Abstract
Synthetic curcuminoid EF24 was studied for its effect on the maturation and inflammatory response in murine bone marrow derived immortalized JAWS II dendritic cells (DCs). EF24 reduced the expression of LPS-induced MHC class II, CD80 and CD86 molecules. It also abrogated the appearance of dendrites, a typical characteristic of mature DCs. These effects were accompanied by the inhibition of LPS-induced activation of transcription factor nuclear factor kappa-light-chain enhancer of activated B cells (NF-κB). Simultaneous reduction of pro-inflammatory cytokines [tumor necrosis factor (TNF)-α, IL-6] both at the mRNA and secreted levels was also observed. To investigate the dependency of LPS effects on MyD88 adaptor protein, we transfected JAWS II DCs with dominant negative MyD88 plasmid construct (MyD88-DN). EF24 reduced NF-κB activity and TNF-α secretion in a MyD88-dependent manner. These results suggest that EF24 modulates DCs by suppressing their maturation and reducing the secretion of inflammatory cytokines. Further, it appears that EF24 acts at or upstream of MyD88 in the LPS-TLR4/MyD88/NF-κB pathway.
Keywords: curcumin, dendritic cells, EF24, inflammation, NF-κB
Introduction
Inflammation is a critical and necessary host defense immune mechanism. However, excessive inflammation can be deleterious to the host. The clinical conditions characterized by excessive inflammation include diseases such as arthritis, asthma, multiple sclerosis, inflammatory bowel disease and atherosclerosis (1). Evidences also suggest that prolonged deregulated inflammation results in oncogenesis (2). A typical immediate host response to inflammatory stimuli involves infiltration of immune cells and the release of pro-inflammatory cytokines, such as tumor necrosis factor (TNF)-α and IL-6. Among various immune cells recruited in the inflammation process, dendritic cells (DCs) serve as a link between the innate and adaptive immunity. Despite being relatively low in number, DCs are more potent antigen presenting cells than macrophages and B cells (3). The recruitment of DCs is accompanied by the up-regulation of antigen presenting MHC class II and T cell co-stimulatory molecules (CD40, CD80 and CD86) and the release of cytokines. As mentioned above, a balanced amount of these cytokines favors stimulation of adaptive immunity, whereas an exaggerated DC response could be detrimental. For instance, myeloid DCs in Crohn’s disease (4), autoimmune diseases (5) and inflammatory lung diseases (6) have been shown to exert an overactive inflammatory response that correlates with severity of diseases.
DCs are responsive to a variety of pro-inflammatory stimuli. LPS originating from the Gram-negative bacteria is a potent stimulant of immune cell infiltration and inflammation. LPS induces secretion of TNF-α and IL-6 in DCs through activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling (7). The LPS signaling is initiated upon its binding to toll-like receptor 4 (TLR4) complex expressed on the cell surface of antigen presenting cells. The TLR-induced inflammatory responses are mediated through a universal adapter protein Myeloid differentiation primary response gene 88 (MyD88) as well as via MyD88-independent pathway (8). It is noteworthy that overstimulation of DCs with LPS has been shown to result in DC-apoptosis that, in turn, may compromise the number of DCs participating in adaptive immunity (9). The exaggerated DC-mediated inflammatory response could be moderated by controlling the receptor-mediated signaling of pro-inflammatory cytokines (10). Curcumin is one such naturally occurring polyphenol that has been shown to inhibit DC maturation and reduce the secretion of pro-inflammatory cytokines TNF-α and IL-6 (11, 12). It’s pleiotropic actions appear to depend on down-regulation of NF-κB both in vitro and in vivo (13). Both MD-2 (14) and the TLR4 receptor complex (15) have been proposed as purported molecular targets for curcumin.
EF24, 3,5-bis(2-fluorobenzylidine)-4-pyperidone, is a synthetic analog of curcumin (16, 17). Synthetic curcuminoids are being developed because they are more potent and better bioavailable than curcumin (18, 19). EF24 was first developed as a tumor suppressant by a group of researchers in Emory University, Atlanta, GA, USA (17). We hypothesize that the effective inhibition of NF-κB signaling by EF24 extends its therapeutic application to a variety of NF-κB-dependent disorders, including inflammation (20). In this work, we investigated the immunopharmacology of EF24 in a DC model. We show that EF24 suppresses LPS-induced DC maturation, NF-κB activity and secretion of pro-inflammatory cytokines. The observations held true in the primary bone marrow derived DCs as well. The results are suggestive of the potential of EF24 as a novel agent for managing DC-mediated exaggerated inflammatory conditions.
Methods
EF24 was synthesized in our laboratory by acid catalyzed Claisen–Schmidt condensation of 4-piperidone and 2-fluorobenzaldehyde and its structural characteristics were determined according to our previous work (21). For all experiments, EF24 was dissolved in endotoxin-free water (Hyclone, Logan, UT, USA). The endotoxin levels were assessed for each batch of EF24 by the Limulus amebocyte lysate (LAL) kit from Charles River Labs (Charleston, SC, USA). The endotoxin content of EF24 solution was ascertained to be <0.1 EU ml−1 (<0.01 ng ml−1). Highly purified LPS (protein content <0.6%) of Escherichia coli O111: B4 was obtained from Calbiochem (Darmstadt, Germany) and used at 100 ng ml−1 concentration.
DC culture
JAWS II DC cell line is an immortalized and immature DC cell line derived from bone marrow of C57BL/6 mice (American Type Culture Collection, Manassas, VA, USA). The cells were maintained in Alpha-modified minimum essential medium (α-MEM; Sigma, St Louis, MO, USA) supplemented with 20% fetal bovine serum (FBS), 4 mM l-glutamine, 100 U/ml penicillin, 100 μg ml−1 streptomycin, 50 μg ml−1 gentamicin (Invitrogen, Grand Island, NY, USA) and 5 ng ml−1 of recombinant murine granulocyte macrophage colony-stimulating factor (GM-CSF; Peprotech, Rocky Hill, NJ, USA) (22). The culture medium was replaced with fresh medium every 48 h.
Isolation and culture of primary bone marrow-derived DCs
Bone marrow-derived primary DCs were harvested from C57BL/6 mice as described elsewhere (22). Briefly, bone marrow from femur and tibia was flushed with RPMI 1640 medium (Gibco Life Sciences, NY, USA), and single cell suspension was obtained by passing the cells through a nylon mesh. The cells were seeded on 6-well tissue culture plates (Nalge-Nunc International Corp, NY, USA) in RPMI 1640 medium containing 10 mM HEPES, 10 μg ml−1 gentamicin, 100 U/ml penicillin, 100 μg ml−1 streptomycin, 10% FBS, 1% MEM nonessential amino acids, 50 μM β-mercaptoethanol, 10 ng ml−1 recombinant murine GM-CSF and 10 ng ml−1 recombinant murine IL-4 (both cytokines from Peprotech). The cells were incubated at 37°C in 5% CO2 atmosphere. The DCs were harvested on day 6 by collecting the nonadherent cells and subjecting them to a density gradient separation (22). The floating cells from the top (density < 1.065 g ml−1) of the Optiprep density gradient (Accurate Chemicals, NY, USA) were harvested as DCs. The morphology and phenotype of primary DCs after harvesting on the 6th day after culture were similar to JAWS II DCs. The characterization of primary DCs has been reported elsewhere (22).
