Abstract
Global deletion of the Igfbp2 gene results in the suppression of bone turnover. To investigate the role of IGFBP-2 in regulating osteoclast differentiation we cultured Igfbp2−/− bone marrow cells and found a reduction in the number of osteoclasts and impaired resorption. Addition of full length IGFBP-2 restored osteoclast differentiation, fusion and resorption. To determine the molecular domains of IGFBP-2 that were required for this effect to be manifest, Igfbp2−/− bone marrow cells mice were transfected with constructs in which the heparin binding (HBD) or the IGF- binding domains of IGFBP-2 were mutated. We found that both domains were necessary for osteoclastogenesis since expression of the mutated forms of either domain failed to support the formation of functionally mature osteoclasts. To discern the mechanism by which IGFBP-2 regulates osteoclast formation, PTEN abundance and phosphorylation status as well as AKT responsiveness to IGF-I were analyzed. Igfbp2−/− cells had elevated levels of PTEN and phospho-PTEN compared with controls. Expression of wild-type IGFBP-2 reduced the level of PTEN to that of wild-type cells. Cells expressing the IGF binding mutant showed suppression of PTEN and phospho-PTEN equivalent to the wild type protein, whereas those expressing the IGFBP-2 HBD mutant showed no PTEN suppression. When the ability of IGF-I to stimulate AKT activation, measured by Thr308 and Ser473 phosphorylation, was analyzed, stimulation of Ser473 in response to IGF-I in pre-osteoclasts required the presence of intact IGFBP-2. This effect was duplicated by the addition of a CK2 inhibitor that prevents the phosphorylation of PTEN. In contrast, in fully differentiated osteoclasts stimulation of Thr308 phosphorylation required the presence of intact IGFBP-2. We conclude that IGFBP-2 is an important regulator of osteoclastogenesis and that both the heparin and the IGF binding domains of IGFBP-2 are essential for the formation of fully differentiated and functional osteoclasts.
Introduction
Local and circulating IGF-I and -II are important regulators of bone metabolism (Canalis, 2009). The bio-availability of IGF I/II is determined by a family of IGF binding proteins (IGFBP 1 – 6). In addition to sequestering the IGFs, IGFBPs have been shown to have both enhancing and inhibiting effects on IGF action depending upon cell type and context (Firth and Baxter 2002). Furthermore, certain IGFBPs have been reported to have IGF independent effects (Wheatcraft and Kerney, 2009).
IGFBP-2 is the second most abundant circulating IGFBP and is expressed in several mammalian tissues including the skeleton (Jones and Clemmons, 1995). IGFBP-2 has been reported to be inhibitory towards bone growth (Hoeflich, et al., 2001). However, other evidence suggests that under certain circumstances, IGFBP-2 can enhance bone formation (Khosla et al., 1998, Conover et al., 2002, Kawai, 2011). To investigate the role of IGFBP-2 in bone remodeling, we characterized mice with a global deletion of the Igfbp2 gene (Igfbp2−/−). Igfbp2−/− mice had low bone mass, reduced bone turnover, and enhanced skeletal fragility, although this was gender and compartment specific (DeMambro, et al., 2008). Histomorphometric analysis of the Igfbp2−/− mice showed fewer osteoblasts and osteoclasts/bone perimeter, reduced bone formation and reduced mineralizing surface/bone surface. Taken together these findings provided genetic evidence that physiological concentrations of IGFBP-2 are required for normal bone acquisition.
Our in vitro studies also demonstrated that there was a defect in both osteoblast and osteoclast formation in bone marrow cells derived from the knockout compared with the control mice (DeMambro. et al., 2008). Furthermore we determined that the reduction in IGFBP-2 was associated with an increase in PTEN (phosphatase and tensin homolog deleted on chromosome 10) protein levels in osteoclasts. PTEN is a lipid phosphatase that is a negative regulator of the PI-3 kinase pathway that functions by converting PIP3 to PIP2. This attenuates signaling via the PI-3 kinase pathway by reducing membrane binding sites for AKT (Cantley, 2002). Since AKT has been shown to play a critical role in the survival and differentiation of osteoclasts (Mandal et al., 2009, Munugalavadla et al., 2008, Kwak et al., 2008, Lee et al, 2002, Lee et al., 2001) we hypothesized that IGFBP-2 mediated regulation of PI3 kinase activation through its ability to control PTEN levels could play an important role in the regulation of osteoclastogenesis.
In order to better understand the cell autonomous effects of IGFBP-2 on osteoclasts, one must consider the molecular structure of IGFBP-2. Although all the IGFBPs bind IGFs at a specific site in the N-terminal domain, there is variability in the linker and C-terminal regions of these proteins. Most of the IGF binding proteins contain a heparin binding domain (HBD) in their Cterminal region. IGFBP-2 is unique in that it also has a second HBD region in the linker domain between the N and C termini. This unique HBD region is required for the localization of IGFBP-2 to extracellular matrix (Russo, et al., 2005) and a peptide containing a sequence has been shown to have positive in vivo effects on bone mass in Igfbp2−/− mice (Kawai, 2011).
Therefore, the aims of this study were to 1) determine at what stage IGFBP-2 regulates osteoclastogenesis; 2) investigate whether IGFBP-2 mediates its effects on osteoclastogenesis in an IGF-dependent or independent manner; and 3) determine whether its ability to modulate PTEN plays a role in regulation of this process.
Materials and Methods
Materials
Alpha MEM medium, penicillin, and streptomycin were purchased from Life Technologies (Grand Island, NY). Recombinant human soluble Receptor Activator for Nuclear Factor κ B Ligand (shRANKL) was purchased from Peprotech Inc (Rockyville, NJ). Recombinant murine macrophage-colony stimulating factor (M-CSF) was purchased from R and D Systems Inc (Minneapolis, MN). Leukocyte Acid Phosphatase (TRAP) Kit was purchased from Sigma Aldrich (St. Louis, MO). Polyvinyl difluoride membranes (Immobilon P) were from Millipore Corporation (Billerica, MA). Autoradiographic film was from Pierce (Rockford, IL). The monoclonal anti-phosphotyrosine (PY99) was from Santa Cruz (Santa Cruz, CA). The anti-phospho-AKT and total AKT as well as the anti–PTEN and phospho-PTEN antibodies were purchased from Cell Signaling Technology, Boston, MA. All other reagents were from Sigma (St. Louis, MO) unless otherwise stated.
Animals
Generation of the B6.129-Igfbp2tm1Jep, referred to as Igfbp2−/− mice, has been described previously (DeMambro et al., 2008; Wood et al., 2000). All analyses were conducted using male and female homozygous mutant mice and same-sex littermate controls. For each experiment, the numbers of mutant and control mice used are provided in Results. All experimental studies and procedures involving mice were reviewed and approved by the Institutional Animal Care and Use Committee of Maine Medical Center Research Institute and The University of North Carolina at Chapel Hill.
Osteoclast cultures
Bone marrow cells were isolated by flushing the femurs from 8 week old Igfbp2−/− and Igfbp2+/+ male and female mice. Osteoclast-like cells were generated by plating the bone marrow cells at 1 × 106 cells per well in 96-well plates in differentiation medium: α-MEM supplemented with 5 % FCS, M-CSF, (30 ng/ml) and RANKL (30–100 ng/ml). Where indicated cells were treated with IGFBP-2 (200 ng/ml), the IGFBP-2 heparin binding domain peptide (200 ng/ml) or the casein kinase 2 (CK2) inhibitor 4,5,6,7-tetrabromobenzotriazole (TBB) at a concentration of 10 μM.
