Abstract
Objective:
To examine domain-specific neurocognitive differences between African American (AA) and Caucasian (CA) patients with pediatric-onset multiple sclerosis (POMS).
Methods:
An extensive battery of neuropsychological tests was given to each subject, including tests in all major domains of cognitive function. Point-biserial correlations between ethnicity and test performance were computed. Significant correlations were followed up with hierarchical multiple regression analysis, accounting for clinical and demographic variables before examining ethnic differences.
Results:
Forty-two patients with POMS including 20 AA and 22 CA subjects were assessed. The cohorts did not differ in age, gender, socioeconomic status, disease duration, disability score, immunoglobulin G index, or number of relapses in the first 2 years of disease. Retaining some of these variables as covariates in the hierarchical regression analysis, the AA cohort performed worse on measures of language (p < 0.001) and complex attention (p < 0.01) than their CA peers.
Conclusion:
AA patients with POMS may be at higher risk for adverse cognitive impact in the areas of language and complex attention. Longitudinal characterization of cognitive pathology is critical for the development of effective intervention strategies to prolong cognitive functioning in POMS cohorts.
Multiple sclerosis (MS) is an inflammatory demyelinating disorder affecting the CNS. Approximately 2%–5% of cases present initially in children under age 18 years.1,2 The clinical course of pediatric-onset MS (POMS) involves symptoms similar to adult-onset MS (AOMS),3 including neurocognitive difficulties.
While the clinical symptoms of POMS are similar to those of AOMS, neurocognitive deficits may differ between the 2 groups. POMS occurs during a time of ongoing myelination in the CNS, so inflammatory demyelination may result in atypical or incomplete formation of white matter pathways crucial for cognitive development. Domains of weaker neurocognitive performance commonly observed in pediatric MS include complex attention, memory, and language.4–6 In one POMS cohort, attention was the most commonly impaired neurocognitive domain, affecting about 30% of subjects.6
In adults, African Americans (AA) have a lower risk of developing MS than Caucasians (CA), but their disease course is typically more aggressive.7 Among adults, one study found that AA with MS had increased risk of severe vs mild neurocognitive impairment (odds ratio = 1.32) compared with CA patients.8 However, these increased odds disappeared with correction for socioeconomic status (SES). Research on ethnic differences among children with MS is limited to a single-center study, which reported higher annualized relapse rates in AA than CA children with MS.9 The present study investigated domain-specific ethnic neurocognitive differences in children and youth with MS.
METHODS
Subjects.
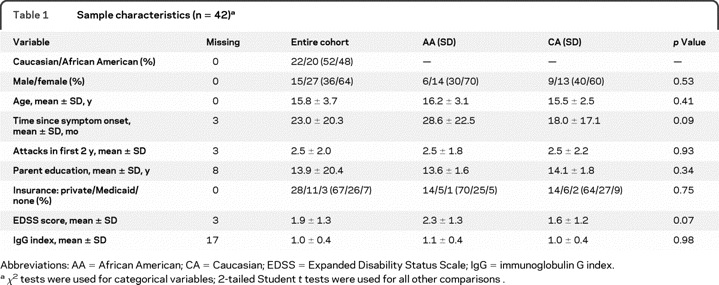
The study cohort included all youth with MS referred to the Center for Pediatric-Onset Demyelinating Disorders clinic at the University of Alabama at Birmingham between April 2006 and September 2009. Inclusion criteria included diagnosis of MS, symptom onset before age 18, and completion of neuropsychological testing. Exclusion criteria included diagnosis at time of study of acute demyelinating encephalomyelitis or neuromyelitis optica. Sample demographics are included in table 1.
Table 1.
Sample characteristics (n = 42)a
Abbreviations: AA = African American; CA = Caucasian; EDSS = Expanded Disability Status Scale; IgG = immunoglobulin G index.
χ2 tests were used for categorical variables; 2-tailed Student t tests were used for all other comparisons.
Standard protocol approvals, registrations, and patient consents.
Approval to conduct this study was granted by the Institutional Review Board at the University of Alabama at Birmingham, and written informed consent was obtained from all legal guardians of patients participating in the study.