Experimental design for the treatment of DCs with LPS and EF24
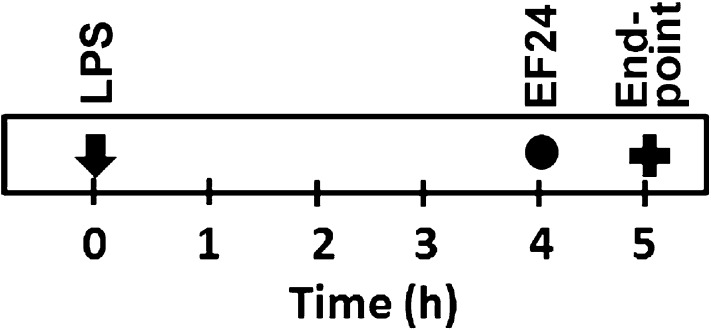
The reported experiments were conducted for 5 h and are illustrated in Fig. 1. The DCs were treated with EF24 (10 μM) for 1 h after 4 h in presence of LPS to generate a post-treatment model. The cumulative duration of LPS treatment was for 5 h. We chose 10 μM as the optimal dose based on the results of a preliminary DC viability study using MTT assay (data not shown).
Fig. 1.
Experimental design to investigate the effect of EF24 on LPS-induced morphologic, phenotypic and functional changes in mouse-derived DCs.
LPS binding assay
The LPS-binding assay was conducted as described by Giacometti et al. (23) using LAL kit from Charles River Labs. Briefly, EF24 or polymyxin B (0, 0.5, 1, 10 and 20 μM) were added together with 0.1 IU ml−1 LPS in a 96-well tissue culture plate. After incubation at 37° C for 40 min, LAL solution (50 μl) was added and the reaction mixture was further incubated 10 min. Finally, 100 μl of substrate-buffer solution was added, and the plate was read immediately and after 12 min at 405 nm. The percent LPS binding was calculated from optical densities (ODs) using the following equation, where ΔOD is the difference in ODs at 12 min and 0 min.
Cytokine measurement
We studied changes in mRNA expression as well as secreted cytokine levels by real-time PCR and ELISA, respectively.
mRNA expression by real-time PCR
Total RNA from DCs was extracted using the RNAeasy Mini Kit (Qiagen, Valencia, CA, USA) and quantified by spectrophotometric absorbance values at 260 nm. Reverse transcriptase reaction was performed for 1 h at 42°C using 2 μg of total RNA, 1 μg of oligo(dT), 200 U of M-MLV reverse transcriptase enzyme, 500 μM dNTP mix and 25 U of RNAase inhibitor (Promega, Madison, WI, USA). The resultant cDNA was stored at −20°C till further used. The primer sequences for various cytokines were:
TNF-α: Forward primer (FP)—5′-AAATGGCCTCCCTCTCATCAGTTC-3′, Reverse primer (RP)—5′-TCCACTTGGTGGTTTGCTACG-3′; IL-6: FP—5′-TCAATTCCAGAAACCGCTATGA-3′, RP—5′-CACCAGCATCAGTCCCAAGA-3′; IL-12p35: FP—5′-CATCGATGAGCTGATGCAGT-3′, RP—5′-CAGATAGCCCATCACCCTGT-3′; IL-12p40: FP—5′-AGGTGCGTTCCTCGTAGAGA-3′, RP—5′-AAAGCCAACCAAGCAGAAGA-3′; MCP-1: FP—5′-CTTCTAGCCCGGAGAGTGTG-3′, RP—5′-GATGCCAACAGCAACTCTGA-3′; IFN-γ: FP—5′-ATCGGCAAAAGGATGGTGAC-3′, RP—5′-TGAGCTCATTGAATGCTTGG-3′; IL-10 FP—5′-CACAAAGCAGCCTTGCAGAA-3′, RP—5′-AGAGCAGGCAGCATAGCAGT-3′.
The primer sequences for the housekeeping gene β-actin were: FP: 5′-CGGGAAATCGTGCGTGACATTAAG-3′ and RP: 5′-TGATCTCCTTCTGCATCCTGTCGG-3′. Primers for all the genes were synthesized by Integrated DNA Technologies (Coralville, IA, USA). The PCR reaction was carried out under identical thermal cycling condition for 40 cycles—15 s at 95° C, 30 s at 54° C, 30 s at 72° C on ABIPrism 7000 sequence detection system (Applied biosytems, Foster City, CA, USA). The reaction was performed using SybrGreen II and the Go Taq Colorless master mix (Promega). Briefly, each PCR reaction was set up in triplicate wells in a total volume of 25 μl. The reaction mixture contained cDNA equivalent of 20 ng of total RNA. Quantitative values of the genes of interest were normalized using β-actin as the endogenous reference, and fold-increase over control was calculated using the relative quantification method of 2−ΔΔ Ct (24).
Measurement of secreted cytokine levels by ELISA
TNF-α levels were measured in cell-free supernatants by ELISA according to the method published earlier (24). Briefly, each well of Immunolon 4 HBX (Thermo Electron Corporation, Milford, MA, USA) were coated with 100 μl of 10 μg ml−1 purified rat anti-mouse TNF-α (BD Biosciences, San Jose, CA, USA) and allowed to incubate overnight at 4°C. After washing the wells 3 times with 0.05% Tween-20-containing PBS (PBST), the wells were incubated at room temperature (RT) for 2 h with 3% BSA, followed by washing with PBST. The cytokine standard solutions (100 μl) used were in the range of 10–1000 pg ml−1, while suitable dilutions of cell-free supernatant samples (100 μl) were added to the wells. All the samples, standards and antibodies were prepared in 3% BSA-PBS. After overnight incubation at 4° C, the wells were washed and incubated with 100 μl of 2.5 μg ml−1 of biotin-labeled anti-mouse TNF-α (BD Biosciences) and incubated at RT for 2 h. Following another washing, 100 μl of 1:200 diluted streptavidin-horseraddish peroxidase (BD Biosciences) was added and the plate was incubated for 1 h at RT. After a final wash, 100 μl of 3,3′,5,5′-tetramethylbenzidine (Sigma) substrate solution was added to each well and allowed to incubate at RT for 30 min. Finally, 50 μl of 0.2 N sulphuric acid was used to stop the reaction and spectrophotometric absorbance was read at 450 nm. The cytokine levels were measured in picograms per milligram. The range of detection for TNF-α ELISA was 10–1000 pg ml−1.
The IL-6 levels were measured in a manner similar to that described for TNF-α and as per manufacturer’s instructions using the rat anti-mouse IL-6 ELISA set (Southern Biotech, Birmingham, AL, USA). The range of detection for IL-6 ELISA was determined to be 7.8–500 pg ml−1.