In cultures treated with 100 ng/ml RANKL, media and treatments were changed at day 3 and day 6, if needed. Between days 5–7, the cells were fixed using 2.5% glutaraldehyde and stained for tartrate-resistant acid phosphatase activity (TRAP5b). TRAP5b-positive multinucleated (more than four nuclei) osteoclasts were counted using light microscopy. The total number of TRAP5b-positive as well as the number of TRAP5b positive multinucleated cells was counted per well. In a separate set of experiments using 30 ng/ml RANKL media, cells and media were changed at days 3, 6 and 9. At day 10, the cells were fixed using 2.5% glutaraldehyde, stained for TRAP5b and counted at predetermined sites of 1 × 1 mm squares. At least five squares were measured in total per treatment. All results are expressed as mean ± SEM. The figure legends indicate the length of time in culture and therefore the RANKL concentration that was used. The figure legends indicate the length of time in culture and therefore the concentration of RANKL used.
Assessment of proliferation
An equal number of bone marrow cells from each genotype (1 × 105) were plated in each well of low attachment plates and incubated in αMEM supplemented with 5% FCS and M-CSF (30 ng/ml). The number of osteoclast precursors recovered after 3 days was counted to compare the responsiveness to M-CSF of the hematopoietic stem cells from the wild-type and Igfbp2−/− mice.
Preparation of the expression vector and virus stocks incorporating the cDNA encoding hemaglutinin (HA) tagged IGFBP-2 and the lacZ control (Con)
Full-length murine IGFBP-2 cDNA was purchased from Open Biosystems (Huntsville, AL) in pCMV-sport6 vector, and after PCR amplification, it was cloned into the pENTR/D-TOPO Gateway entry vector according to the manufacturer’s instructions (Invitrogen). The forward and reverse primers used to generate the PCR product were: forward primer, 5′-CACCATGCTGCCGAGATTGGGCGGCCC -3′; and reverse primer, 5′-TTAAGCGTA ATCTGGAACATCCTGCACACTTTGGGCATGGGCCC -3′. The forward primer includes a KOZAK sequence (boldface) placed 5 prime to the ATG (underlined) start site. The reverse primer contains the sequence encoding the hemagglutinin epitope (italicized) followed by the stop codon (boldface). The resultant product was sequenced (University of North Carolina Genome Analysis Facility, Chapel Hill, NC) to confirm the correct full-length murine IGFBP-2 sequence using M13 forward and reverse primers.
The wild-type IGFBP-2 inserted into the pENTR/D-TOPO vector was used as a template to make two substitution mutants. The first incorporated substitution of amino acids within the IGF binding domain of IGFBP-2 (IGFBP2ΔIGF). The wild-type sequence is 101ELPLKALV108 and the substitutions highlighted in bold were as follows: AAAKAAA. The second IGFBP-2 mutant (IGFBP2 ΔHBD) incorporated substitution of amino acids within the linker region of IGFBP-2 which encodes a heparin binding domain (HBD) which is unique to IGFBP-2. The wild-type sequence is 188KHLSLEEPKKLR 199 the substitutions, highlighted in bold, were as follows: AALSLEEPAALA. The Quick Change Site-Directed Mutagenesis kit by Stratagene (Agilent Technologies, Santa Clara, CA) was used to incorporate the base changes needed to encode these substitutions (shown in bold below).
To generate the IGFBP2ΔIGF construct the following primer was used: 5′-ACCCGGGCCGAGGCGGCCGCGGCTCCCAGAGCCGCGGGTACC – 3′ and to generate the IGFBP2 ΔHBD construct the following primer was used: 5′-AAGGGTGCCGCAGCCTCAGTCTGGAGGAGCCCGCGGCGTTGGCGCCGCCTCCC-3′. After selection of the correct clone based on sequencing, the cDNA encoding the wild-type protein and the two mutated forms of IGFBP-2 were transferred from the entry-vector into pLenti6-V5 DEST vector using the LR clonase reaction following the manufacturer’s instructions (Invitrogen). The pLenti6-V5 Dest control (LacZ) construct was obtained from Invitrogen.
Preparation of viral stocks
Viral stocks were generated with 293FT cells (Invitrogen) for each individual pLenti-construct. Cells were plated at 5 × 106 per 75-cm2 plate (Corning Glassworks, Corning, NY) the day before transfection in growth medium (DMEM-H with 10% FBS, streptomycin at 100 μg/ml, and penicillin at 100 U/ml). On the day of transfection, the culture medium was replaced with 5 ml of Opti-modified Eagle’s medium-I (Invitrogen) without antibiotics or serum. DNA-Lipofectamine 2000 complexes for each transfection were prepared according to the manufacturer’s protocol (Invitrogen). The next day, the medium containing the DNA-Lipofectamine 2000 complexes was removed and replaced with the growth medium. The virus-containing supernatants were harvested 48–72 h after transfection and centrifuged at 3000 rpm for 15 min at 4 C to pellet the cell debris. The supernatants were filtered and stored as 1-ml aliquots at −80 C.
Expression of IGFBP-2 protein in bone marrow cells from Igfbp2−/− mice
Bone marrow cells were isolated and plated in low attachment plates and enriched for osteoclast precursors by incubation with M-CSF (30 ng/ml). An equal number of cells were plated in wells of a 96 well plate and then incubated with 100μl of virus containing supernatant containing 4 μg/ml polybrene overnight. The virus supernatant was removed and the medium was replaced with osteoclast differentiation medium with 30 ng/ml RANKL as described above and incubated for 10 days. Parallel cultures were stained for TRAP5b activity or lysed to analyze HA/IGFBP-2 expression by western immunoblotting.
Bone resorption assay
Bone marrow cells were cultured as described above in Corning Osteo Assay Surface 96-well plates (Corning Life Sciences, Lowell, MA). Media and treatments were changed every 3 days until the end of a 14 day culture period. Wells were then washed with PBS, and half were fixed with 10% Formalin at 37° for 15 minutes. Following fixation these cells were then stained for TRAP5b and counted. The remaining wells were then incubated in 10% sodium hypochlorite for 5 minutes, washed twice with water, and stained with a modified Von Kossa Stain [5% (w/v) aqueous silver nitrate solution]. Wells were then incubated for 30 minutes in the dark, soaked in water for 5 minutes and treated with 100ul of 5% sodium carbonate (w/v in 10% formalin). Incubation for 5 minutes at room temperature reduces Ionic silver to metallic silver and the unresorbed mineralized surface turns black while the resorbed areas are white. Digital images were captured and % resorbed area/well analyzed using NIH Image Version 1.61. Three wells were assessed per treatment, in three different experiments.
Cell lysis and biochemical analysis
For the direct detection of protein osteoclast-like cells were induced by culturing bone marrow cells with M-CSF (30 ng/ml) RANKL (30 ng/ml) and other treatments as indicated. After either 5 or 10 days cultures were placed in serum-free medium overnight followed by short term (5 – 20 minutes) treatment with IGF-I (50 ng/ml). Cells were lysed in a modified RIPA buffer (the lysates from three wells were pooled). After centrifugation, equal amounts of cellular protein were mixed with non-reducing gel loading buffer, heated to 70 C for 10 min, and separated by SDS-PAGE (8%). To detect association between proteins or to analyze the tyrosine phosphorylation status, immunoprecipitation studies were performed as previously described (Maile, et al., 2003). After SDS-PAGE, the proteins were visualized by immunoblotting as we have previously described (Maile, et al., 2003). For immunoblotting, the antibodies were used at concentrations between 1:500 and 1:1000. Chemiluminescent images were scanned using a DuoScan T1200 (AGFA Brussels, Belgium) and band intensities of the scanned images were analyzed using NIH Image, version 1.61.
