Abstract
ATP7A is a copper-transporting ATPase critical for central and peripheral nervous system function. Mutations in ATP7A cause Menkes disease and occipital horn syndrome (OHS), allelic X-linked recessive conditions that feature vascular abnormalities ascribed to low activity of lysyl oxidase, a copper-dependent enzyme. From a recently created Menkes disease/OHS patient registry, we identified 4 of 95 subjects with major congenital heart defects (4.2%), a proportion exceeding the general population prevalence (≈1%). In conjunction with mouse models of Menkes disease, OHS, and lysyl oxidase deficiency, which feature aortic aneurysms, irregular attachment between vascular endothelium and mesoderm and other defects of embryological development, our observation suggests an important role of copper metabolism in cardiac development. Congenital heart disease may be an under-appreciated abnormality in Menkes disease, and should be considered in a broad differential diagnosis of cardiac defects found prenatally in male fetuses. Conversely, newborn infants with suspected or confirmed Menkes disease should be evaluated for heart disease by careful clinical examination and echocardiography, if indicated.
Keywords: ATP7A, congenital heart disease, lysyl oxidase, Menkes disease, occipital horn syndrome
Introduction
Menkes disease is an infantile neurodegenerative disorder caused by mutations in ATP7A, an X-chromosomal gene that encodes a copper-transporting P-type ATPase (Kaler 2011; Kaler et al., 2008). Deficiency of ATP7A results in abnormal copper transport and reduced activities of copper-dependent enzymes, including dopamine-beta-hydroxylase (Kaler 2011) and lysyl oxidase (Royce et al., 1980). The clinical phenotype of Menkes disease includes neurodevelopmental delay, cerebral and cerebellar degeneration, seizures, growth retardation, and connective tissue abnormalities such as arterial and venous aneurysms, bladder diverticula, gastrointestinal polyps, and joint and skin laxity (Desai and Kaler 2008; Godwin et al., 2006; Kaler et al., 1993; Liu et al., 2005; Price et al., 2007; White et al., 1993). In occipital horn syndrome (OHS), a milder allelic variant caused by less severe ATP7A defects, such connective tissue manifestations predominate (Kaler et al., 1994). The impact of this predisposition on cardiac development has not been evaluated for either of these allelic conditions.
Lysyl oxidases are extracellular copper enzymes that play critical roles in the formation, stability, and repair of extracellular matrix, through oxidative deamination of lysine and hydoxylysine residues in elastin and collagen (Mäki 2009; Rodríguez et al., 2008). In the absence of normal lysyl oxidase activity, formation of covalent cross-linkages that provide tensile and elastic character to skeletal, pulmonary and cardiovascular connective tissues is reduced. Five distinct lysyl oxidase isozymes exist which are encoded on different chromosomes and vary in tissue distribution (Hornstra et al., 2003). All require copper and lysyl tyrosyl quinone as cofactors (Alcudia et al., 2008).
Cells in patients with Menkes/OHS are deficient in delivering copper to the trans-Golgi compartment of cells where pro-lysyl oxidases are normally metallated (Alcudia et al., 2008; Kosonen et al., 1997; Ashino et al., 2010). Mouse models of these conditions, mottledviable brindled, mottledtohm and mottledblotchy, are caused by mutations in Atp7a, the murine homolog of ATP7A, and illustrate the effects of lysyl oxidase deficiency. These include aortic aneurysms, yolk sac hemorrhage, and irregular attachment between vascular endothelium and mesoderm (Kaler 2011). The phenotype of lysyl oxidase knockout mice is similar, with a high incidence of aortic aneurysms and tortuosity (Rodríguez et al., 2008; Hornstra et al., 2003; Mäki et al., 2002). Cardiac-specific knockout of the copper import gene Ctr1 in mice (Ctr1hrt/hrt), results in cardiac copper deficiency, severe cardiomyopathy, and premature death (Kim et al., 2010). Dietary copper deficiency in rodents also predisposes to cardiac abnormalities (Keen et al., 2003). Rat embryos exposed to copper deficiency in vitro are characterized by a swollen pericardium, protruding heart, blood pooling, a small bulbus cordis (conotruncus), and distended anterior cardinal veins (Hawk et al., 1998). Copper-deficient mouse embryos show an increased incidence of total heart defects, including abnormal looping or heart swelling (Beckers-Trapp et al., 2006). Given these cumulative findings, we evaluated the frequency of congenital heart disease associated with ATP7A mutations in a cohort of 95 Menkes disease/OHS patients.
