expanded tissue: a possible mechanism of angiogenesis in tissue expansion. Plast Reconstr Surg. 1998;101:392–8. doi: 10.1097/00006534-199802000-00020. [DOI] [PubMed] [Google Scholar]
- 18.Takei T, Mills I, Arai K, Sumpio BE. Molecular basis for tissue expansion: clinical implications for the surgeon. Plast Reconstr Surg. 1998;102:247–58. doi: 10.1097/00006534-199807000-00044. [DOI] [PubMed] [Google Scholar]
- 19.Brobmann G, Huber J. Effect of different shape tissue expanders on transluminal pressure, oxygen tension, histopathologic changes and skin expansion in pigs. Plast Reconstr Surg. 1985;76:731–6. doi: 10.1097/00006534-198511000-00013. [DOI] [PubMed] [Google Scholar]
- 20.DeFilippo RE, Atala A. Stretch and growth: the molecular and physiologic influences of tissue expansion. Plast Reconstr Surg. 2001;109:2450–61. doi: 10.1097/00006534-200206000-00043. [DOI] [PubMed] [Google Scholar]
- 21.Austad ED, Thomas SB, Pasyk K. Tissue expansion: dividend or loan? Plast Reconstr Surg. 1986;78:63–7. doi: 10.1097/00006534-198607000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Stark GB, Hong C, Futrell JW. Rapid elongation of arteries and veins in rats with a tissue expander. Plast Reconstr Surg. 1987;80:567–78. doi: 10.1097/00006534-198710000-00016. [DOI] [PubMed] [Google Scholar]
- 23.Manders E, Sasaki G, Austad E. Tissue expansion today. A symposium sponsored by the Plastic Surgery Educational foundation, Hershey, Pa. 1986 Sep 23; (Referred: Austad ED, Pasyk KA. Discussion on Rapid elongation of arteries and veins in rats with a tissue expander. Plast Reconstr Surg 1987;80:579-81.) [Google Scholar]
- 24.Martini DV, Har-el G, Mcphee J, Lucente F. Rapid intraoperative facial nerve expansion. Otolaryngol Head Neck Surg. 1996;114:602–12. doi: 10.1016/s0194-5998(96)70254-1. [DOI] [PubMed] [Google Scholar]
- 25.Ikeguch EF, Stifelman MD, Hensle TW. Ureteral tissue expansion for bladder augmentation. J Urol. 1998;159:1665–8. doi: 10.1097/00005392-199805000-00086. [DOI] [PubMed] [Google Scholar]
- 26.Tse DT, Abdulhafez M, Orozco MA, Tse JD, Azab AO, Pinchuk L. Evaluation of an integrated orbital tissue expander in congenital anophthalmos: report of preliminary clinical experience. Am J Ophthalmol. 2011;151:470–82. doi: 10.1016/j.ajo.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 27.Chudacoff RM, Alexander J, Alvero R, Segars JH. Tissue expansion vaginoplasty for treatment of congenital vaginal agenesis. Obstet Gynecol. 1996;87:865–8. [PubMed] [Google Scholar]
- 28.Shively RE. Skin-Expander volume estimator. Plast Reconstr Surg. 1986;77:482–3. doi: 10.1097/00006534-198603000-00031. [DOI] [PubMed] [Google Scholar]
- 29.Raposio E, Santi P. Computer-aided preoperative planning of tissue expansion. Ann Plast Surg. 1997;39:416–7. doi: 10.1097/00000637-199710000-00013. [DOI] [PubMed] [Google Scholar]
- 30.Patel PK. Estimating the tissue-expander volume: a poor man's recipe. Plast Reconstr Surg. 1986;78:426. doi: 10.1097/00006534-198609000-00035. (Letter) [DOI] [PubMed] [Google Scholar]
- 31.Duits EHA, Molenaar J, van Rappard JHA. The modeling of skin expanders. Plast Reconstr Surg. 1989;83:362–5. doi: 10.1097/00006534-198902000-00031. [DOI] [PubMed] [Google Scholar]
- 32.Nordstrom RE. “Stretch back” in scalp redirections for male pattern baldness. Plast Reconstr Surg. 1984;73:422–6. [PubMed] [Google Scholar]
- 33.van Rappard JHA, Molenaar J, van Doorn K, Sonneveld GJ, Borghouts JMHM. Surface-area increase in tissue expansion. Plast Reconstr Surg. 1988;82:833–7. doi: 10.1097/00006534-198811000-00016. [DOI] [PubMed] [Google Scholar]
- 34.Bhandari PS. Mathematical calculations in a spherical tissue expander. Ann Plast Surg. 2009;62:200–4. doi: 10.1097/SAP.0b013e31817fe464. [DOI] [PubMed] [Google Scholar]
- 35.Jayaratne YS, Lo J, Zwahlen RA, Cheung LK. Three-dimensional photogrammetry for surgical planning of tissue expansion in hemifacial microsomia. Head Neck. 2010;32:1728–36. doi: 10.1002/hed.21258. [DOI] [PubMed] [Google Scholar]
- 36.Hallock GG. Maximum overinflation of tissue expanders. Plast Reconstr Surg. 1987;80:567–9. doi: 10.1097/00006534-198710000-00015. [DOI] [PubMed] [Google Scholar]