Statistics
ANOVA or the Student’s t test was used to compare differences between groups. Data presented for all the cell culture experiments correspond to three independent experiments with at least three replicate wells within each experiment. Data are expressed as mean ± SEM in tables and figures. Differences were considered significant when p < 0.05 unless otherwise noted.
Results
IGFBP-2 is required for osteoclast maturation
Analysis of bone marrow cells from male Igfbp2−/− and Igfbp2+/+ mice cultured in M-CSF and RANKL, revealed that the numbers of TRAP5b+ osteoclasts with > 4 nuclei were significantly lower in the Igfbp2−/− cultures after 7 days compared to Igfbp2+/+ (p <0.05; Table 1). There was a 32% decrease in osteoclast formation in the Igfbp2−/− cultures consistent with our previous observations (DeMambro, et al., 2008). This defect was restored by the addition of recombinant IGFBP-2 (200ng/ml) such that osteoclast formation in Igfbp2−/− cells increased to numbers that were comparable to cells from Igfbp2+/+ mice (Table 1).
Table 1.
Osteoclast formation in Igfbp2−/− and Igfbp2+/+bone marrow cultures
| Source | # of Osteoclasts/well | # of Osteoclasts/well + IGFBP-2 (200ng/ml) |
|---|---|---|
| Male Igfbp2 +/+ | 554.0 ± 16.4 | 584.7 ± 23.4 |
| Male Igfbp2 −/− | 372.3 ± 14.9a | 559.7 ± 36.1b |
| Female Igfbp2 +/+ | 452.0 ± 16.6 | 431.3 ± 9.4 |
| Female Igfbp2 −/− | 397.7 ± 9.5a | 458.3 ± 12.2b |
p<0.05 Igfbp2 +/+ versus Igfbp2 −/−
p<0.05 control versus IGFBP-2 treatment
Igfbp2−/− and Igfbp2+/+ bone marrow cells from female mice cultured under the same conditions revealed a modest but significant (12%) decrease in osteoclastogenesis (p <0.05; Table 1). This defect was also rescued with treatment of IGFBP-2 (200 ng/ml) (Table 1).
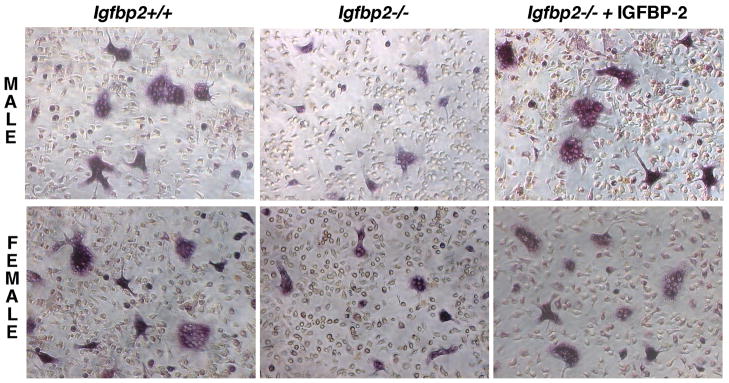
A representative image is shown in figure 1.
Fig. 1. In vitro osteoclastogenesis.
Representative images of TRAP5b+ osteoclast cells in the male and female Igfbp2−/− and Igfbp2+/+ from 7 day bone marrow cultures (left and middle panels). Igfbp2−/− cultures were also treated with 200 ng/ml IGFBP-2 (right) rescuing the null phenotype; all images captured at 20X magnification.
Proliferative response to M-CSF
An equal number of bone marrow cells were obtained from the femurs of Igfbp2+/+ and Igfbp2−/− mice and plated in low attachment plates (to assist in recovery of cells after culture). Cultures were treated with M-CSF for 3 days in the presence of 5 % FCS. In contrast to the significant increase in cell number in the Igfbp2+/+ cultures, there was no significant increase in cell number in the Igfbp2−/− cultures (1.7 ± 0.06 vs. 1.2 ± 0.14 fold increase; Table 2).
Table 2.
Proliferation of Igfbp2 +/+and Igfbp2−/− bone marrow cells treated with M-CSF
| Source | Total number of cells plated | Total number of cells harvested after M-CSF treatment |
|---|---|---|
| Igfbp2 +/+ | 1.9 ± 0.04 × 106 | 3.4 ± 0.13 × 106 |
| Igfbp2 −/− | 1.9 ± 0.04 × 106 | 2.1± 0.07 × 106 a |
p<0.05 Igfbp2 +/+ versus Igfbp2 −/−
Analysis of bone resorption capacity
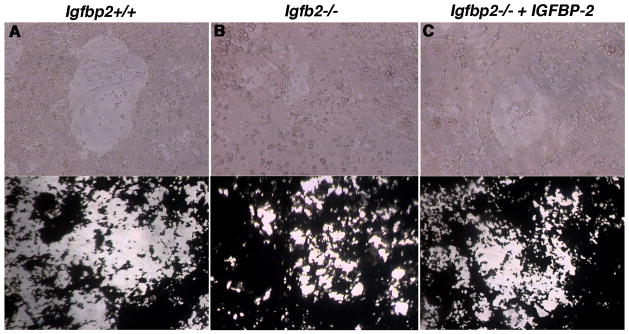
As a measure of function, we analyzed the bone resorption capacity of osteoclasts derived from Igfbp2+/+ and Igfbp2−/− bone marrow cells in vitro using the Corning Osteo Assay Surface. A mean of 38 ± 5 % of the surface area of each well was resorbed when the Igfbp2+/+ wild-type cultures were analyzed. In contrast a mean of 19 ± 2 % of the surface area of each well was resorbed following analysis of the Igfbp2−/− cultures (p <0.03, Fig. 2). The addition of recombinant IGFBP-2 to the Igfbp2−/− cultures resulted in a significant increase in bone resorption (37.0 ± 2.8 % of the total surface area), which was comparable to that observed when Igfbp2+/+ cells were used.
Fig. 2. Bone resorption in vitro.
Igfbp2−/− and Igfbp2+/+ osteoclast cultures plated on the Corning Osteo Assay Surface 96 well plate. Top panels are representative images, at 20X magnification, of osteoclasts in resorbed areas of the plate. A) Igfbp2+/+ B) Igfbp2−/− or C) Igfbp2−/− treated with IGFBP-2 (200 ng/ml). Bottom panels are examples of von Kossa stained wells (taken at 10X magnification) after 14 days in culture. The unresorbed matrix is black while the resorbed surface is white.