Effect of EF24 on DC morphology
The morphological changes in cells were observed by light microscopy under an inverted compound microscope (Jenco International Inc, Portland, OR, USA). Digital images were taken using the Canon Power shot A630 (Canon USA Inc, Lake Success, NY, USA) at ×160 magnification. In order to observe any changes in intracellular structures, we performed transmission electron microscopy (TEM) after 5 h treatment with EF24 with or without LPS. The cells were fixed in 0.1 M sodium cacodylate buffer containing 4% PFA and 2% gluteraldehyde for 4 h at RT. The samples were post-fixed in 1% osmium tetroxide for 1.5 h and washed with 0.1 M sodium cacodylate buffer, followed by dehydration in an ethanol series of 50, 60, 75, 85 and 95% ethanol for 15 min each. The cells were washed twice in 100% ethanol and passed through epon-araldite (6.2 g epon + 4.4 g araldite + 12.4 g of dodecenyl succinic anhydride + 0.8 g N,N-dimethylbenzylamine) solution in ethanol. The cells were embedded in an epon-araldite resin. Finally, 100 nm sections were cut using a microtome, and the sections were placed on glow-discharged 300 mesh copper grids. The ultrasections were stained with Sato’s lead (mixture of calcinated lead citrate, lead nitrate, lead acetate and sodium citrate) and observed by a Hitachi H-7600 transmission electron microscope at 2500–4000× (Imaging Core Facility, Oklahoma Medical Research Foundation, Oklahoma City, OK, USA).
Immunophenotyping of DCs
DCs were treated with EF24 (10 μM) and LPS (100 ng ml−1) and after the end of the indicated time period, both adherent and nonadherent cells were harvested. The harvested DCs were suspended in 100 μl DPBS containing 1% FBS and 0.09% sodium azide. Previously titrated anti-mouse antibodies to myeloid DC-specific markers: PE-conjugated CD11c (clone HL3) and CD80 (clone 16-10A1), FITC-conjugated CD14 (clone RMC5-3) and CD86 (clone GL1), and biotin-conjugated MHC class II (IA/IE) (clone 2G9) and CD40 (clone 3/23) (BD Biosciences) were added to the DCs at the ratio of 1 μg antibody per one million cells. The mixture of cells and antibodies was incubated for 30 min on ice in dark. The cells were washed thrice with PBS containing 1% FBS and 0.09% sodium azide. Finally, 0.125 μg strepdavidin-allophycocyanin (BD Biosciences) was added to the appropriate tubes containing cells stained with biotin-conjugated antibodies and incubated for 20 min on ice in dark. The cells were washed, fixed in freshly prepared 0.5% paraformaldehyde and run through an automated dual laser excited FACS Calibur at the Flow and Imaging Core Facility, University of Oklahoma Health Science Center (OUHSC, Oklahoma City, OK, USA). The histogram and dot-plot charts were obtained and analyzed using Summit V4.3 software (Dako Colorado Inc, Carpinteria, CA, USA). The isotype control antibody-stained cells served as controls for the background staining.
Electrophoretic mobility shift assay for DNA-binding ability of NF-κB
The DCs were washed once with ice-cold PBS and nuclear proteins were extracted with nuclear and cytoplasmic extraction reagents (CER) (Thermo Scientific, Rockford, IL, USA) according to the manufacturer’s protocol. Briefly, 1 ml of ice-cold CER-I was added to the cell pellet and incubated on ice for 10 min after vigorous vortexing. CER-II (55 μl) was added to the tubes and centrifuged at 16 000 × g. The supernatant was discarded and the pellet was resuspended in 500 μl of nuclear extraction reagent and incubated on ice for 40 min. The total protein in the nuclear extract was determined by BCA protein Assay (Thermo Scientific Pierce). For electrophoretic mobility shift assay (EMSA), a double-stranded oligonucleotide containing a tandem repeat of the consensus sequence of 5′-GGG GAC TTT CC-3′ was end-labeled with [c-32P]-ATP (Amersham, Arlington Heights, IL, USA) using T4 polynucleotide kinase (Promega). Free and unbound radioisotope was separated using a push column device (Stratagene, La Jolla, CA, USA). Binding reaction was performed by mixing the nuclear extract (10 μg), 0.1 μg of poly (dI-dC) (Sigma–Aldrich) and 0.5 ng of [γ-32P]-ATP-labeled NF-κB-specific oligonucleotide probe in a buffer containing 10 mM Tris–HCl (pH 7.5), 100 mM NaCl, 1 mM dithiothreitol, 1 mM EDTA, and 20% (v/v) glycerol. For the competition assay, the nuclear extract was pre-incubated with unlabeled homologous NF-κB oligonucleotide followed by addition of [γ-32P]-ATP-labeled NF-κB probe. All the samples were electrophoresed on 6% polyacrylamide gels in Tris-glycine buffer (25 mM Tris, 0.19 M glycine and 1 mM EDTA). The gels were dried and autoradiographed (Eastman Kodak Co., Rochester, NY, USA). Quantitative analysis was performed using one-dimensional gel analysis (Image Quant TL; Amersham Biosciences) software.
Luciferase reporter assay for NF-κB activity
The NF-κB luciferase gene reporter (pGL4.32 [Luc2p/NFκB-RE/Hygro], Promega) was a gift from Dr Kelly Standifer (University of Oklahoma Health Sciences Center, Oklahoma City, OK, USA) and the MyD88 dominant negative construct (MyD88-DN) lacking the death domain was provided by Dr Ruslan Medzhitov (Yale University School of Medicine, New Haven, CT, USA) (25). JAWS II DCs were co-transfected with pGL4.32 [Luc2p/NF-κB-RE/Hygro] plasmid DNA with MyD88 dominant negative plasmid in pcDNA3 vector as described earlier (26). Briefly, around 0.5–1 × 106 cells per well were allowed to grow overnight in a 24-well tissue culture plate (Greiner Bio-one). Serum-free DMEM (Invitrogen, Carlsbad, CA, USA) was used as the transfection medium. The transfection mixture was prepared by adding 4 μl of TransIT-TKO transfection reagent (Mirus, Madison, WI, USA) to 150 μl of DMEM. Each plasmid DNA was added at the ratio of 2 μg DNA to 4 μl of transfection reagent. The cells were allowed to remain in transfection mixture for 4 h at 37° C in a 5% CO2 incubator, before addition of 250 μl of serum-containing α-MEM and culturing for additional 20 h. The transfected cells were treated with EF24 or LPS using the 5 h post-treatment model. For the luciferase activity assay, the cells were washed with PBS and the cell lysates were prepared in the reporter assay cell lysis buffer (Promega). The firefly luciferase activity was measured with 20 μl of lysate in BioTek Synergy HT plate reader (BioTek Instruments, Winooski, VT, USA). The total protein was determined using BCA protein assay reagent kit (Pierce). The luminescence units were normalized with total protein content. The cell-free supernatants collected in these experiments were used for measuring secreted TNF-α levels. The experimental controls included cells transfected with pcDNA3 vector in place of MyD88-DN construct.
Statistical analysis
The results were analyzed by one-way analysis of variance applying the Bonferroni post-test using Prism software (GraphPad, San Diego, CA, USA). Unless specified differently, P value < 0.05 was considered as statistically significant.