Clinical and neurocognitive assessment.
Each subject was evaluated by an interdisciplinary team of neurologists, a psychiatrist, and a neuropsychologist. In addition to demographic information, data collected on each participant included disability as rated on the Expanded Disability Status Scale (EDSS10), number of relapses experienced in the first 2 years of disease course, months since symptom onset, and CSF levels of immunoglobulin G (IgG), when available.
The following neurocognitive tests were administered to each participant in a single session:
Global verbal and nonverbal functioning, assessed by the Wechsler Abbreviated Scale of Intelligence (WASI) verbal and performance IQs11
Complex attention, assessed by the Delis-Kaplan Executive Function System (D-KEFS) Letter-Number Trail Making Task12
Phonemic and semantic verbal fluencies, assessed by the D-KEFS Letter and Category Fluency Tests, respectively12
Confrontation naming, assessed by the Expressive One-Word Picture Vocabulary Test (EOWPVT), a non-literacy– based measure of vocabulary and expressive language13
Verbal memory, assessed by the California Verbal Learning Test–Children's Version (CVLT-C), in which the participant must recall a “shopping list” of items presented across 5 learning trials, with an intervening word list presented as a distractor14
Statistical analysis.
AA and CA cohorts were compared on all demographic variables using 2-tailed Student t tests. To measure SES in subsequent analyses, a composite variable was created by averaging standardized measures of presence and type of insurance and parental years of education. Insurance type was categorized ordinally as private insurance, Medicaid, or no insurance.15 If more than one parent's education level was listed, the highest education attained by either parent who resided with the child was utilized.
A small number of data points (6%) were missing and replaced through Expectation Maximization estimation.16 Bivariate correlation coefficients were obtained between standardized neurocognitive test scores and clinical/demographic variables selected on the basis of their theoretical importance to neurocognitive function. Neurocognitive variables significantly correlated with ethnicity were followed up in hierarchical regression analyses to ascertain the unique contribution of ethnicity to test scores after controlling for relevant covariates. All analyses were conducted in SPSS version 15.0.
RESULTS
Demographic data.
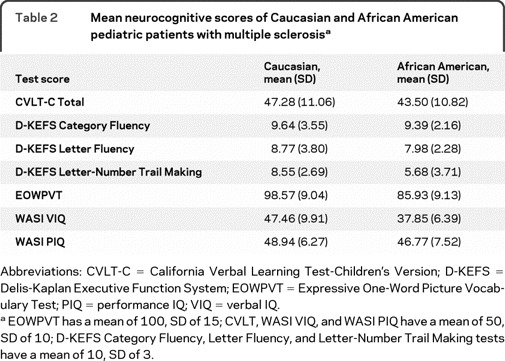
Forty-two subjects aged 6–21 years comprised the sample, including 20 AAs and 22 CAs. Descriptive data are presented in table 1. AA and CA cohorts did not differ on any demographic or clinical variables, although there were trends for AA children to have higher EDSS scores and longer time since disease onset. Neurocognitive mean scores and standard deviations for AA and CA cohorts are displayed in table 2.
Table 2.
Mean neurocognitive scores of Caucasian and African American pediatric patients with multiple sclerosisa
Abbreviations: CVLT-C = California Verbal Learning Test-Children's Version; D-KEFS = Delis-Kaplan Executive Function System; EOWPVT = Expressive One-Word Picture Vocabulary Test; PIQ = performance IQ; VIQ = verbal IQ.
EOWPVT has a mean of 100, SD of 15; CVLT, WASI VIQ, and WASI PIQ have a mean of 50, SD of 10; D-KEFS Category Fluency, Letter Fluency, and Letter-Number Trail Making tests have a mean of 10, SD of 3.