Rescue of osteoclastogenesis by recombinant IGFBP-2 is both IGF dependent and independent
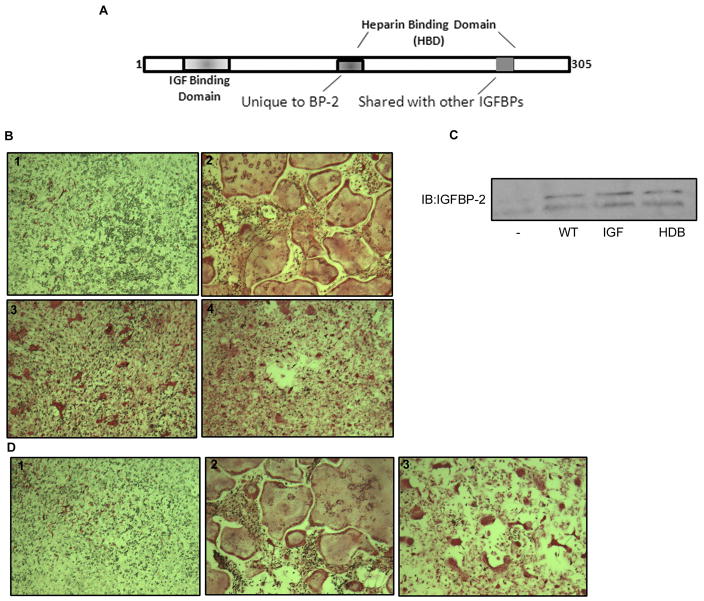
To compare the contribution of the IGF binding domain and the unique HBD region of the IGFBP-2 protein on osteoclastogenesis we made two mutant forms of IGFBP-2 (Fig. 3A). In one, 5 amino acids between amino acid 101 and 108, within the IGF binding domain were substituted and in a second mutant, 6 amino acids within the unique HBD region were substituted (i.e. between amino acids 188–199). Igfbp2 −/− bone marrow cells transduced to express either wild-type or one of the two mutant forms of IGFBP-2 were then compared with IGFBP-2 null bone marrow cells transduced with a control construct. Expression of the control construct did not result in an increase in mature osteoclast number following incubation with RANKL and MCSF (Table 3). In contrast expression of wild-type IGFBP-2 resulted in a significant increase in osteoclast formation compared with control cultures (p <0.05) However, expression of neither the IGF binding site nor the HBD substitution mutants was sufficient to increase osteoclast formation in the cells from the Igfbp2−/− mice resulting in a similar number of osteoclasts as those from the control construct (Table 3 and Fig. 3B). The lack of an effect was not due to differences in transduction efficiencies since secretion of IGFBP-2 was equivalent when the cells expressing wild-type and the two mutant forms of the protein were compared (Fig. 3C). Taken together these results suggest that both the IGF binding domain and the HBD region of IGFBP-2 contribute to mature osteoclast formation.
Fig. 3. In vitro osteoclastogenesis following expression of wild-type and mutant forms of IGFBP-2.
A) Diagram showing regions of IGFBP-2 including the two regions (IGF binding and unique HBD) in which amino acid substitutions were performed. B) Induction of osteoclasts in bone marrow cultures from Igfbp2−/− mice transduced with either a control construct (#1) or constructs expressing wild-type (#2) or the non-IGF binding mutant of IGFBP-2 (#3) or the heparin binding domain substitution mutant (#4). At day 10, the cells were fixed and stained for TRAP5b. C) Equal amounts of cell lysates from Igfbp2−/− bone marrow cultures expressing the different forms of IGFBP-2 obtained at day 10, were separated by SDS-PAGE and immunoblotted with the indicated antibody. D) Induction of osteoclast cells in bone marrow cultures of Igfbp2−/− and treated with vehicle (#1), IGFBP-2 (#2) or heparin binding domain peptide (#3). At day 10, the cells were fixed and stained for TRAP5b.
Table 3.
OC formation in cells derived from bone marrow cells from Igfbp-2 −/− mice
| IGFBP-2 construct | No of OC/1mm2 |
|---|---|
| Control | 10 ± 3 |
| IGFBP-2 (WT) | 60 ± 7 a |
| Δ IGF | 10 ± 4 |
| ΔHBD | 9 ± 3 |
p<0.05 +IGFBP-2 versus −IGFBP-2
Direct effect of the heparin binding domain (HBD) on Osteoclast maturation
To examine whether the HBD region exerted a direct effect on osteoclastogenesis we synthesized and treated Igfbp2−/− bone marrow cells with a synthetic peptide homologous to the unique HBD region of IGFBP-2 (Kawai, 2011). Treatment of cells with the HBD peptide induced a significant increase in TRAP5b+ cells to an extent that was not significantly different to the addition of wild-type IGFBP-2 (55 ± 6 vs. 31 ± 8) (Table 4A). However, the cells appeared smaller in size and contained fewer fused cells (as determined by counting the number of nuclei/TRAP5b+ cell) compared with cells from wild-type mice (Fig. 3D). Consistent with this result when we counted mature osteoclasts with greater than 4 nuclei per cell wild type IGFBP-2 stimulated a 4.5 fold increase but the HBD peptide had no effect (Table 4B).
Table 4A.
TRAP5b+ cells derived from bone marrow cells from Igfbp-2 −/− mice
p<0.05 +treatment versus −treatment
Table 4B.
OC formation in cells derived from bone marrow cells from Igfbp-2 −/− mice
| Treatment | No of OC/1mm2 |
|---|---|
| Control | 8 ± 3 |
| IGFBP-2 (WT) | 37 ± 4 a |
| HBD | 12 ± 8 |
p<0.05 +IGFBP-2 versus −IGFBP-2
To determine whether these smaller osteoclasts were fully functional we performed a resorption assay. As described above there was a significant decrease in the resorptive capacity of the Igfbp2−/− cells compared with the Igfbp2+/+ cells when both were incubated with M-CSF and RANKL (0.07 ± 0.06 versus 19.3 ± 2.1 % of the total surface area was digested). The addition of the HBD peptide to the Igfbp2−/− cells did not result in a significant increase in bone resorption compared with the Igfbp2+/+ cells treated with M-CSF and RANKL alone (0.08 ±0.02 %). This suggests that the smaller osteoclasts observed following incubation with the HBD peptide were not fully mature, functional osteoclasts.
Modulation of PTEN plays a role in the regulation of osteoclastogenesis by IGFBP-2
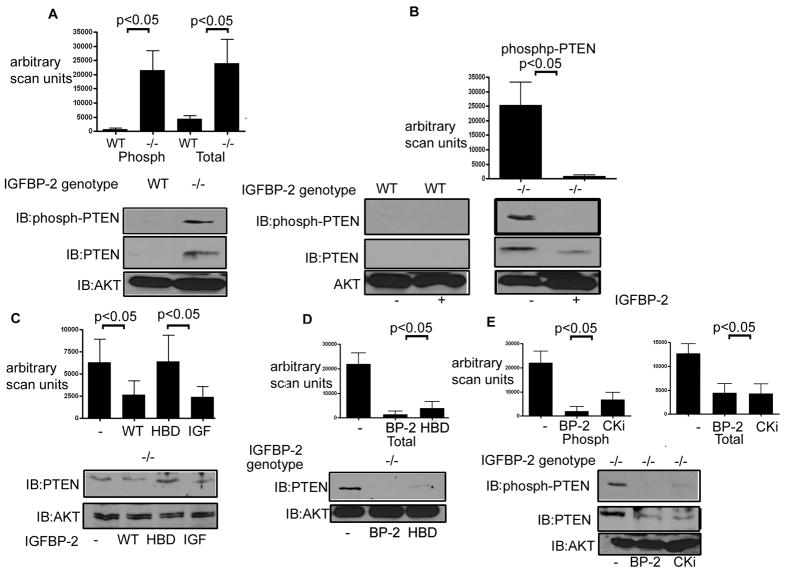
Previous studies from our laboratory and others have shown an inverse correlation between the level of IGFBP-2 and PTEN (DeMambro, et al., 2008, Perks, et al., 2007, Mehrian-Shai, et al., 2007). Consistent with our previous studies we determined that the level of PTEN was significantly elevated (i.e.10 ± 2 fold increase) in the cultures from Igfbp-2−/− mice compared with Igfbp-2 +/+ (p <0.05 Fig. 4A middle panel). The level of PTEN is controlled, in part, by its phosphorylation which functions to stabilize PTEN (Vazquez, et al., 2000). Therefore we compared the levels of phospho-PTEN using an antibody specific for phosphorylation of residues Ser380, Thr382/383 and Ser385. When equal amounts of total protein were examined we determined that the PTEN protein in the lysates from the Igfbp2−/− cultures exhibited a 10.4 ± 1.9 fold increase in phosphorylation compared to the lysates from the Igfbp2 +/+ cultures where there was no detectable phospho-PTEN (p <0.05, Fig. 4A top panel). Addition of IGFBP-2 reduced both phospho and total PTEN levels in the Igfbp-2−/− derived cultures (Fig. 4B) to a level that was indistinguishable from that in the Igfbp2 +/+ cultures.