Methods
Subjects
A registry of 95 patients with Menkes disease or occipital horn syndrome (http://menkesohs.nichd.nih.gov) was retrospectively reviewed for subjects with congenital heart disease. Criteria for entry in the registry included clinical findings consistent with the diagnosis of Menkes disease or occipital horn syndrome, and biochemical or molecular confirmation (Kaler 2011; Kaler et al. 2008; Liu et al. 2002). Ninety patients from 74 families were diagnosed as having Menkes disease, and five patients from four families as having OHS. The patients reviewed were born between 1978 and 2010. A majority (n=85) resided in the United States. Ten infants were born in countries outside the US, including Australia, England, Germany, Italy (n=2), Jordan, New Zealand (n=2), and Trinidad (n=2). The study was conducted under protocols approved by Institutional Review Boards within the Division of Intramural Research, National Institutes of Health.
Cardiac Evaluation
The patients included in this analysis had medical reports of newborn physical examinations, with specific reference to auscultation of the heart and lungs, available for review. Subjects noted as having cardiac murmurs (n=7) underwent echocardiographic evaluation, the records of which were reviewed. All echocardiograms were obtained by trained echocardiographers, and written interpretations of the attending cardiologists were accepted as accurate. Patients with patent foramen ovale noted were not considered to have congenital heart disease.
Results
Evidence of major congenital heart disease was detected in four of 95 subjects, each with a diagnosis of Menkes disease and mutation at the ATP7A locus. Individual case histories are presented below, and summarized in Table 1. There was no known history of prior congenital heart disease in these families. Diagnostic tests such as array comparative genomic hybridization or whole exome sequencing to formally exclude alternative molecular bases for congenital heart disease had not been performed.
Table 1.
Characteristics of Four Menkes Disease Subjects with Congenital Heart Disease
| Patient | Cardiac Defect | ATP7A Mutation | Family History of Menkes disease | Clinical Phenotype | Early Copper* | Cardiac Outcome | Neurological Outcome |
|---|---|---|---|---|---|---|---|
| 1 | Tetralogy of Fallot | S637L | Positive | Mild Menkes | No | Surgical repair at 1 yr of age | Moderate neurodevelopmental delaysa |
| 2 | Transposition of the Great Arteries | IVS15 AS, −1, g→a | Negative | Classical Menkes | No | Rashkind balloon atrial septostomy at birth; Surgical repair at 2 weeks of age | Seizures and severe neurodevelopmental delays; died at 5 mosof age |
| 3 | Pulmonic Stenosis | L625X | Positive | Classical Menkes | Yes | Balloon valvuloplasty as neonate; tricuspid insufficiency; died from R heart failure at age 2.5 yr | EEG abnormalities and moderate neurodevelopmental delaysb |
| 4 | Coarctation of Aorta | IVS21 DS,+1, g→a | Negative | Classical Menkes | No | Surgical repair at 1 mo of age | Epilepsy; global neurodevelopmental delays at age 10 mos |
Copper histidine 250 μg sc b.i.d. beginning within 22 days of age (Kaler et al. 2008)
Described in Donsante et al., 2007
Described in Kaler et al., 2010
Case 1
This Hispanic male with a mild Menkes disease phenotype had tetralogy of Fallot (TOF) diagnosed shortly after birth and repaired at one year of age (Donsante et al., 2007). There was a family history of one older sibling and one younger sibling with Menkes disease but no cardiac disease. ATP7A mutation analysis for all three disclosed a missense mutation (S637L) in an exon 8 splicing enhancer that dramatically reduces the gene transcript and product (Donsante et al., 2007). This subject walked at 3 years of age and, at his current age of 9 years, shows severe speech delay.
Case 2
This patient was diagnosed prenatally as having transposition of the great vessels (TGV). At birth, he underwent emergency Rashkind balloon atrial septostomy to enlarge the atrial communication, thereby allowing for adequate mixing of blood and improved oxygenation. At two weeks of age, surgical correction of the transposition was performed. He experienced seizures at 7 weeks of age, showed poor neurodevelopment and was suspected as having Menkes disease at 4 ½ months of age. There was no prior family history. He died at 5 months of age and postmortem ATP7A gene analysis indicated mutation of a canonical splice junction position (IVS15 AS,-1,g→a), predicted to severely impair proper RNA splicing.