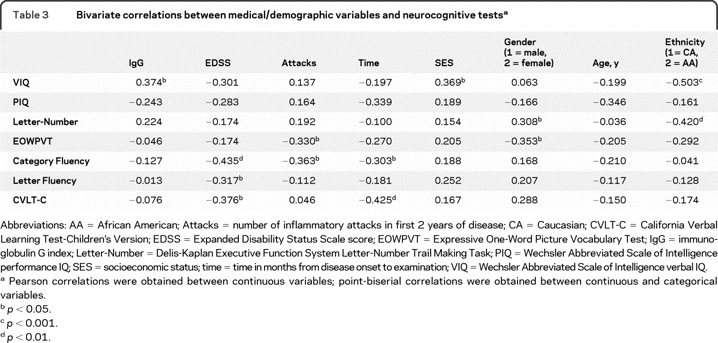
Correlations between neurocognitive performance and ethnicity.
Table 3 shows point-biserial correlation coefficients between ethnicity and neurocognitive test scores. Ethnicity correlated with WASI verbal IQ (VIQ) (p < 0.001), D-KEFS Letter-Number Trail Making Task (p < 0.01), and approached significance with EOWPVT (p = 0.06). WASI VIQ also correlated with SES and IgG index (p < 0.05), and D-KEFS Letter-Number Trail Making Task and EOWPVT correlated with gender (p < 0.05). Thus, SES, IgG index, and gender were retained as covariates in the multivariate hierarchical regression analyses.
Table 3.
Bivariate correlations between medical/demographic variables and neurocognitive testsa
Abbreviations: AA = African American; Attacks = number of inflammatory attacks in first 2 years of disease; CA = Caucasian; CVLT-C = California Verbal Learning Test-Children's Version; EDSS = Expanded Disability Status Scale score; EOWPVT = Expressive One-Word Picture Vocabulary Test; IgG = immunoglobulin G index; Letter-Number = Delis-Kaplan Executive Function System Letter-Number Trail Making Task; PIQ = Wechsler Abbreviated Scale of Intelligence performance IQ; SES = socioeconomic status; time = time in months from disease onset to examination; VIQ = Wechsler Abbreviated Scale of Intelligence verbal IQ.
Pearson correlations were obtained between continuous variables; point-biserial correlations were obtained between continuous and categorical variables.
p< 0.05.
p< 0.001.
p< 0.01.
Hierarchical regression analysis.
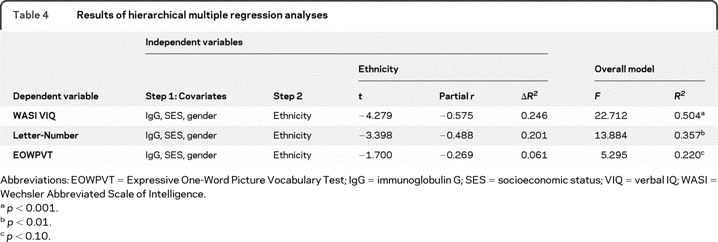
Assumptions of the linear regression model were examined, including normality, linearity, homoscedasticity, absence of multicollinearity, absence of univariate outliers, and the ratio of subjects to predictors. All variables met these assumptions. Hierarchical regression models were conducted to determine the unique contribution of ethnicity to neurocognitive test score. Variables included in the models were chosen based on both theoretical and empirical rationale. SES was included as a covariate based upon previous research in ethnic neurocognitive differences in adult MS8 as well as its significant correlation with WASI VIQ in this sample. IgG index and gender were included on the basis of their significant correlations with neurocognitive variables of interest, as well as our theoretical understanding of possible contributory factors to cognitive dysfunction in pediatric MS. Previous pediatric MS research has reported gender differences in physical disability status17 and IgG index is a marker of immunologic response that has been shown to differ between AAs and CAs with MS.18 The first step of each hierarchical regression model included the SES, IgG index, and gender covariates. The second step included ethnicity to assess its unique contribution to the model. The dependent variable in each separate hierarchical regression was the neurocognitive test score shown to correlate with ethnicity (i.e., VIQ, Letter-Number Trail Making Task, and EOWPVT scores).