Fig. 4. Regulation of PTEN by IGFBP-2.
A) Equal amounts of cell lysates from Igfbp2 −/− (−/−) and Igfbp2+/+ (WT) derived bone marrow cultures obtained 10 days after stimulation with M-CSF and RANKL (+) were separated by SDS-PAGE and immunoblotted with the indicated antibody. AKT was immunoblotted as a loading control.
B) Equal amounts of cell lysates from Igfbp2 −/− (−/−) and Igfbp2+/+(WT) derived bone marrow cultures treated with IGFBP-2 obtained 10 days after stimulation with M-CSF and RANKL (+) were separated by SDS-PAGE and immunoblotted with the indicated antibody. Some cultures received IGFBP-2 (200 ng/ml for 10 days). C) Equal amounts of cell lysates from Igfbp2 −/− derived bone marrow cultures treated with IGFBP-2 or HBD peptide for 10 days with M-CSF and RANKL (+) were separated by SDS-PAGE and immunoblotted for PTEN. Immunoblotting for total AKT was used to demonstrate that there was no significant difference in the total amount of protein in each lysate sample.
D) Equal amounts of cell lysates from the M-CSF primed bone marrow cultures of Igfbp2−/− cells that had been transduced with either a control construct (−) or constructs expressing wild-type (WT), heparin binding domain substitution (HBD) or the non-IGF binding mutant of IGFBP-2 (IGF) and treated for 10 days with RANKL and M-CSF were separated by SDS-PAGE and immunoblotted for PTEN. E) Lysates from Igfbp2−/− bone marrow cultures that were exposed to IGFBP-2 (BP-2) or the caseine kinase inhibitor (CK2) and RANKL+ M-CSF for 10 days were separated by SDS-PAGE and immunoblotted with the indicated antibody. The bar graphs show the results of scanning densitometry units obtained for 3 individual experiments.
To determine the contribution of the HBD region of IGFBP-2 to the regulation of PTEN Igfbp2−/− osteoclast cultures were incubated with recombinant wild-type IGFBP-2 and the two mutant forms of IGFBP-2. Exposure to wild type IGFBP-2 suppressed PTEN by 2.9 ± 1.3 fold (p<0.05) and the IGF binding domain mutant was equally effective whereas exposure to the HBD mutant form of IGFBP-2 had no effect (Fig. 4C). Since these data suggested that the HBD region of IGFBP-2 was responsible for the regulation of PTEN levels, we examined the ability of the HBD peptide to decrease the elevated levels of PTEN in Igfbp2−/− bone marrow cells. The addition of the heparin binding domain peptide was equally effective in reducing PTEN protein (Fig. 4D).
Regulation of PTEN phosphorylation contributes to its protein level
Although several different kinases have been reported to phosphorylate PTEN, casein kinase 2 (CK2) is believed to mediate the phosphorylation of Ser380, Thr382/383 and Ser385 (Al-Khouri, et al., 2005). To determine whether CK2 is involved in the phosphorylation of PTEN in the absence of IGFBP-2 we examined PTEN phosphorylation in Igfbp2−/− bone marrow cells in the presence or absence of the CK2 inhibitor TBB. In the presence of the CK2 inhibitor there was a significant decrease in PTEN phosphorylation (Figure 4E top panel) in the Igfbp2−/− bone marrow cells and the change was equivalent to cultures that had been exposed to IGFBP-2. Exposure to TBB also resulted in a similar degree of suppression of the PTEN protein level (Fig 4E middle panel).
IGFBP-2 is required for IGF-I stimulated ATK phosphorylation in maturing osteoclasts
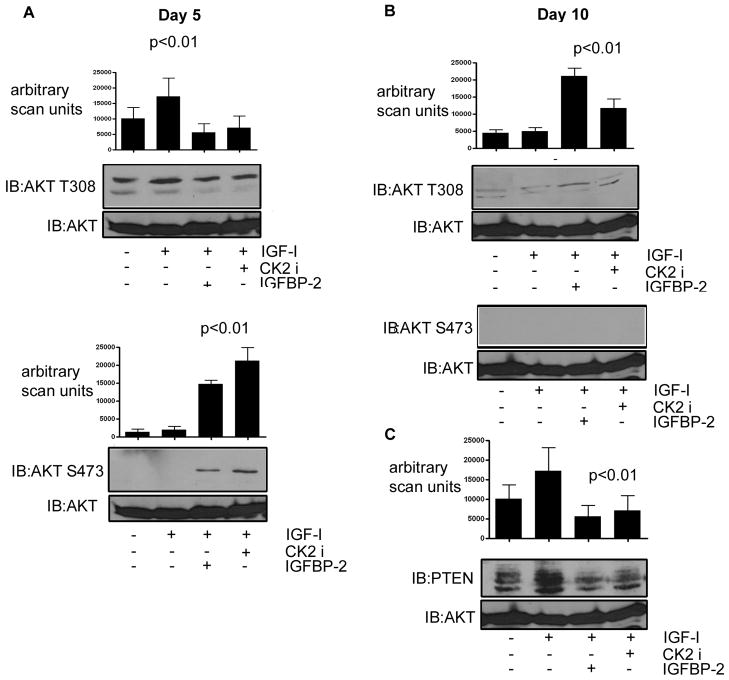
To understand the significance of the regulation of total and phosphorylated PTEN levels we examined AKT phosphorylation in response to IGF-I. Bone marrow cells from the Igfbp2 −/− mice that had been induced to undergo osteoclastogenesis in the presence or absence of exogenously added recombinant IGFBP-2 were serum-starved overnight prior to stimulation with IGF-I. We first examined AKT phosphorylation after 5 days in culture with M-CSF and RANKL, i.e. prior to osteoclast fusion and formation of mature osteoclasts. At that time point we detected robust phosphorylation of AKT Thr308 in the basal state which was increased, but not significantly, by IGF-I (Fig. 5A). In the presence of IGFBP-2 or the CK2 inhibitor there was no detectable increase in AKT Thr308 phosphorylation (Fig. 5A). This is likely due to the fact that at this time point the cells, which are grown in serum medium containing IGF-I, are highly proliferative and thus further stimulation does not elicit an increased response in terms of AKT Thr308 phosphorylation. However, when we examined phosphorylation of AKT Ser473 we determined that in contrast to Thr308, basal phosphorylation was undetectable (Fig. 5A lower panel). While IGF-I alone was unable to stimulate a significant increase in Ser473 phosphorylation (Fig. 5A lower panel), when the Igfbp2 −/− cells were incubated with IGFBP-2 or the CK2 inhibitor IGF-I stimulated robust phosphorylation of Ser473 [a 7.5 ± 0.6 and 9.8 ± 1.2 fold increase respectively compared with no IGF-I treatment (p<0.01)].
Fig. 5. Regulation of AKT phosphorylation by IGFBP-2.
Equal amounts of cell lysate from Igfbp2 −/− derived bone marrow cultures 5 (A) and 10 (B&C) days after stimulation with RANKL and M-CSF followed by short-term treatment with IGF-I (50ng/ml) (10 min) were separated by SDS-PAGE and immunoblotted with the indicated antibody. IGFBP-2 or the CK2 inhibitor were added for 10 days where indicated. The graph shows the results of the phospho-AKT immunoblots. The blots shown are derived from discontinuous lanes on the same blot. Immunoblotting for total AKT was used to demonstrate that there was no significant difference in the total amount of protein in each lysate sample.