Results
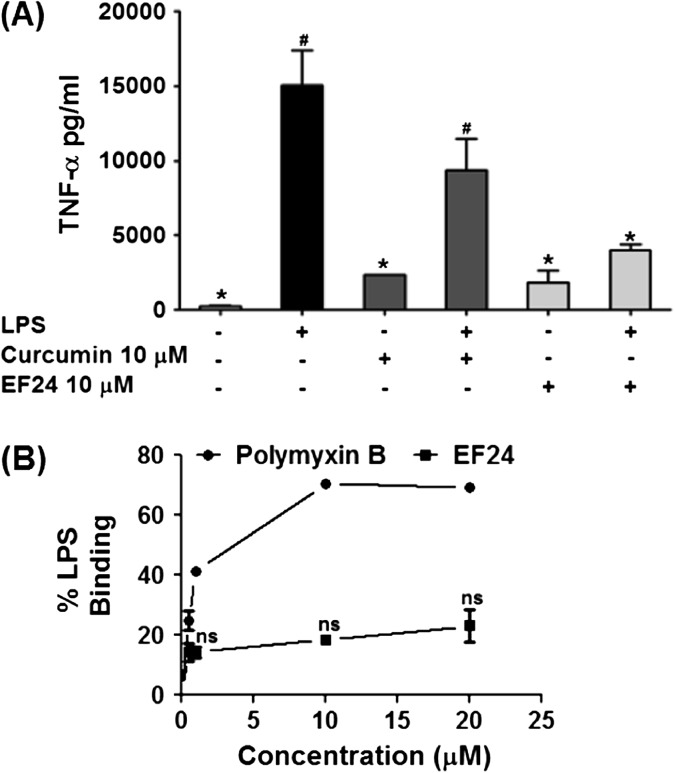
EF24 is a synthetic analog of a naturally occurring polyphenol curcumin. Our laboratory has recently shown that like curcumin, EF24 also possesses tumor suppressive properties (16). In this work, we investigated the modulation of DC function by EF24. As shown in Fig. 2(A), we found that EF24 was effective in inhibiting the TNF-α release, but equivalent concentration of curcumin could not significantly suppress LPS-induced TNF-α secretion. In order to rule out direct interaction of EF24 and LPS, we performed an LPS-binding assay. It is clear from Fig. 2(B) that EF24 does not bind to LPS. With increasing concentration, the % LPS binding with polymyxin B increased up to 60%, whereas there was no change in the % LPS binding with EF24 over all the concentration ranges. The results suggest that the functional inhibition of LPS-mediated effects in DCs is not through a mechanism involving extracellular binding of EF24 and LPS in the culture medium. These preliminary experiments formed a platform for further investigation of effects of EF24 on DC functions.
Fig. 2.
(A) EF24 is more potent than curcumin in reducing LPS-induced TNF-α secretion from JAWS II DCs. LPS-stimulated DCs were treated with 10 μM EF24 or curcumin and secreted amounts of TNF-α (picograms per milligram, mean ± SEM) were measured by ELISA. (B) Percent binding of EF24 (0–20 μM) and polymyxin B (0–20 μM) with LPS (0.1 IU ml−1) in an in vitro microwell-based assay. Polymyxin B served as a positive control. The results are representative of two experiments performed in triplicate.
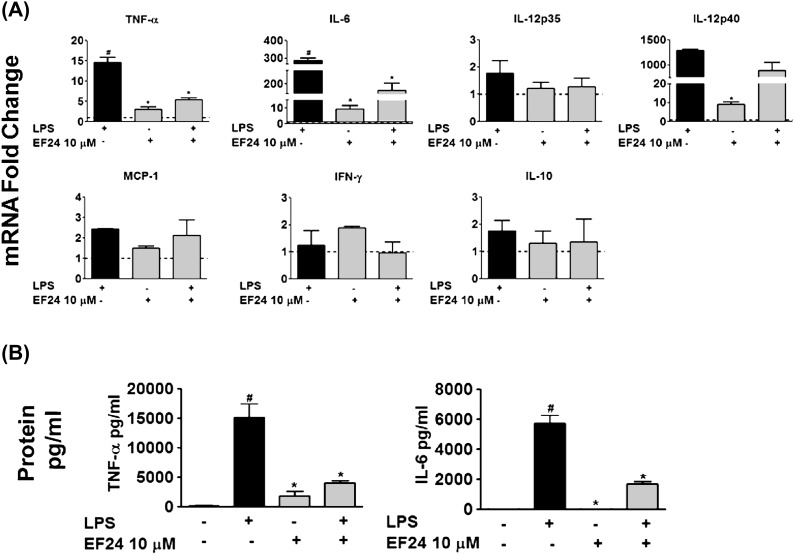
EF24 inhibits the mRNA expression and secretion of TNF-α and IL-6
To verify that EF24-mediated inhibition of cytokines is at transcriptional level, we determined the effect of EF24 on LPS-induced mRNA expression of several cytokines by quantitative real-time PCR. Treatment with EF24 significantly suppressed the LPS-induced TNF-α and IL-6 mRNA (P < 0.05; Fig. 3A). The mRNA levels of cytokines IL-12, monocyte chemotactic protein (MCP)-1, IFN-γ and IL-10 were not significantly affected by the addition of EF24. We further confirmed this observation at the secreted levels of TNF-α and IL-6 (Fig. 3B). Compared with the basal levels, LPS stimulated the release of TNF-α and IL-6 in large amounts. EF24 by itself did not induce any marked release of these cytokines, but it significantly reduced the LPS-induced secretion of both TNF-α and IL-6 (Fig. 3B). Secreted cytokine levels of IL-12 and MCP-1 were not significantly reduced by EF24, whereas secreted cytokine levels of IFN-γ and IL-10 were undetected at all times (data not shown).
Fig. 3.
(A) Quantitative-polymerase chain reaction for the measurement of TNF-α, IL-6, IL-12 (IL-12p35 and IL-12p40), MCP-1, IFN-γ, and IL-10 mRNA expression in presence of 10 μM EF24 (fold changes as compared with control) in JAWS II DCs. The results are representative of three experiments performed in triplicate. (B) Cytokine levels of TNF-α and IL-6 measured in picograms per milligram (mean ± SEM) by ELISA in JAWS II DCs. The results shown are representative of two independent experiments performed in triplicate # P < 0.05 versus control cells and * P < 0.05 versus LPS-treated cells.