Case 3
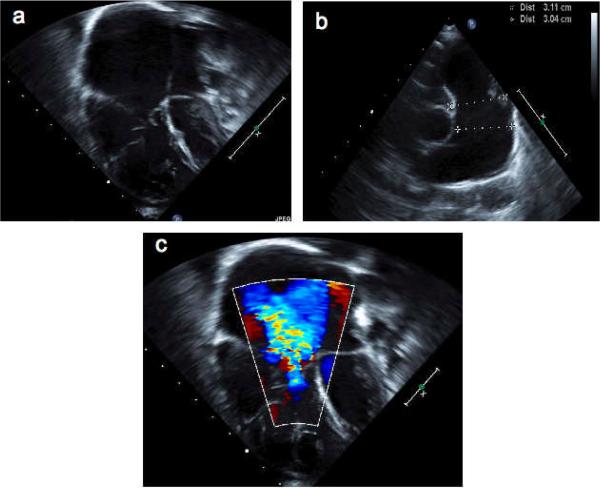
The patient's older brother had been affected by Menkes disease and died at three years of age. This older brother did not have known congenital heart disease. Neonatal screening by plasma catecholamine analysis was performed and indicated the diagnosis of Menkes disease in this infant. Treatment with copper injections, was begun at 16 days of age. Pulmonic stenosis, identified shortly after birth, was treated by balloon valvuloplasty. ATP7A gene analysis showed a nonsense mutation, L625X. He had EEG abnormalities and moderate neurodevelopmental delays, gastrointestinal bleeding from gastric polyps (surgically excised at 20 months of age), and a cardiac murmur that became progressively louder due to residual pulmonic stenosis and tricuspid regurgitation (Fig. 1). He developed right heart failure requiring digoxin and diuretics and died at home suddenly, at 2½ years of age.
Figure 1.
Echocardiographic abnormalities in Menkes disease. A series of echocardiographic images from patient #3 at age two years who underwent balloon valvuloplasty in the newborn period for severe pulmonary valve stenosis. By age two years, the right ventricle, atrium (panel A), and pulmonary artery (panel B) were massively dilated, and there was severe tricuspid valve regurgitation (panel C). Although valvular insufficiency is not uncommon following valvuloplasty, the degree of right heart dilation and dysfunction in this patient with Menkes disease was more severe, and developed more rapidly than expected.
Case 4
A three week old male infant of Pakistani heritage presented with tachypnea, congestion and decreased feeding. He was born at 35 weeks gestation with a birth weight of 2,925g. Blood pressure was 110/57 in the right arm, 113/55 in the left arm, 51/35 in the right leg, and 50/35 in the left leg. Brachial artery pulses were 2+ bilaterally, whereas femoral artery pulses were not palpable. Cardiovascular examination was significant for a grade II/VI systolic murmur heard over the back. A transthoracic echocardiogram showed juxtaductal coarctation of the aorta (peak coarctation pressure = 56 mm Hg), left-to-right shunting via a patent foramen ovale, physiological branch pulmonary stenosis, and a mildly dilated right atrium with borderline left ventricular hypertrophy. At 1 month of age, he underwent resection of the coarctation and end-to-end anastomosis.
At three months of age, he developed seizures. Brain MRI revealed abnormal T2 hyperintensity within the periventricular white matter region, as well as multiple small foci of restricted diffusion in the bilateral basal ganglia, internal capsules, and the right thalamus with decreased signal on apparent diffusion coefficient mapping, compatible with acute infarctions. He was started on levetiracetam and phenobarbital for management of seizures. Over the next three months, failure to thrive, and developmental delays were noted. On physical exam at six months of age, he showed pale hair, a high-arched palate, axial hypotonia, and appendicular hypertonia with hyperactive deep tendon reflexes. This constellation of findings and his clinical course suggested a diagnosis of Menkes disease which was confirmed by light microscopy of hair (showing pili torti), low serum copper and ceruloplasmin levels, and a splice junction mutation (IVS21 DS, +1 g→a) in ATP7A. He was begun on copper treatment after detailed discussions with his parents (Sheela et al. 2005).