After 10 days incubation in the presence of M-CSF and RANKL, (i.e. Igfbp2 −/− cells as mature fully fused osteoclasts) the cellular responses were significantly altered. The level of basal AKT phosphorylation as measured by Thr308 phosphorylation was much less robust (Fig 5B), consistent with the cells being less proliferative at this time point compared with day 5. Furthermore, while there was no significant increase in AKT Thr308 phosphorylation in response to IGF-I, incubation with either IGFBP-2 or the CK2 inhibitor plus IGF-I resulted in a 4.2 ± 0.7 fold increase in AKT Thr308 phosphorylation (p< 0.01). At the 10 day time point there was no detectable AKT Ser473 phosphorylation with any of the treatments (Fig. 5B middle panel). To relate these changes to the ability of IGFBP-2 to regulate PTEN levels we also examined PTEN levels in response to IGF-I in the 10 day cultures. IGF-I was unable to change the high level of PTEN in the Igfbp2 −/− cells (Fig. 5C). However, in the presence of IGFBP-2, IGF-I stimulated a significant 3.0 ± 0.2 fold (p<0.01) decrease in PTEN presumably permitting the increase in AKT Thr308 phosphorylation.
Discussion
In the current study we demonstrate that IGFBP-2 plays an important role in regulating the differentiation of hematopoietic precursors into mature, functional osteoclasts. Previously we reported that mice which had the Igfbp2 gene deleted had fewer osteoclasts per bone perimeter and markedly impaired bone resorption parameters (DeMambro et al., 2008). In the present study we found that during pre-osteoclastic growth, stimulation of Igfbp2−/− cells with M-CSF was not sufficient to promote full proliferation. Furthermore, when Igfbp2−/− bone marrow cells were exposed to M-CSF and RANKL, differentiation of TRAP5b+ osteoclasts and their fusion into mature osteoclasts was impaired.
The conventional differentiation medium used for generating osteoclasts is composed of either 5 or 10% fetal calf serum, which contains 10–20 ng/ml of IGF-I and 30–50 ng/ml of IGFBP-2. Therefore it is difficult to assess in vitro the relative importance of the IGF regulatory system in determining the pace of osteoclastogenesis. Importantly prior findings from Wang et al., using osteoclast precursors derived from Igf1−/− mice showed that IGF-I was clearly required to obtain adequate numbers of multinucleated osteoclasts (Wang, 2006). Our findings add to those studies and suggest that IGFBP-2 is critical for stimulating an optimal number of pre-osteoclasts, whereas both IGF-I and IGFBP-2 are required to generate fully functional osteoclasts.
To determine the molecular mechanism that mediates IGFBP-2 actions on osteoclasts, we examined the role of the heparin and IGF binding domains of the IGFBP-2 molecule. In the presence of M-CSF, RANKL and 5% FCS, the HBD peptide was adequate to achieve normal numbers of TRAP5b+ osteoclasts in Igfbp2−/− cultures. However the cells were small and the percentage of cells that contained <4 nuclei per cell was much greater than the Igfbp2+/+ controls (Fig. 3). In contrast when Igfbp2−/− cultures were treated with intact IGFBP-2, similar numbers of TRAP5b+ cells were obtained and many of the cells had greater than 10 nuclei per cell. Importantly there was a major functional difference between these two populations of osteoclasts. The cells that were generated following stimulation with the HBD peptide, most of which had less than 4 nuclei/cell showed reduced osteolytic activity in the pit resorption assay whereas osteoclasts generated with intact IGFBP-2 had robust osteolytic activity and multiple nuclei.
Next, we employed site directed mutagenesis to further define the importance of the various IGFBP-2 domains. Cells transfected with a mutant form of IGFBP-2 that did not bind to IGF-I had minimal ability to differentiate into osteoclasts. Similarly, a mutant in which the heparin binding domain was substituted with alanine residues such that the charge density was markedly altered also had minimal ability to induce differentiation into mature, functional osteoclasts. These findings support the conclusion that the HBD is absolutely essential for the formation of TRAP5b+ cells from bone marrow cells, but the IGF-I binding domain is also required for IGFBP-2 to induce fully functional osteoclasts.
It is conceivable that IGF-I binding to IGFBP-2 alters the function of the HBD, but this possibility was not addressed in this study. Other studies with IGF binding proteins have shown that heparin binding domains can enhance binding to cell surface proteins (Jones and Clemmons, 1995; Parker et al, 1998; Russo et al., 2005; Yang et al., 1996). This suggests that IGFBP-2 may be attaching either to a cell surface glycosaminoglycan or proteoglycan entity, thereby mediating its effect or that the charge density configuration of the HBD region is required for binding to a cell surface specific receptor. Our findings in this study and the work of others (Arai et al., 1996; Kuang et al., 2006) suggest that IGF-I binding to IGFBP-2 alters these interactions. Thus it is conceivable that a major role of IGF-I in osteoclastogenesis may not only be through activation of the Type I IGF receptor but also through its ability to bind IGFPBP-2 and thereby augment its activity on bone cells (Wang, 2006).
To further determine the downstream mechanisms for these two IGFBP-2 domains we examined the ability of IGFBP-2 to suppress PTEN levels in osteoclasts derived from Igfbp2+/+ and Igfbp2−/− bone marrow cells. At baseline levels of both phosphorylated and total PTEN were 4–5 fold higher in osteoclasts derived from Igfbp2−/− mice compared to Igfbp2+/+ littermates. When intact IGFBP-2 was added exogenously to osteoclasts from the Igfbp2−/− mice, it completely suppressed phosphorylated PTEN. Similarly, following transduction of the Igfbp2−/− cells with wild type IGFBP-2, PTEN was totally suppressed whereas transduction with the HBD mutant resulted in no change in PTEN. In contrast, transduction with the IGF binding mutant was capable of fully suppressing PTEN suggesting that this activity was retained in the absence of IGF-I binding to IGFBP-2. When either intact IGFBP-2 or the HBD peptide was added PTEN was also suppressed. However despite a reduction in PTEN levels neither the IGF binding domain mutant nor the HBD peptide were able to support the formation of mature, fully functional osteoclasts. These findings suggest that addition of HBD alone is adequate to suppress PTEN and that the HBD sequence within full length IGFBP-2 is the region that accounts for this function but that the IGF binding domain of IGFBP-2 is also required for the formation of mature fully functional osteoclasts.
Previously, Russo et al. showed that overexpression of IGFBP-2 in a neuroblastoma cell line led to enhanced proliferation and migration (Russo, 2005). Furthermore, they demonstrated that the heparin binding domain in IGFBP-2 mediated its ability to attach to extracellular matrix and that transfection of a heparin binding domain mutant into this cell line was not associated with increased proliferation. Other investigators have shown that IGFBP-2 can suppress PTEN in breast cancer cells and suggested that this was mediated through IGFBP-2 binding to integrins (Perks et al, 2008). Mehrian-Shar, et al., demonstrated that PTEN regulated IGFBP-2 bioactivity and that mouse embryo fibroblasts derived from Igfbp2−/− mice had elevated PTEN levels and defective AKT dependent cellular transformation (Mehrian-Shar,2007). Since Ser/Thr phosphorylation stabilizes PTEN and caseine kinase 2 is a kinase that phosphorylates PTEN in osteoclasts, we determined the effect of modulating CK2 activity. The CK2 inhibitor caused PTEN suppression that was equal to the effect of IGFBP-2, which is consistent with other reports that have shown that CK2-dependent PTEN phosphorylation stabilizes the protein (Torres and Pulido, 2001; Shehata et al., 2010). Thus, IGFBP-2 appears to be functioning in part by regulating PTEN phosphorylation status. Since CK2-mediated PTEN phosphorylation stabilizes PTEN these findings suggest that one mechanism by which IGFBP-2 regulates PTEN is through its ability to regulate CK2 binding and/or access to PTEN, or by inhibition of CK2 enzymatic activity.