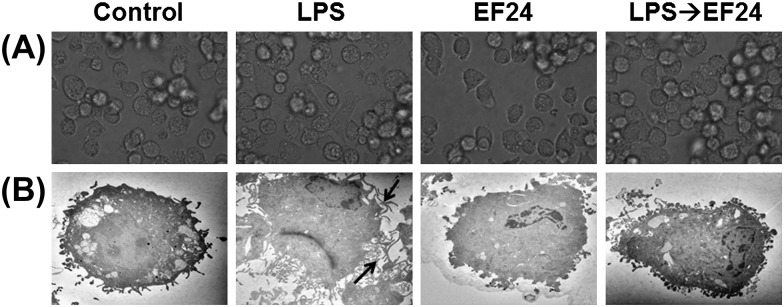
EF24 inhibits LPS-induced maturation of DCs
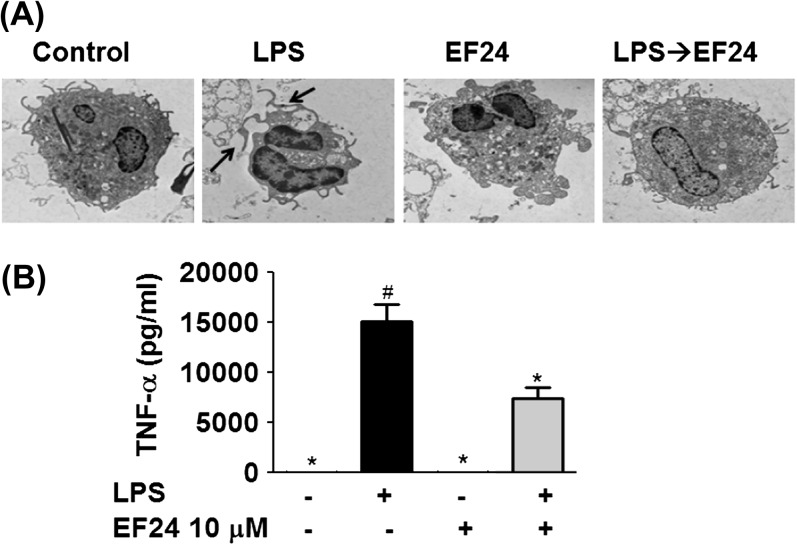
First, we investigated the effect of EF24 on phenotypic maturation of JAWS II DCs and to determine whether EF24 has any apparent deleterious effects on the morphological characteristics of these cells. Under light microscope, the LPS-treated cells appeared elongated as compared with a typical round morphology of immature JAWS II DCs (Fig. 4A). Qualitatively, the DCs also tended to adhere to the plastic surface more after LPS treatment than the normal immature DCs. To ultrastructurally elucidate the morphological changes (magnified scale of ×3000), we examined the JAWS II DCs by TEM (Fig. 4B). Normal immature DCs were characterized by the absence of tentacles-like structures. In presence of LPS, however, conspicuous dendrites emerged within 5 h (arrows), suggestive of DC maturation. EF24 treatment by itself had no effect on DC morphology, but it abolished the LPS-stimulated morphologic change (the appearance of conspicuous dendrites). These observations suggest that EF24 does not allow LPS-induced morphologic maturation of DCs.
Fig. 4.
Morphological changes induced by LPS treatment on JAWS II DCs are abrogated by EF24. (A) Light microscopic pictures of DCs treated with vehicle-control, 100 ng ml−1 LPS, 10 μM EF24 and 100 ng ml−1 LPS followed by 10 μM EF24 (LPS→EF24). The pictures were acquired after 5 h of treatment. (B) Transmission electron photomicrographs (×3000) of JAWS II DCs treated similarly. The arrows indicate the conspicuous dendrites on the mature JAWS II DCs that are subdued by EF24 treatment. The electron micrographs are representative of two independent experiments.
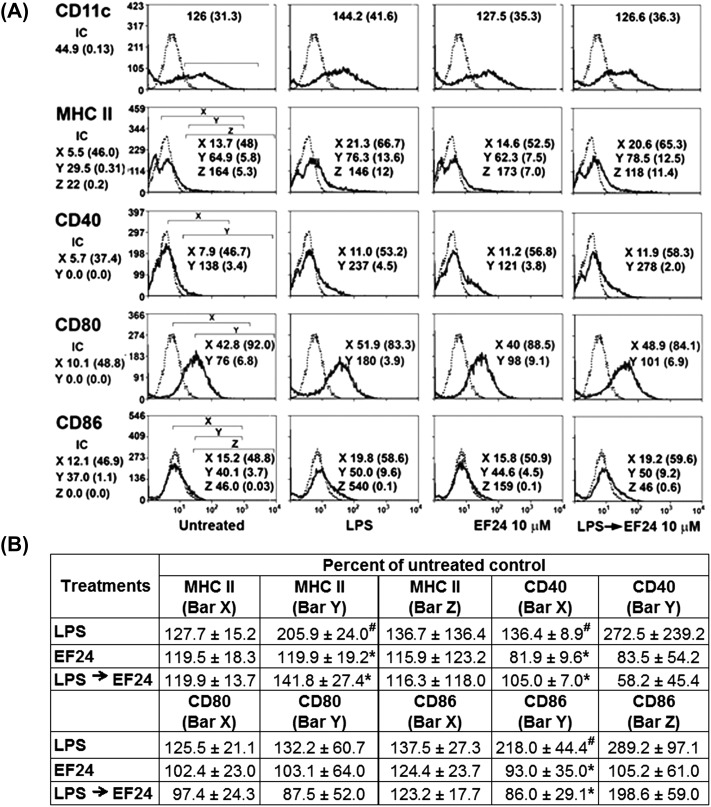
Next, we determined the expression of immunophenotypic markers of DCs, such as CD11c, MHC class II, CD40, CD80 and CD86. LPS was found to induce expression of DC maturation markers CD40, CD80 and CD86 (Fig. 5A) over untreated control. The treatment with EF24 (LPS→EF24) reduced the LPS-stimulated MFI values and percent number of cells positive for MHC class II, CD80 and CD86 (Fig. 5B). In essence, the EF24-mediated morphologic and phenotypic alterations in LPS-stimulated cells indicate that EF24 inhibits maturation of DCs.
Fig. 5.
Effect of EF24 on expression of DC-specific cell-surface markers. JAWS II DCs were gated based on forward scatter and side scatter on the dot plot. (A) The fluorochrome stained cells were analyzed within the selected regions (bars X, Y, Z) of the flow cytometric histograms. The dotted histograms indicate the isotype control. Mean fluorescence intensity (MFI) and percent number of cells (in parenthesis) are shown for the respective DC markers. The first panel of histograms (untreated) has the regions marked with square brackets, and the same regions have been applied to the rest of the histograms for measurement of MFI and percent positive cells. The JAWS II DCs were treated for 5 h with 100 ng ml−1 LPS, 10 μM EF24 and 10 μM EF24 added after LPS (LPS→EF24). (B) The change in number of cells (±SEM) positive for the given markers is shown as percent of untreated control. Results are derived from four independent experiments. # P < 0.05 versus untreated control and * P < 0.05 versus LPS-treated cells.
EF24 reduces the LPS-induced activation of NF-κB
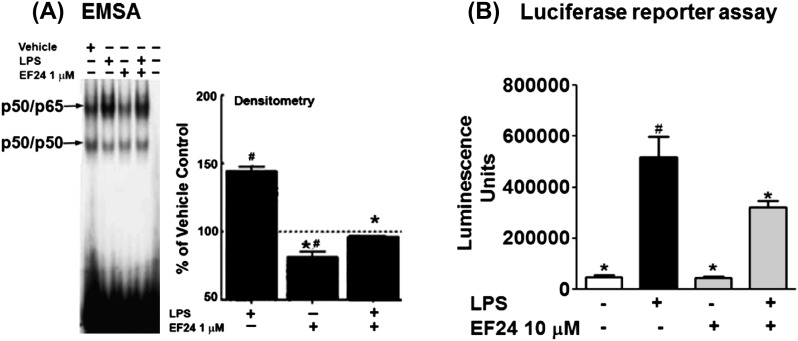
The results so far imply that EF24 not only inhibits secretion of effector molecules, but also alters the early inducible cell surface expression of the maturation markers in DCs. Because NF-κB is pivotal to the inflammation signaling, we investigated whether EF24 affects the activation of NF-κB in DCs. We measured DNA-binding activity (p50/65) in the nuclear extracts (by EMSA, Fig. 6A) and NF-κB activation by a luciferase reporter assay (Fig. 6B). DCs treated with LPS showed remarkable increase in DNA binding (around 50% over vehicle control) as well as the activation of NF-κB (Fig. 6A). However, the presence of EF24 significantly attenuated the LPS-induced effects on NF-κB.