Ethnicity explained 24.6% of the sample's variance in WASI VIQ after accounting for SES, IgG index, and gender (table 4), with AAs scoring an average of 1 SD lower than CAs (AA mean = 37.85, SD = 6.39; CA mean = 47.76, SD = 9.91). Ethnicity explained 20.1% of variance in Letter-Number Trail Making Test scores, with AAs scoring lower than CAs (AA mean = 5.68, SD = 3.71; CA mean = 8.55, SD = 2.69). Finally, ethnicity approached significance as a predictor of EOWPVT (p = 0.06), and explained 6.1% of the sample's performance, with AAs scoring lower than CAs (AA mean = 85.93, SD = 9.13; CA mean = 98.57, SD = 9.04).
Table 4.
Results of hierarchical multiple regression analyses
Abbreviations: EOWPVT = Expressive One-Word Picture Vocabulary Test; IgG = immunoglobulin G; SES = socioeconomic status; VIQ = verbal IQ; WASI = Wechsler Abbreviated Scale of Intelligence.
p< 0.001.
p< 0.01.
p < 0.10.
DISCUSSION
AA pediatric patients with MS performed poorer than CA peers in 2 domains: complex attention and language. Complex attention involves focused attention combined with other demands such as visuomotor or visuospatial processing and may affect learning and school performance, as complex attention is necessary for many classroom tasks, e.g., taking notes while simultaneously listening to a lecture. In a previous study,5 children with MS performed poorly in complex attention tasks when compared with healthy controls. Moreover, the range for the MS group's performance in that study was much wider than the 2 control groups' ranges, suggesting differential impairment within the MS cohort. Given that previous studies with POMS cohorts have noted deficits in these areas, AA children and adolescents with MS may not show unique cognitive difficulties when compared with CA peers, but may demonstrate greater or faster decline in the cognitive capacities typically affected in pediatric MS.
Language is typically conceptualized as either verbal fluency or crystallized verbal knowledge. Pediatric MS cohorts have deficits in both domains.5,6 Language skills lay groundwork for much of academic learning, and may continue to develop well into adulthood. In adults with degenerative disorders, language tends to be a relatively preserved function, but a different pattern has emerged in the pediatric population. Because language is still maturing during childhood and adolescence, demyelination may preclude typical acquisition of language skills, accounting for the language deficits described in POMS but not in adult-onset MS.19
Currently, little is known about neurocognitive differences among pediatric patients with MS of different ethnic backgrounds. While more aggressive disease in AA children may result in greater neurocognitive impairment, reliance on disability measures such as the EDSS, which derives primarily from physical impairment, does not adequately address neurocognitive domains.
The present study utilized a cross-sectional design, revealing a “snapshot” of subjects' neurocognitive functioning at one point in time. How the inflammatory demyelinating process of MS affects ongoing myelination in the developing brain is poorly understood. The sequelae of concurrent myelination and demyelination may differ from the effects of demyelinating processes on fully developed white matter pathways, as studied in adults with MS. Future research should consider longitudinal observation of neurocognitive function in children and youth with MS to better comprehend the implications of developing MS at a young age, and how demyelination due to MS in childhood may influence adult cognitive functioning, after natural myelination typically concludes in healthy individuals.
Also complicating development of youth with MS is the fact that patients must overcome obstacles to learning such as chronic fatigue and prolonged school absences due to illness. Longitudinal monitoring of neurocognitive function in pediatric MS cohorts would help characterize the pathologic neurodevelopment of this population, and would educate development of early interventions to address relevant areas of neurocognitive function.
Though the AA and CA cohorts did not differ on disease duration, the developmental neurocognitive course of each cohort is unclear. We do not know if the observed differences continue over time; for example, it is possible that the rate and progression of neurocognitive deficits varies between the 2 ethnic groups. Consequently, the observed differences may not be maintained over time, may be maintained but at a less significant level, or may increase.
Although the sample size of the present study was comparable to many pediatric MS cohorts in the literature, statistical power may have been insufficient to detect all differences. Furthermore, as these analyses were correlational, no causal inferences can be made regarding the observed differences in cognitive functioning between the 2 ethnically defined groups. Observed ethnic differences may not be due to ethnicity itself, but to a third factor such as depression, culture, or access to educational resources that was not covaried in the present analyses. Further, while we attempted to account for factors such as SES and IgG index that might confound with ethnicity, it is possible that the neurocognitive tests themselves have some cultural bias that contributed partially or fully to the neurocognitive disparities between the ethnic cohorts. Finally, the cross-sectional design constrains interpretation and generalizability of our findings.