To determine the significance of these changes in PTEN, we analyzed the AKT phosphorylation response to IGF-I. Pre-osteoclast cultures of Igfbp2−/− cells had constitutively high levels of phospho-AKT at Thr308 which, were slightly but not significantly increased with IGF-I; this response was inhibited with IGFBP-2 or the CK2 inhibitor. In contrast there was no AKT Ser473 phosphorylation when IGF-I alone was added but it was significantly increased with the addition of IGFBP-2 or the CK2 inhibitor. When fully differentiated cultures were examined, the results were very different. IGF-I could not stimulate Thr308 phosphorylation unless IGFBP-2 was present and the CK2 inhibitor partially restored this effect. The fully differentiated cells did not express phospho-AKT Ser473 in spite of adequate suppression of PTEN even in the presence of IGF-I and IGFBP-2. These findings suggest that during the preosteoclast growth phase AKT Ser473 phosphorylation requires both IGF-I and IGFBP-2 exposure, whereas phosphorylation of Thr308 can be minimally stimulated by IGF-I and this response is not enhanced with IGFBP-2. In contrast in fully differentiated cells, AKT Ser473 can no longer be stimulated but Thr308 phosphorylation is stimulated by IGF-I and this response is enhanced by the presence of IGFBP- 2. These findings support the conclusion that IGF-I and IGFBP-2 play complementary roles in signaling osteoclast differentiation and function.
Prior reports have defined IGF-I independent effects of IGFBPs to be those actions that are completely independent of IGF-I (Firth and Baxter, 2002). However, authors often utilize this term to mean those actions which do not require IGFBP binding to IGFs. In our studies it appears that IGFBP-2 acting through its HBD can suppress PTEN independently of IGF-I. Although this effect appears to be adequate for IGF-I to stimulate AKT Ser473 phosphorylation, stimulation of AKT Thr308 phosphorylation in fully differentiated cells requires the presence of an intact IGF-I binding domain. More importantly, since our IGF binding deficient form of IGFBP-2 was capable of suppressing PTEN but could not fully rescue the cellular phenotype, this finding suggests that IGF-I binding to IGFBP-2 as well as to the IGF-I receptor is required for, fully differentiated osteoclast function.
The importance of differential regulation of Ser473 and Thr308 phosphorylation for osteoclast differentiation has not been addressed in previous studies. Our findings suggest that growing osteoclasts require IGF-I induction of Ser473 phosphorylation and that Thr308 is principally regulated by constitutive activation during this phase. In contrast in fully mature differentiated cells, Ser473 phosphorylation is not present whereas Thr308 phosphorylation is regulated by IGF-I and maximal stimulation requires the presence of IGFBP-2. Prior studies have suggested that Thr308 phosphorylation is important for FOXO3A phosphorylation and transport out of the nucleus which subsequently alters FOXO3A regulated gene transcription (Zeng et al., 2009). Those studies revealed that inhibition of this process even with maintenance of Ser473 phosphorylation markedly accelerated apoptosis. Our studies in vascular smooth muscle have shown that selective inhibition of AKT Thr308 phosphorylation by downregulation of PDK1, the kinase that phosphorylates this residue, results in acceleration of apoptosis and an inability of IGF-I to inhibit this process (Shen et al., 2010). This suggests that maintenance of viable, differentiated osteoclasts and prevention of apoptosis in this cell type may require Thr308 phosphorylation and that IGFBP-2 may be important for osteoclast survival.
Prior studies have emphasized that the most important function of IGFBPs is to limit IGF-I access to receptors thus inhibiting IGF-I actions. Our study demonstrates that two distinct domains within IGFBP-2 are necessary for IGFBP-2 to enhance IGF-I actions in osteoclasts and that the two domains alter signaling through the PI-3 kinase pathway in a complementary manner. Although IGF-I binding to IGFBP-2 may simply be enhancing the amount of IGFBP-2 that binds to a cell surface glycosaminoglycan or a receptor, it is also possible that it alters this interaction in a qualitative manner and thus further enhances downstream signaling. In summary, we found that both IGF-I and IGFBP-2 are required for osteoclast proliferation and differentiation. Formation of functional osteoclasts requires not only IGF-I and the HBD region of IGFBP-2, but also the presence of an intact IGF-I binding site on IGFBP-2. Further elucidation of the molecular mechanism by which these two proteins function in a complementary manner may provide novel information for understanding the importance of the IGFs in the regulation of osteoclastogenesis in vivo.
Acknowledgments
The authors wish to thank Laura Lindsey for her help in preparing the manuscript. This work was supported by grants from the National Institutes of Health AR061164, AG02331 and AR053853
Abbreviations list
- IGF-I
Insulin-like growth factor-I
- IGFBP-2
Insulin-like growth factor binding protein-2
- HBD
Heparin binding domain
- TRAP
Tartrate resistant acid phosphatase
- CSF
Colony stimulating factor
- RANKL
Receptor activation nuclear factor K ligand
- HA
Hemagglutinin
Footnotes
VED was involved in acquisition of data, data analysis, drafting and revising of manuscript and approved of the final version
LAM contributed to the conception and design, was involved in acquisition of data, data analysis and interpretation, drafting and revising of manuscript and approved of the final version
CW, MK, TC were involved in acquisition of data and approved the final version
CR and DRC contributed to the conception and design, data interpretation, drafting and revising of manuscript and approved of the final version
LAM and DRC accept responsibility for the integrity of the data analysis
All authors state that they have nothing to disclose and no conflict on interest
References
- Arai T, Busby W, Jr, Clemmons DR. Binding of insulin-like growth factor (IGF) I or II to IGF-binding protein-2 enables it to bind to heparin and extracellular matrix. Endocrinol. 1996;137:4571–4575. doi: 10.1210/endo.137.11.8895319. [DOI] [PubMed] [Google Scholar]
- Al-Khouri AM, Ma Y, Togo SH, Williams S, Mustelin T. Cooperative phosphorylation of the tumor suppressor phosphatase and tensin homologue (PTEN) by casein kinases and glycogen synthase kinase 3beta. J Biol Chem. 2005;280:35192–35202. doi: 10.1074/jbc.M503045200. [DOI] [PubMed] [Google Scholar]
- Canalis E. Growth factor control of bone mass. J Cell Biochem. 2009;108:769–777. doi: 10.1002/jcb.22322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover CA, Johnstone EW, Turner RT, Evans GL, Ballard FJ, Doran PM, Khosla S. Subcutaneous administration of insulin-like growth factor (IGF)-II/IGF binding protein-2 complex stimulates bone formation and prevents loss of bone mineral density in a rat model of disuse osteoporosis. Growth Horm IGF Res. 2002;12:178–183. doi: 10.1016/s1096-6374(02)00044-8. [DOI] [PubMed] [Google Scholar]