Fig. 6.
Inhibition of LPS-induced NF-κB by EF24. (A) NF-κB-DNA binding activity in JAWS II DCs treated with vehicle, 100 ng ml−1 LPS, 1 μM EF24 and post-treatment with 1 μM EF24 was investigated by EMSA. The values were normalized with those of vehicle-treated cells shown as the dotted line. * P < 0.001 versus LPS-treated cells and # P < 0.001 versus vehicle-treated cells. (B) The effect of EF24 treatment (10 μM) on LPS-induced NF-κB activity was also confirmed by luciferase reporter assay (# P < 0.05 versus control and * P < 0.05 versus LPS treatment). The results are representative of three independent experiments performed in triplicate.
EF24 does not further reduce LPS-induced effects in MyD88-DN DCs
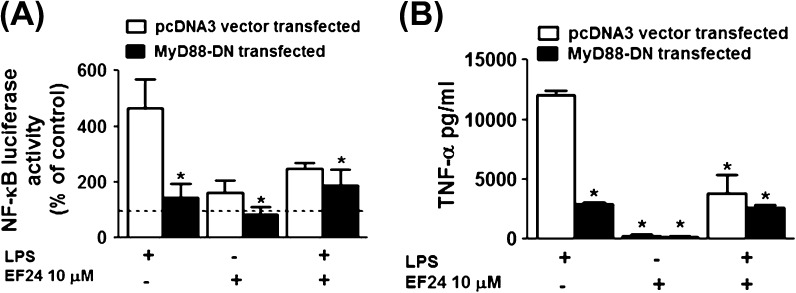
To delineate the molecular mechanism of action of EF24, we knocked down the expression of MyD88 (an adaptor protein associated with TLR4 signaling) in immortalized JAWS II DCs by transfection with a MyD88 dominant negative construct (MyD88-DN). We used NF-κB activity and TNF-α secretion as two endpoints for this experiment. In order to determine NF-κB activity as an endpoint, the cells were co-transfected with pGL4.32 [Luc2p/NF-κB-RE/Hygro] (luciferase) reporter and MyD88-DN plasmid DNA. As shown in Fig. 7(A) (empty bars), almost two-third of LPS-mediated induction of NF-κB in DCs occurred via MyD88-dependent pathway, and this induction was inhibited by EF24 treatment. In MyD88-DN cells Fig. 7(A) (filled bars), LPS-mediated NF-κB activation is significantly reduced, but this residual LPS-effect is not influenced by the presence of EF24. This finding suggests that EF24 most-likely acts on MyD88-dependent pathway and that the action of EF24 could be at or upstream of MyD88 in the LPS-TLR4-NF-κB nexus. As a second endpoint, we assayed TNF-α levels in the cell-free supernatants. As demonstrated in Fig. 7(B), we found that the inhibition of TNF-α levels followed the pattern observed in the case of NF-κB activity in similarly treated cells.
Fig. 7.
(A) NF-κB luciferase activity measured in the cell lysate of JAWS II DCs transfected with the MyD88 dominant negative (MyD88-DN) or pcDNA3 plasmid DNA (control) constructs. The results are expressed as percent of control and represented as mean (±SEM) of four independent experiments performed at different times in triplicate. Dotted line indicates the control value. (B) The secreted TNF-α levels (in picograms per milligram) in cell-free supernatants of JAWS II DCs transfected with the MyD88-DN or pcDNA3 plasmid DNA constructs. The results are a mean (±SEM) of two experiments performed in triplicate. * P < 0.05 versus LPS-treated pcDNA3 plasmid transfected JAWS II DCs.
The anti-inflammatory actions of EF24 are also observed in primary DCs
JAWS II cells are immortalized cell line of mouse bone marrow origin. In order to confirm that EF24 also influences LPS-induced inflammatory pathway in primary cells, we isolated and cultured primary DCs from mouse bone marrow. Like in JAWS II cells, EF24 was found to recover the normal morphology of immature primary DCs in LPS-stimulated cells (Fig. 8A). On similar lines, EF24 also inhibited the LPS-induced TNF-α secretion from primary DCs (Fig. 8B).
Fig. 8.
The effect of EF24 on primary bone marrow derived DCs. (A) Transmission electron photomicrographs (×3000) of primary bone marrow-derived DCs treated with control, 100 ng ml−1 LPS, 1 μM EF24 and 100 ng ml−1 LPS followed by 1 μM EF24 (LPS→EF24). The arrows indicate the presence of conspicuous dendrites on the primary DCs. The electron micrographs are representative of two independent experiments. (B) The cytokine levels of TNF-α (picograms per milligram, mean ± SEM) measured by ELISA secreted by primary DC. The results shown are a representative of three independent experiments each performed in triplicate. # P < 0.05 versus control cells and * P < 0.05 versus LPS-treated cells.
Overall, the results are demonstrative of anti-inflammatory properties of EF24 in DCs. The action of EF24 appears to be dependent on MyD88; however, its anti-inflammatory action might be a composite result of its action on multiple molecular pathways such as DC maturation, NF-κB activity and subsequent transcriptional control of inflammatory cytokines.
Discussion
Immunity is broadly categorized as adaptive and innate immune response. Adaptive immunity is dependent upon clonally distributed T and B cells, whereas innate immunity is characterized by engulfment and digestion of microbes and foreign substances by macrophages and leukocytes. DCs are at the crossroad of adaptive and innate immunity; they capture antigens and present them to the adaptive arm consisting of T and B cells. DCs use instructive cytokines and several co-stimulatory molecules (like CD80 and CD86) to interact with T cells and signal clonal expansion of antigen-specific T cells. An insufficient or perturbed innate immune response and constant exposure to pathogenic ligands or infectious organisms can lead to accumulation of deleterious inflammatory cytokines (27). Although macrophages and other immune cells are also important in overall host response, the polarization of DC mechanisms can be crucial to the eventual outcome in the host (4). In DC-mediated response, the maturation of DCs is the first step that is regulated by a variety of extracellular stimuli and adjuvants, including cytokines, endogenous stress-related ligands and bacterial products. Gram-negative bacteria-derived LPS serves as a stimulus by interacting with a pathogen-recognition and antigen-sensing receptor TLR4 (28). LPS binding to cell surface TLR4 and signaling via MyD88-dependent or -independent pathways results in DC maturation. The maturation of DCs is followed by the production of pro-inflammatory cytokines (IL-12, IL-6 and TNF-α) and also stimulated expression of co-stimulatory molecules CD40, CD80 and CD86 (8). An exaggerated TLR4-NF-κB signaling can result in deleterious levels of cytokines. Thus, immunomodulators are being investigated to optimally inhibit TLR4-NF-κB for suppression of harmful inflammation while maintaining the beneficial adaptive arm of the immune response. Curcumin is one such immunomodulator that has been shown to affect functional maturation of DCs and release of inflammatory cytokines (11, 12).