Less severe cardiac malformations were noted in two other subjects. A Black male born at 36.5 weeks gestation to a G4P3 healthy mother with no family history of Menkes disease was noted to have differential hair pigmentation (Kaler, 1994) and a harsh grade II–III/VI systolic murmur at birth. Echocardiography revealed thickened pulmonic valve leaflets and mild pulmonary stenosis, which was not considered hemodynamically significant. The murmur persisted and prophylaxis against subacute bacterial endocarditis was advised. The infant was diagnosed subsequently as having Menkes disease based on plasma neurochemical levels (Kaler et al., 2008) and began copper treatment at 10 days of age. A nonsense mutation (W1187X) was detected in the ATP7A gene. While clinical seizure activity was never noted, he showed EEG abnormalities, poor neurodevelop-mental outcome, and died at 17 months of age (Kaler et al., 2010).
Another patient had clinical, radiographic and biochemical features consistent with OHS (Tang et al., 2006). There was no prior family history of copper transport disorders. During a childhood evaluation for dizziness, echocardiography disclosed an atrial septal defect, considered functionally insignificant. He is now 20 years old and attends college.
Discussion
The population prevalence of congenital heart disease is estimated at 1% (Brown et al., 2009). It is known that mutations in various transcription factor genes can disrupt proper mammalian cardiac development (Nemer 2008), and that certain genetic disorders, such as trisomy 18, Marfan syndrome, and Williams syndrome commonly manifest cardiac abnormalities (Zeigler 2008). However, the prevalence of congenital heart disease associated with Menkes disease and its variants (Kaler et al., 1994; Kennerson et al., 2010) has not been estimated previously. Although we found a four-fold higher proportion in our large Menkes/OHS cohort, we cannot exclude biased ascertainment as a contributory factor. In addition, the present findings need to be replicated in future independent cohorts of patients with ATP7A mutations.
We propose that an increased frequency of congenital heart disease in these conditions may relate to prenatal deficiency of lysyl oxidase, the cuproenzyme required for proper formation and stability of extracellular matrix (Mäki 2009; Rodríguez et al., 2008; Alcudia et al., 2008). Embryonic myocardial cells secrete a specialized extracellular matrix interposed between the endocardium and myocardium (Moon 2008), a process required for cardiac development and which coincides with initial expression of lysyl oxidase in rat embryos (Mäki 2009). The similar cardiovascular problems found among the lysyl oxidase knockout mouse (Hornstra et al., 2003; Mäki et al., 2002) and Menkes/OHS mouse models (Kaler 2011; Mototani et al., 2006) lend further credence to this hypothesis. The abnormalities of the right ventricular conal region in TOF and persistence of the aortic subconus with resorption of the subpulmonic conus in TGV may correlate with the conotruncal abnormalities documented in rodent embryonic copper deficiency (Hawk et al., 1998). On the other hand, among seven subjects who underwent echocardiography, none were noted to have aortic aneurysms, a relatively common abnormality in the mottled mouse series of defective copper transport (Kaler 2011).
Postnatal copper treatment would not be expected to influence cardiac developmental problems in either mouse models or affected humans, however prenatal copper repletion or gene addition (Mototani et al., 2006; Donsante et al., 2011) could be effective, depending on the timing of intervention.
In summary, congenital heart disease may be an under-appreciated abnormality in Menkes disease, which should be considered in a broad differential diagnosis when cardiac defects are found prenatally in male fetuses. Conversely, newborn infants with suspected or confirmed Menkes disease should be evaluated for heart disease by careful clinical examination and echocardiography, if indicated.
Acknowledgements
We gratefully acknowledge Maryellen Rechen for expert care of the patients and their families. This work was supported by the Division of Intramural Research, National Institutes of Health.