Why ethnic-based differences occur in pediatric MS remains largely unknown. The ethnic groups in this study did not differ on SES, age, gender, or disease variables, so it is unlikely the neurocognitive disparities are due to demographic or readily observable physical factors. Replication of these findings and sequential neuropsychological testing over time will be critical before conclusive statements can be made regarding the current findings.
Ethnic differences in immunologic disease are not without precedent. More severe disability and greater immunologic response have been associated with AA ethnicity in genetic and immunologic research with adult MS cohorts.17,20 In systemic lupus erythematosus, AAs have a disproportionately higher mortality risk than CAs, suggesting pathologic differences between ethnicities.21,22 Further research should explore associations between cognitive decline and biological factors in order to identify underlying explanations for why cognitive difficulties occur in MS, and particularly why adverse cognitive impact may be more severe for AA children with MS.
The present results suggest cognitive interventions focused on language and complex attention skills may help youth with MS, particularly those of AA ethnicity, who may be more vulnerable to loss of those cognitive skills. We are just beginning to gain some understanding of the cognitive impact of MS in children and youth, and there have been no prior studies examining ethnic differences in the neurocognitive functioning of individuals with POMS. It is hoped that these findings may spur further research on ethnic-based risk for adverse cognitive impact in POMS.
Editorial, page 2054.
- AA
- African American
- AOMS
- adult-onset multiple sclerosis
- CA
- Caucasian
- CVLT-C
- California Verbal Learning Test–Children's Version
- D-KEFS
- Delis-Kaplan Executive Function System
- EDSS
- Expanded Disability Status Scale
- EOWPVT
- Expressive One-Word Picture Vocabulary Test
- IgG
- immunoglobulin G
- POMS
- pediatric-onset multiple sclerosis
- SES
- socioeconomic status
- VIQ
- verbal IQ
- WASI
- Wechsler Abbreviated Scale of Intelligence
AUTHOR CONTRIBUTIONS
Statistical analysis was conducted by Kelly Ross.
DISCLOSURE
K.A. Ross received salary support from the Ida B. Boyd Foundation. Dr. Schwebel serves on a scientific advisory board for Sociometrics Corporation; serves on the editorial boards of the Journal of Injury and Violence Research, the Journal of Pediatric Psychology, and the Journal of Safety Research; receives royalties from the publication of The Student Teacher's Handbook, 4th ed. (Erlbaum, 2002); has served as a consultant to Ohio State University (NIH grant), ORCAS (NIH grants), Guemmer & Ritt (Law firm, Tampa, FL), and Alexander Hawes, LLP (Law firm, San Jose, CA); and receives research support from the NIH (5R01HD058573-02 [PI] and 3R01HD058573-01A1S1 [PI]), the CDC (CCU409679 [Investigator]), and The Blue Dog Foundation (UK). Dr. Rinker II serves on speakers' bureaus for and has received speaker honoraria from Biogen Idec, EMD Serono, Inc., Pfizer Inc., and Teva Pharmaceutical Industries Ltd.; and has received research support from EMD Serono, Biogen Idec, the NIH (5R01HD30149-11 [subinvestigator], and the National Multiple Sclerosis Society, Pediatric MS Center of Excellence. Dr. Ness has served as a consultant for Merck Serono and has received research support from the NIH (NINDS K08NS43220 [primary investigator]) and the National Multiple Sclerosis Society Network of Pediatric MS Centers of Excellence. Dr. Ackerson has served as a consultant for the Alabama Head Injury Foundation and has received research support from the National Multiple Sclerosis Society and the Health Resources and Service Administration.