Synthetic curcuminoids, such as EF24, have enhanced pharmacological effect and bioavailability as compared with curcumin (29, 30). In cancer cells, EF24 inhibits NF-κB by down-regulating IκB kinase β (20). However, the cellular targets of EF24 in the immune system, in general, and DC systems, in particular, are not known. In this work, we investigated the influence of EF24 in the modulation of LPS-stimulated maturation and cytokine secretion in DCs. Cytokines are the effector molecules in the immunoregulation by DCs, only when the latter attain maturation characteristics. Morphologic changes, together with surface expression of immunophenotypic markers, are hallmark of this process. Although originally developed as an anti-proliferative agent, EF24 does not appear to have any deleterious effects on DCs in the 1–10 μM concentration range used in this study (data not shown).
We have previously shown that LPS induces maturation of immortalized JAWS II DCs (26) as well as primary bone marrow-derived DCs (24). The maturation of DCs is characterized by increase in the expression levels of antigen presentation markers (MHC class II) and co-stimulatory molecules (CD40, CD80 and CD86). Immature DCs express these markers at low level. Here, we found that EF24 potently reduced LPS-stimulated DC maturation. We observed that treatment with EF24 reduced LPS-stimulated MHC class II, CD80 and CD86 expression in DCs.
It is well established that most of the LPS-stimulated inflammatory effect is mediated by TLR4-signaling pathway (24, 31). TLR4 signaling occurs via MyD88-dependent or -independent pathways. The effects of LPS spanning TLR4 and NF-κB are manifested through secretion of pro-inflammatory cytokines (32). TLR4 up-regulation results in the activation of transcription factor NF-κB (7, 33, 34), which in turn leads to multifaceted effects, including inflammation (35). Using EMSA as well as a luciferase reporter assay, we found that EF24 reduces the LPS-stimulated activation of NF-κB. Previous studies have shown that inhibition of NF-κB down-regulates MHC II, co-stimulatory molecules like CD80, CD86 and CD40 (36). We also show that EF24 reduces the LPS-stimulated secretion of inflammatory cytokines TNF-α and IL-6, suggesting that EF24 may be used in modulation of inflammatory conditions. These findings are consistent with those of curcumin that has also been shown to reduce the secretion of TNF-α and IL-6 (13). As shown in Fig. 2(A), EF24 is more potent in controlling the secretion of TNF-α than curcumin. At the same time, it does not significantly affect the LPS-stimulated IL-12 (data not shown). Because IL-12 is responsible for activation of Th1 response, our finding suggests that EF24 may not affect the Th1 response. Although not addressed here, our future studies will be directed toward investigating the effects of EF24 on the DC-induced adaptive Th1-cell response.
The adapter protein MyD88 is the key intermediate signaling adaptor molecule in TLR-NF-κB pathway. Based on our results in MyD88 knock down experiments, it appears that EF24 may be acting via a pathway dependent on MyD88 adapter to reduce NF-κB activation. These results further confirm that EF24 targets LPS-stimulated TLR4-NF-κB signaling. Interestingly, curcumin has been shown to reduce TLR4 downstream signaling by direct interaction with MD-2, another component of TLR4 complex (14), and by preventing the homodimerization of the receptor (15). It will be of further interest to investigate the molecular and spacio-chemical characteristics of interaction between EF24 and TLR4 or its partners.
In summary, we provide evidence in support of an anti-inflammatory role of curcuminoid EF24. These effects of EF24 may be of utility in controlling ‘cytokine storm’ (37) originating after microbial or non-infectious inflammatory insult in DCs, macrophages and other immune cells. The experimental results described in this article point toward the possibility of EF24 affecting several different functions of DCs. Besides inhibiting expression of MHC class II and co-stimulatory molecules, it reduces NF-κB activation and suppresses pro-inflammatory cytokine secretion. At the same time, it prevents morphologic and phenotypic maturation of DCs. The possibility that EF24 may be involved in affecting a complex cross talk between the TLR4-NF-κB, Jak1/Stat1 and the MAPK pathways cannot be ruled out. There exists a growing awareness of the role of inflammation in oncogenesis (2). TLR4-stimulated inflammatory response by immune cells not only plays an important role in the pathogenesis of cancer but some cancer cells also express increased levels of TLR4 (38). Curcumin has been historically known to suppress inflammatory process, and curcumin as well as some of the curcuminoids are under clinical trials for both inflammatory disorders (39) and cancers (40).
Funding
National Institutes of Health (1R03 CA143614-01 to V.A.); Oklahoma Center for the Advancement of Science and Technology (to S.A.).
Acknowledgments
The authors acknowledge the technical help from Cathy King and synthetic work of Pallavi Lagisetty. Part of the work was been presented in the American Association of Immunologists meeting, Baltimore, MD, USA (2010).
References
- 1.Nathan C. Points of control in inflammation. Nature. 2002;420:846. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 2.Rook GA, Dalgleish A. Infection, immunoregulation, and cancer. Immunol. Rev. 2011;240:141. doi: 10.1111/j.1600-065X.2010.00987.x. [DOI] [PubMed] [Google Scholar]
- 3.Reis e Sousa C. Dendritic cells in a mature age. Nat. Rev. Immunol. 2006;6:476. doi: 10.1038/nri1845. [DOI] [PubMed] [Google Scholar]
- 4.Baumgart DC, Thomas S, Przesdzing I, et al. Exaggerated inflammatory response of primary human myeloid dendritic cells to lipopolysaccharide in patients with inflammatory bowel disease. Clin. Exp. Immunol. 2009;157:423. doi: 10.1111/j.1365-2249.2009.03981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanco P, Palucka AK, Pascual V, Banchereau J. Dendritic cells and cytokines in human inflammatory and autoimmune diseases. Cytokine Growth Factor. Rev. 2008;19:41. doi: 10.1016/j.cytogfr.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lommatzsch M, Bratke K, Bier A, et al. Airway dendritic cell phenotypes in inflammatory diseases of the human lung. Eur. Respir. J. 2007;30:878. doi: 10.1183/09031936.00036307. [DOI] [PubMed] [Google Scholar]
- 7.Fujihara M, Muroi M, Tanamoto K, Suzuki T, Azuma H, Ikeda H. Molecular mechanisms of macrophage activation and deactivation by lipopolysaccharide: roles of the receptor complex. Pharmacol. Ther. 2003;100:171. doi: 10.1016/j.pharmthera.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Takeda K, Akira S. Toll receptors and pathogen resistance. Cell. Microbiol. 2003;5:143. doi: 10.1046/j.1462-5822.2003.00264.x. [DOI] [PubMed] [Google Scholar]
- 9.De Smedt T, Pajak B, Klaus GG, et al. Antigen-specific T lymphocytes regulate lipopolysaccharide-induced apoptosis of dendritic cells in vivo. J. Immunol. 1998;161:4476. [PubMed] [Google Scholar]