- DeMambro VE, Clemmons DR, Horton LG, Bouxsein ML, Wood TL, Beamer WG, Canalis E, Rosen CJ. Gender-specific changes in bone turnover and skeletal architecture in IGFBP-2 null mice. Endocrinol. 2008;149:2051–2061. doi: 10.1210/en.2007-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev. 2002;23:824–854. doi: 10.1210/er.2001-0033. [DOI] [PubMed] [Google Scholar]
- Hoeflich A, Nedbal S, Blum WF, Erhard M, Lahm H, Brem G, Kolb HJ, Wanke R, Wolf E. Growth inhibition in giant growth hormone transgenic mice by overexpression of insulin-like growth factor-binding protein-2. Endocrinol. 2001;142:1869–1898. doi: 10.1210/endo.142.5.8149. [DOI] [PubMed] [Google Scholar]
- Jones JL, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- Khosla S, Hassoun AA, Baker BK, Liu F, Zein NN, Whyte MP, Reasner CA, Nippoldt TB, Tiegs RD, Hintz RL, Conover CA. Insulin-like growth factor system abnormalities in hepatitis C-associated osteosclerosis. Potential insights into increasing bone mass in adults. J Clin Invest. 1998;101:2165–2173. doi: 10.1172/JCI1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang Z, Yao S, Keizer DW, Wang CC, Bach LA, Forbes BE, Wallace JC, Norton RS. Structure, dynamics and heparin binding of the C-terminal domain of insulin-like growth factor-binding protein-2 (IGFBP-2) J Mol Biol. 2006;364:690–704. doi: 10.1016/j.jmb.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Kwak HB, Lee MS, Kim HS, Cho HJ, Kim JW, Lee ZH, Oh J. Proteasome inhibitors induce osteoclast survival by activating the Akt pathway. Biochem Biophys Res Commun. 2008;377:1–6. doi: 10.1016/j.bbrc.2008.05.048. [DOI] [PubMed] [Google Scholar]
- Lee SE, Woo KM, Kim SY, Kim HM, Kwack K, Lee ZH, Kim HH. The phosphatidylinositol 3-kinase, p38, and extracellular signal-regulated kinase pathways are involved in osteoclast differentiation. Bone. 2002;30:71–77. doi: 10.1016/s8756-3282(01)00657-3. [DOI] [PubMed] [Google Scholar]
- Lee SE, Chung WJ, Kwak HB, Chung CH, Kwack KB, Lee ZH, Kim HH. Tumor necrosis factor-alpha supports the survival of osteoclasts through the activaiton of Akt and ERK. doi: 10.1074/jbc.M103642200. [DOI] [PubMed] [Google Scholar]
- Maile LA, Badley-Clarke J, Clemmons DR. The association between integrin-associated protein and SHPS-1 regulates insulin-like growth factor-I receptor signaling in vascular smooth muscle cells. Mol Biol Cell. 2003;14:3519–3528. doi: 10.1091/mbc.E03-04-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel CC, Ghosh Choudhury G, Ghosh-Choudhury N. Phosphatidylinositol 3 kinase/Akt signaling relay cooperates with smad in bone morphogenetic protein-2-induced colony stimulating factor-1 (CSF-1) expression and osteoclast differentiation. Endocrinol. 2009;150:4989–4998. doi: 10.1210/en.2009-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrian-Shai R, Chen CD, Shi T, Horvath S, Nelson SF, Reichardt JK, Sawyers CL. Insulin growth factor-binding protein 2 is an candidate biomarker for PTEN status and PI3K/Akt pathway activation in glioblastoma and prostate cancer. Proc Natl Acad Sci USA. 2007;104:5563–5568. doi: 10.1073/pnas.0609139104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munugalavadla V, Vemula S, Sims EC, Krishnan S, Chen S, Yan J, Li H, Niziolek PJ, Takemoto C, Robling AG, Yang FC, Kapur R. The p85alpha subunit of class IA phosphatidylinositol 3-kinase regulates the expression of multiple genes involved in osteoclast maturation and migration. Mol Cell Biol. 2008;28:7182–7198. doi: 10.1128/MCB.00920-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan S, Mukhopadhyay A, Narasimhan SD, Tesz G, Czech MP, Tissenbaum HA. A PP2A regulatory subunit regulates C. Elegans insulin/IGF-1 signaling by modulating AKT-1 phosphorylation. 2009;136:939–951. doi: 10.1016/j.cell.2009.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker A, Rees C, Clarke J, Busby WH, Jr, Clemmons DR. Binding of insulin-like growth factor (IGF)-binding protein-5 to smooth-muscle cell extracellular matrix is a major determinant of the cellular response to IGF-I. Mol Biol Cell. 1998;9:2383–2392. doi: 10.1091/mbc.9.9.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perks CM, Vernon EG, Rosendhal AH, Tonge D, Holly JM. IGF-II and IGFBP-2 differentially regulate PTEN in human breast cancer cells. Oncogene. 2007;26:5966–5977. doi: 10.1038/sj.onc.1210397. [DOI] [PubMed] [Google Scholar]
- Russo VC, Schutt BS, Andaloro E, Ymer SI, Hoeflich A, Ranke MB, Bach LA, Werther GA. Insulin-like growth factor binding protein-2 to extracellular matrix plays a critical role in neuroblastoma cell proliferation, migrationa nd invasion. Endocrinol. 2005;146:4445–4455. doi: 10.1210/en.2005-0467. [DOI] [PubMed] [Google Scholar]
- Shen X, Xi G, Radhakrishnan Y, Clemmons DR. PDK1 recruitment to the SHPS-1 signaling complex enhances insulin-like growth factor-I-stimulated AKT activation and vascular smooth muscle cell survival. J Biol Chem. 2010;285:29416–29424. doi: 10.1074/jbc.M110.155325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehata M, Schnabl S, Demirtas D, Hilgarth M, Hubmann R, Ponath E, Badmya S, Lehner C, Hoelbl A, Duechler M, Gaiger A, Zielinski C, Schwarzmeier JD, Jaeger U. Reconstitution of PTEN activity by CK2 inhibitors and interference with the PI3-K/Akt cascade counteract the antiapoptotic effect of human stromal cells in chronic lymphocytic leukemia. Blood. 2010;116:2513–2521. doi: 10.1182/blood-2009-10-248054. [DOI] [PubMed] [Google Scholar]
- Sugatani T, Alvarez U, Hruska KA. PTEN regulates RANKL and osteopontin-stimulated signal transduction during osteoclast differentiation and cell motility. J Biol Chem. 2003;278:5001–5008. doi: 10.1074/jbc.M209299200. [DOI] [PubMed] [Google Scholar]
- Torres J, Pulido R. The tumor suppressor PTEN is phosphorylated by the protein kinase CK2 at its C terminus. Implications for PTEN stability to proteasome-mediated degradation. J Biol Chem. 2001;276:993–996. doi: 10.1074/jbc.M009134200. [DOI] [PubMed] [Google Scholar]
- Vazquez F, Ramaswamy S, Nakamura N, Sellers WR. Phosphorylation of the PTEN tail regulates protein stability and function. Mol Cell Biol. 2000;20:5010–5018. doi: 10.1128/mcb.20.14.5010-5018.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatcroft SB, Kearney MT. IGF-dependent and IGF-I independent actions of IGF-binding protein-1 and -2: implications for metabolic homeostasis. Trends Endocrinol Metab. 2009;20:153–162. doi: 10.1016/j.tem.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Wheatcroft SB, Kearney MT, Shah AM, Ezzat VA, Miell JR, Modo M, Williams SC, Cawthorn WP, Medina-Gomez G, Vidal-Puig A, Sethi JK, Crossey PA. Diabetes. 56:285–294. doi: 10.2337/db06-0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YW, Yanagishita M, Rechler MM. Heparin inhibition of insulin-like growth factor-binding protein-3 binding to human fibroblasts and rat glioma cells: role of heparan sulfate proteoglycans. Endocrinol. 137:4363–4371. doi: 10.1210/endo.137.10.8828497. [DOI] [PubMed] [Google Scholar]