This work was supported by the National Institutes of Health Division of Intramural Research, Grant HD008768 (NICHD)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alcudia JF, Martinez-Gonzalez J, Guadall A, Gonzalez-Diez M, Badimon L, Rodríguez C. Lysyl oxidase and endothelial dysfunction: mechanisms of lysyl oxidase down-regulation by pro-inflammatory cytokines. Front Biosci. 2008;13:2721–2727. doi: 10.2741/2879. [DOI] [PubMed] [Google Scholar]
- Ashino T, Sudhahar V, Urao N, et al. Unexpected Role of the Copper Transporter ATP7A in PDGF-Induced Vascular Smooth Muscle Cell Migration. Circ Res. 2010;107:787–799. doi: 10.1161/CIRCRESAHA.110.225334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckers-Trapp ME, Lanoue L, Keen CL, Rucker RB, Uriu-Adams JY. Abnormal development and increased 3-nitrotyrosine in copper-deficient mouse embryos. Free Radic Biol Med. 2006;40:35–44. doi: 10.1016/j.freeradbiomed.2005.08.020. [DOI] [PubMed] [Google Scholar]
- Brown ML, Dearani JA, Burkhart HM. The adult with congenital heart disease: medical and surgical considerations for management. Curr Opin Pediatr. 2009;21:561–564. doi: 10.1097/MOP.0b013e3283302685. [DOI] [PubMed] [Google Scholar]
- Desai V, Kaler SG. Role of copper in human neurological disorders. Am J Clin Nutr. 2008;88:855S–858S. doi: 10.1093/ajcn/88.3.855S. [DOI] [PubMed] [Google Scholar]
- Donsante A, Tang JR, Godwin SC, Holmes CS, Goldstein DS, Bassuk A, Kaler SG. Differences in ATP7A gene expression underlie intra-familial variability in Menkes disease/occipital horn syndrome. J Med Genet. 2007;44:492–497. doi: 10.1136/jmg.2007.050013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donsante A, Yi L, Zerfas P, et al. ATP7A gene addition to the choroid plexus results in long-term rescue of the lethal copper transport defect in a Menkes disease mouse model. Mol Ther. 2011 doi: 10.1038/mt.2011.143. advance online publication 30 August 2011. doi:10.1038/mt.2011.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godwin SC, Shawker T, Chang M, Kaler SG. Brachial artery aneurysms in Menkes disease. J Pediatr. 2006;149:412–415. doi: 10.1016/j.jpeds.2006.05.041. [DOI] [PubMed] [Google Scholar]
- Hawk SN, Uriu-Hare JY, Daston GP, Jankowski MA, Kwik-Uribe C, Rucker RB, Keen CL. Rat embryos cultured under copper-deficient conditions develop abnormally and are characterized by an impaired oxidant defense system. Teratology. 1998;57:310–320. doi: 10.1002/(SICI)1096-9926(199806)57:6<310::AID-TERA4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Hornstra IK, Birge S, Starcher B, Bailey AJ, Mecham RP, Shapiro SD. Lysyl oxidase is required for vascular and diaphragmatic development in mice. J Biol Chem. 2003;278:14387–14393. doi: 10.1074/jbc.M210144200. [DOI] [PubMed] [Google Scholar]
- Kaler SG. ATP7A copper transport diseases: emerging concepts and future trends. Nat Rev Neurol. 2011;7:15–29. doi: 10.1038/nrneurol.2010.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaler SG, Westman JA, Bernes SM, et al. Gastrointestinal hemorrhage associated with gastric polyps in Menkes disease. J Pediatr. 1993;122:93–95. doi: 10.1016/s0022-3476(05)83496-1. [DOI] [PubMed] [Google Scholar]
- Kaler SG, Gallo LK, Proud VK, et al. Occipital horn syndrome and a mild Menkes phenotype associated with splice site mutations at the MNK locus. Nat Genet. 1994;8:195–202. doi: 10.1038/ng1094-195. [DOI] [PubMed] [Google Scholar]
- Kaler SG, Holmes CS, Goldstein DS, et al. Neonatal diagnosis and treatment of Menkes disease. N Engl J Med. 2008;358:605–614. doi: 10.1056/NEJMoa070613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaler SG, Liew CJ, Donsante A, Hicks JD, Sato S, Greenfield JC. Molecular correlates of epilepsy in early diagnosed and treated Menkes disease. J Inher Metab Dis. 2010;33:583–589. doi: 10.1007/s10545-010-9118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen CL, Hanna LA, Lanoue L, Uriu-Adams JY, Rucker RB, Clegg MS. Developmental consequences of trace mineral deficiencies in rodents: acute and long-term effects. J Nutr. 2003;133(5 Suppl 1):1477S–1480S. doi: 10.1093/jn/133.5.1477S. [DOI] [PubMed] [Google Scholar]