- 10.Flohe SB, Agrawal H, Schmitz D, Gertz M, Flohe S, Schade FU. Dendritic cells during polymicrobial sepsis rapidly mature but fail to initiate a protective Th1-type immune response. J. Leukoc. Biol. 2006;79:473. doi: 10.1189/jlb.0705413. [DOI] [PubMed] [Google Scholar]
- 11.Kim GY, Kim KH, Lee SH, et al. Curcumin inhibits immunostimulatory function of dendritic cells: mAPKs and translocation of NF-kappa B as potential targets. J. Immunol. 2005;174:8116. doi: 10.4049/jimmunol.174.12.8116. [DOI] [PubMed] [Google Scholar]
- 12.Shirley SA, Montpetit AJ, Lockey RF, Mohapatra SS. Curcumin prevents human dendritic cell response to immune stimulants. Biochem. Biophys. Res. Commun. 2008;374:431. doi: 10.1016/j.bbrc.2008.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou H, Beevers CS, Huang S. The targets of curcumin. Curr. Drug Targets. 2011;12:332. doi: 10.2174/138945011794815356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gradisar H, Keber MM, Pristovsek P, Jerala R. MD-2 as the target of curcumin in the inhibition of response to LPS. J. Leukoc. Biol. 2007;82:968. doi: 10.1189/jlb.1206727. [DOI] [PubMed] [Google Scholar]
- 15.Youn HS, Saitoh SI, Miyake K, Hwang DH. Inhibition of homodimerization of Toll-like receptor 4 by curcumin. Biochem. Pharmacol. 2006;72:62. doi: 10.1016/j.bcp.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 16.Lagisetty P, Vilekar P, Sahoo K, Anant S, Awasthi V. CLEFMA-an anti-proliferative curcuminoid from structure-activity relationship studies on 3,5-bis(benzylidene)-4-piperidones. Bioorg. Med. Chem. 2010;18:6109. doi: 10.1016/j.bmc.2010.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adams BK, Ferstl EM, Davis MC, et al. Synthesis and biological evaluation of novel curcumin analogs as anti-cancer and anti-angiogenesis agents. Bioorg. Med. Chem. 2004;12:3871. doi: 10.1016/j.bmc.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Sun D, Zhuang X, Xiang X, et al. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol. Ther. 2010;18:1606. doi: 10.1038/mt.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anand P, Thomas SG, Kunnumakkara AB, et al. Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochem. Pharmacol. 2008;76:1590. doi: 10.1016/j.bcp.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Kasinski AL, Du Y, Thomas SL, et al. Inhibition of IkappaB kinase-nuclear factor-kappaB signaling pathway by 3,5-bis(2-flurobenzylidene)piperidin-4-one (EF24), a novel monoketone analog of curcumin. Mol. Pharmacol. 2008;74:654. doi: 10.1124/mol.108.046201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lagisetty P, Powell DR, Awasthi V. Synthesis and structural determination of 3 3,5-bis(2-fluorobenzylidene)-4-piperidone analogs of curcumin. J. Mol. Str. 2009 936:23. [Google Scholar]
- 22.Vilekar P, Awasthi V, Lagisetty P, King C, Shankar N, Awasthi S. In vivo trafficking and immunostimulatory potential of an intranasally-administered primary dendritic cell-based vaccine. BMC Immunol. 2010;11:60. doi: 10.1186/1471-2172-11-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giacometti A, Cirioni O, Ghiselli R, et al. Cathelicidin peptide sheep myeloid antimicrobial peptide-29 prevents endotoxin-induced mortality in rat models of septic shock. Am. J. Respir. Crit. Care Med. 2004;169:187. doi: 10.1164/rccm.200307-971OC. [DOI] [PubMed] [Google Scholar]
- 24.Awasthi S, Magee DM. Differences in expression of cell surface co-stimulatory molecules, Toll-like receptor genes and secretion of IL-12 by bone marrow-derived dendritic cells from susceptible and resistant mouse strains in response to Coccidioides posadasii. Cell. Immunol. 2004;231:49. doi: 10.1016/j.cellimm.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Medzhitov R, Preston-Hurlburt P, Kopp E, et al. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol. Cell. 1998;2:253. doi: 10.1016/s1097-2765(00)80136-7. [DOI] [PubMed] [Google Scholar]
- 26.Awasthi S, Cox RA. Transfection of murine dendritic cell line (JAWS II) by a nonviral transfection reagent. Biotechniques. 2003;35:600. doi: 10.2144/03353dd03. , 604. [DOI] [PubMed] [Google Scholar]
- 27.Brown KL, Cosseau C, Gardy JL, Hancock RE. Complexities of targeting innate immunity to treat infection. Trends Immunol. 2007;28:260. doi: 10.1016/j.it.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 28.Adema GJ. Dendritic cells from bench to bedside and back. Immunol. Lett. 2009;122:128. doi: 10.1016/j.imlet.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 29.Mosley CA, Liotta DC, Snyder JP. Highly active anticancer curcumin analogues. Adv. Exp. Med. Biol. 2007;595:77. doi: 10.1007/978-0-387-46401-5_2. [DOI] [PubMed] [Google Scholar]
- 30.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol. Pharm. 2007;4:807. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 31.Carpenter S, O'Neill LA. Recent insights into the structure of Toll-like receptors and post-translational modifications of their associated signalling proteins. Biochem. J. 2009;422:1. doi: 10.1042/BJ20090616. [DOI] [PubMed] [Google Scholar]
- 32.Bottero V, Withoff S, Verma IM. NF-kappaB and the regulation of hematopoiesis. Cell Death Differ. 2006;13:785. doi: 10.1038/sj.cdd.4401888. [DOI] [PubMed] [Google Scholar]
- 33.Jiang W, Sun R, Wei H, Tian Z. Toll-like receptor 3 ligand attenuates LPS-induced liver injury by down-regulation of toll-like receptor 4 expression on macrophages. Proc. Natl Acad. Sci. USA. 2005;102:17077. doi: 10.1073/pnas.0504570102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uronen-Hansson H, Allen J, Osman M, Squires G, Klein N, Callard RE. Toll-like receptor 2 (TLR2) and TLR4 are present inside human dendritic cells, associated with microtubules and the Golgi apparatus but are not detectable on the cell surface: integrity of microtubules is required for interleukin-12 production in response to internalized bacteria. Immunology. 2004;111:173. doi: 10.1111/j.0019-2805.2003.01803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kluwe J, Mencin A, Schwabe RF. Toll-like receptors, wound healing, and carcinogenesis. J. Mol. Med. 2009;87:125. doi: 10.1007/s00109-008-0426-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshimura S, Bondeson J, Foxwell BM, Brennan FM, Feldmann M. Effective antigen presentation by dendritic cells is NF-kappaB dependent: coordinate regulation of MHC, co-stimulatory molecules and cytokines. Int. Immunol. 2001;13:675. doi: 10.1093/intimm/13.5.675. [DOI] [PubMed] [Google Scholar]
- 37.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N. Engl. J. Med. 2003;348:138. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 38.Wang EL, Qian ZR, Nakasono M, et al. High expression of Toll-like receptor 4/myeloid differentiation factor 88 signals correlates with poor prognosis in colorectal cancer. Br. J. Cancer. 2010;102:908. doi: 10.1038/sj.bjc.6605558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern. Med. Rev. 2009;14:141. [PubMed] [Google Scholar]
- 40.Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. Curcumin: from ancient medicine to current clinical trials. Cell. Mol. Life Sci. 2008;65:1631. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]