REFERENCES
- 1. Duquette P, Murray TJ, Pleines J, et al. Multiple sclerosis in childhood: clinical profile in 125 patients. Pediatrics 1987;111:359–363 [DOI] [PubMed] [Google Scholar]
- 2. Klein NP, Ray P, Carpenter D, et al. Rates of autoimmune diseases in Kaiser Permanente for use in vaccine adverse event safety studies. Vaccine 2010;28:1062–1068 [DOI] [PubMed] [Google Scholar]
- 3. Gorman MP, Healy BC, Polgar-Turcsanyi M, Chitnis T. Increased relapse rates in pediatric-onset compared with adult-onset multiple sclerosis. Arch Neurol 2009;66:54–55 [DOI] [PubMed] [Google Scholar]
- 4. Amato MP, Goretti B, Ghezzi A, et al. Cognitive and psychosocial features of childhood and juvenile MS. Neurology 2008;70:1891–1897 [DOI] [PubMed] [Google Scholar]
- 5. Banwell B, Anderson P. The cognitive burden of multiple sclerosis in children. Neurology 2005;64:891–894 [DOI] [PubMed] [Google Scholar]
- 6. MacAllister WS, Belman AL, Milazzo M, et al. Cognitive functioning in children and adolescents with multiple sclerosis. Neurology 2005;64:1422–1425 [DOI] [PubMed] [Google Scholar]
- 7. Cree BAC, Reich DE, Khan O, et al. Modification of multiple sclerosis phenotypes by African ancestry at HLA. Arch Neurol 2004;66:226–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marrie RA, Cutter G, Tyry T, Vollmer T, Campagnolo D. Does multiple sclerosis–associated disability differ between races? Neurology 2006;66:1235–1240 [DOI] [PubMed] [Google Scholar]
- 9. Boster AL, Endress CF, Hreha SA, Caon C, Perumal JS, Khan OA. Pediatric-onset multiple sclerosis in African-American black and European-Origin white patients. Pediatr Neurol 1009;40:31–33 [DOI] [PubMed] [Google Scholar]
- 10. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33:1444–1452 [DOI] [PubMed] [Google Scholar]
- 11. Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI). San Antonio: Harcourt Assessment; 1999. [Google Scholar]
- 12. Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System (D-KEFS). San Antonio: The Psychological Corporation; 2001. [Google Scholar]
- 13. Gardner MF. Manual for Expressive One-Word Picture Vocabulary Test. Novato: Academic Therapy Publication; 1985. [Google Scholar]
- 14. Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test–Children's Version. San Antonio: The Psychological Corporation; 1994. [Google Scholar]
- 15. Ward MM. Medical insurance, socioeconomic status, and age of onset of end-stage renal disease in patients with lupus nephritis. J Rheumatol 2007;34:2024–2027 [PubMed] [Google Scholar]
- 16. Niu T, Ding AA, Kreutz R, Lindpaintner K. An expectation-maximization-likelihood-ratio test for handling missing data. Genetics 2005;169:1021–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Renoux C, Vikusic S, Mikaeloff Y, et al. Natural history of multiple sclerosis with childhood onset. N Engl J Med 2007;356:2603–2613 [DOI] [PubMed] [Google Scholar]
- 18. Rinker JR, II, Trinkaus K, Naismith RT, Cross AH. Higher IgG index found in African Americans versus Caucasians with multiple sclerosis. Neurology 1997;69:68–72 [DOI] [PubMed] [Google Scholar]
- 19. Rao SM, Leo GJ, Bernardin L, Unverzagt F. Cognitive dysfunction in multiple sclerosis, I: frequency, patterns, and predictions Neurology 1991;41:685–691 [DOI] [PubMed] [Google Scholar]
- 20. Cree BAC, Reich DE, Khan O, et al. Modification of multiple sclerosis phenotypes by African ancestry at HLA. Arch Neurol 2009;66:226–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Anderson E, Nietert PJ, Kamen DL, Gilkeson GS. Ethnic disparities among patients with systemic lupus erythematosus in South Carolina. J Rheumatol 2008;35:819–825 [PMC free article] [PubMed] [Google Scholar]
- 22. Krishnan E, Hubert HB. Ethnicity and mortality from systemic lupus erythematosus in the U.S. Ann Rheum Dis 2006;65:1500–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]


