Abstract
Cognitive impairment affects a large proportion of patients with multiple sclerosis (MS) and has a profound impact on their daily-life activities. Improving the knowledge of the pathophysiology of cognitive impairment in MS and of the mechanisms responsible for its evolution over time might contribute to development of better outcome measures and targets for innovative treatment strategies. Due to their ability to detect MS-related abnormalities, MRI techniques are a valuable tool to achieve these goals. Following an updated overview of the assessment methods and profile of cognitive impairment in patients with MS, this review provides a state-of-the-art summary of the main results obtained from the application of conventional and modern magnetic resonance– based techniques to quantify MS-related damage, in terms of macroscopic lesions, as well as involvement of the normal-appearing white matter and gray matter and their association with cognitive impairment. The possible role of brain cortical reorganization in limiting the clinical consequences of disease-related damage is also discussed. Finally, the utility of the previous techniques to monitor the progression of cognitive deficits over time and the efficacy of possible therapeutic strategies is considered.
Cognitive impairment affects a large proportion of patients with multiple sclerosis (MS), with a prevalence rate ranging from 40% to 70%.1,2 Although cognitive deficits have been observed from the early stages of the disease, they are more frequent and pronounced in chronic progressive MS and tend to worsen over time. Cognitive capacity is critical for a range of activities such as work, driving, and adherence to medication regimen, but in diseases such as MS, where physical disability is prominent, cognitive impairment is sometimes overlooked or even disregarded. The definition of the mechanisms underlying its development and the identification of markers useful to monitor its progression might contribute to drive future pharmacologic and rehabilitative strategies.
MRI is the most used paraclinical tool to investigate in vivo the pathobiology of MS and to monitor disease evolution.3 After providing a clinical background of the main cognitive deficits encountered in patients with MS, and of the most suitable tests for their assessment, this review summarizes the contribution provided by conventional and quantitative magnetic resonance (MR)–based techniques to improve the understanding of the factors associated with cognitive impairment in MS. Since the efficiency of brain cortical reorganization in the different stages of the disease might play a major role in explaining interindividual heterogeneity of the clinical manifestations, the main results obtained from the application of fMRI to study cognitive network functions in these patients are also discussed. Finally, the utility of MRI techniques to monitor cognitive impairment progression over time as well as their promises to assess treatment are considered.
CLINICAL BACKGROUND
In cross-sectional studies comparing MS and matched controls, patients with MS commonly exhibit impairment on a wide array of tests ranging from processing speed tasks to measures of complex executive functions. However, in large sample studies including a full spectrum of cognitive domains,2,4 2 areas are proven to be particularly sensitive to MS-associated impairment. The first domain is information processing speed. Tests measuring the speed of mental processing without demand for new learning, language expression, or complex executive abilities have proven to be very sensitive in several studies.5 The second domain is episodic memory.2,6 Measures here typically require the repetition or recall of verbal or visual information presented over successive learning trials. Then, roughly 20–30 minutes later, patients are asked to recall again the same information without another exposure.
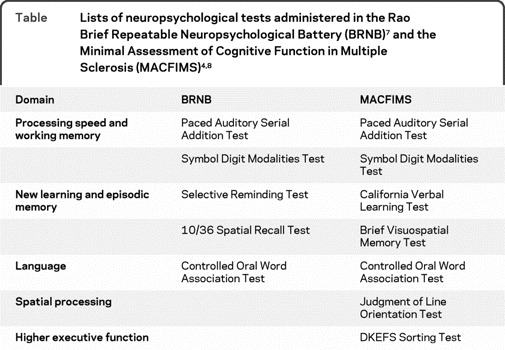
One of the impediments to comparing the many studies of cognition in MS is the difficulty of weighting the results of one test to another purportedly measuring the same cognitive domain. There are 2 validated test batteries that have reached a threshold of wide acceptance and replicability in the literature: the Rao Brief Repeatable Neuropsychological Battery (BRNB)7 and the Minimal Assessment of Cognitive Function in Multiple Sclerosis (MACFIMS).4,8 The tests from each battery are listed in the table and there is considerable overlap. Yet there are advantages to each approach—the BRNB requires less time and has been translated into multiple European languages, while the MACFIMS has a stronger psychometric foundation and includes assessment of spatial processing and higher executive function abilities. The Symbol Digit Modalities Test (SDMT) is a traditional, person-administered test that requires only 5 minutes to be completed. It has been proposed as a promising alternative to the Paced Auditory Serial Addition Task (PASAT) to form a reliable version of the Multiple Sclerosis Functional Composite (MSFC). A study in a large group of patients with MS and controls demonstrated the validity of both the PASAT and SDMT version of the MSFC, with slightly better ability of the SDMT version in predicting diagnosis, disease course, and work disability.9 The SDMT is also among the more reliable and sensitive10 tests in the MS literature and is a robust correlate of several brain imaging findings.