- Kennerson ML, Nicholson GA, Kaler SG, et al. Missense mutations in the copper transporter gene ATP7A cause X-linked distal hereditary motor neuropathy. Am J Hum Genet. 2010;86:343–352. doi: 10.1016/j.ajhg.2010.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BE, Turski ML, Nose Y, Casad M, Rockman HA, Thiele DJ. Cardiac copper deficiency activates a systemic signaling mechanism that communicates with the copper acquisition and storage organs. Cell Metab. 2010;11:353–363. doi: 10.1016/j.cmet.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosonen T, Uriu-Hare JY, Clegg MS, Keen CL, Rucker RB. Incorporation of copper into lysyl oxidase. Biochem J. 1997;327(Pt 1):283–289. doi: 10.1042/bj3270283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P-C, McAndrew PE, Kaler SG. Rapid and robust screening of the Menkes disease/occipital horn syndrome gene. Genet Test. 2002;6:255–260. doi: 10.1089/10906570260471778. [DOI] [PubMed] [Google Scholar]
- Liu PC, Chen YW, Centeno J, Quesado M, Lem KE, Kaler SG. Downregulation of myelination, energy, and translational genes in Menkes disease brain. Molec Genet Metab. 2005;85:291–300. doi: 10.1016/j.ymgme.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Mäki JM. Lysyl oxidases in mammalian development and certain pathological conditions. Histol Histopathol. 2009;24:651–660. [Google Scholar]
- Mäki JM, Räsänen J, Tikkanen H, Sormunen R, Mäkikallio K, Kivirikko KI, Soininen R. Inactivation of the lysyl oxidase gene Lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice. Circulation. 2002;106:2503–2509. doi: 10.1161/01.cir.0000038109.84500.1e. [DOI] [PubMed] [Google Scholar]
- Moon A. Mouse models of congenital cardiovascular disease. Curr Top Dev Biol. 2008;84:171–248. doi: 10.1016/S0070-2153(08)00604-2. [DOI] [PubMed] [Google Scholar]
- Mototani Y, Miyoshib I, Okamura T, et al. Phenotypic and genetic characterization of the Atp7a Mo-Tohm mottled mouse: A new murine model of Menkes disease. Genomics. 2006;87:191–199. doi: 10.1016/j.ygeno.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Nemer M. Genetic insights into normal and abnormal heart development. Cardiovascular Pathology. 2008;17:48–54. doi: 10.1016/j.carpath.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Price D, Ravindranath T, Kaler SG. Internal jugular phlebectasia in Menkes Disease. Int J Pediatr Otorhinolarygol. 2007;71:1145–1148. doi: 10.1016/j.ijporl.2007.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez C, Martínez-González J, Raposo B, Alcudia JF, Guadall A, Badimon L. Regulation of lysyl oxidase in vascular cells: lysyl oxidase as a new player in cardiovascular diseases. Cardiovasc Res. 2008;79:7–13. doi: 10.1093/cvr/cvn102. [DOI] [PubMed] [Google Scholar]
- Royce PM, Camakaris J, Danks DM. Reduced lysyl oxidase activity in skin fibroblasts from patients with Menkes' syndrome. Biochem J. 1980;192:579–586. doi: 10.1042/bj1920579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheela SR, Manoj L, Liu P-C, Lem KE, Kaler SG. Copper replacement treatment for symptomatic Menkes disease: ethical considerations. Clin Genet. 2005;68:278–283. doi: 10.1111/j.1399-0004.2005.00496.x. [DOI] [PubMed] [Google Scholar]
- Tang J, Robertson SP, Lem KE, Godwin SC, Kaler SG. Functional copper transport explains neurologic sparing in occipital horn syndrome. Genet Med. 2006;8:711–718. doi: 10.1097/01.gim.0000245578.94312.1e. [DOI] [PubMed] [Google Scholar]
- White SR, Reese K, Sato S, Kaler SG. Spectrum of EEG findings in Menkes disease. Electroenceph Clin Neurophysiol. 1993;87:57–61. doi: 10.1016/0013-4694(93)90175-u. [DOI] [PubMed] [Google Scholar]
- Zeigler VL. Congenital heart disease and genetics. Crit Care Nurs Clin North Am. 2008;20:159–169. doi: 10.1016/j.ccell.2008.01.008. [DOI] [PubMed] [Google Scholar]