Table.
Lists of neuropsychological tests administered in the Rao Brief Repeatable Neuropsychological Battery (BRNB)7 and the Minimal Assessment of Cognitive Function in Multiple Sclerosis (MACFIMS)4,8
MACROSCOPIC LESIONS AND ATROPHY ASSESSED WITH MRI
Seminal studies of patients with MS found only a modest association between T2 lesion burden of the whole brain or in specific sites of the cerebral white matter (WM) and neuropsychological test performance.11 This supported the early notion of a functional disconnection between cortical and deep gray matter (GM) structures secondary to damage to the WM. T2 hyperintensities reflect heterogeneous pathologic substrates, including edema, inflammation, demyelination, remyelination, gliosis, and axonal loss. The assessment of T1 hypointensities, which reflect changes in extent of demyelination and axonal density, has not substantially improved clinico-radiologic associations, whereas the quantification of volume decrease (atrophy) of the whole brain or of selected brain regions (i.e., third ventricle, corpus callosum, bicaudate ratio) provides robust correlates of MS-associated cognitive dysfunction.12 These measures of brain atrophy are thought to be markers of the most destructive aspects of MS pathology. In most studies, brain volume measures are correlated better with patients' cognitive performance than T2 and T1 lesion volumes, both in cross-sectional11 and longitudinal13 studies.
More recently, GM involvement in MS has received increased interest. In a comprehensive postmortem MS study, Kutzelnigg et al.14 showed that, with progression of disease, WM abnormalities change from predominantly focal and periventricular to more subtle and diffuse. They also showed that such WM changes are accompanied by a large increase in the extent of demyelination in the GM. These histopathologic findings were confirmed later by in vivo atrophy studies, which demonstrated that although GM atrophy can already be found in early disease, it only becomes prominent in the chronic phase.15 The assessment of GM atrophy16 and the topographic distribution of such damage17 can help to differentiate cognitively impaired from cognitively preserved patients. Interestingly, the rate of GM atrophy accelerates around the conversion point from relapsing-remitting (RR) to secondary progressive (SP) MS.18 What causes this disproportionate increase in GM damage when compared to WM damage18 is currently unknown. Whether cortical GM demyelination may be solely responsible for GM volume loss or whether other pathologic processes also play a role is an important research question that needs to be addressed by future studies. Although dendritic and synaptic pathology and changes of number and shape of neurons in the MS cortex have been described,19 they are generally relatively subtle.
The effect of focal GM lesions on the development of GM atrophy or of cognitive impairment has been difficult to study. With the introduction of new imaging techniques such as double inversion recovery (DIR) and phase-sensitive inversion recovery, an improved visualization of MS cortical lesions has been achieved. However, only a fraction of the GM lesions seen on histopathologic analysis is detected with DIR sequences.20 An increase of cortical lesions over time was shown to correlate with impaired neuropsychological test performance21 and cortical lesions and atrophy independently predicted cognitive impairment.22 On the contrary, T2 hyperintense WM lesion volume and contrast-enhancing WM lesion number did not differ between cognitively impaired and cognitively unimpaired patients.22 Several factors might contribute to explain between-study discrepancies of the relationships between T2 lesion burden and neuropsychological performance, including the numbers and clinical characteristics of the subjects enrolled and the methods used to measure T2 lesion burden (e.g., number vs volume of lesions, global vs regional T2 lesion load).
Similarly, GM atrophy, which starts early and in distinct cortical regions in MS, could be reliably related to cognitive impairment.23 Furthermore, assessment of lesions and atrophy of GM structures critical for specific cognitive domains could provide additional pieces of information. In line with this, hippocampal atrophy has been associated with deficits in memory encoding and retrieval,24 whereas the formation of new hippocampal lesions over a 3-year period was related to impairment of visuospatial memory.21
DAMAGE TO THE NORMAL-APPEARING WM AND “DIFFUSE” INJURY TO GM ASSESSED WITH QUANTITATIVE MRI TECHNIQUES
Abnormalities of the normal-appearing (NA) WM and GM injury are important in determining cognitive impairment in patients with MS, in addition to the role played by WM demyelinating lesions. The detection of NAWM abnormalities has been hitherto easier than that in the GM through the use of quantitative MRI techniques such as magnetization transfer (MT) MRI, diffusion tensor (DT) MRI, and proton MR spectroscopy (1H-MRS).3 More recently, using high-field scanners, our ability to detect and quantify such abnormalities has improved further.
Changes in MT ratio (MTR), a measure based on the exchange of magnetization between free and bound protons and principally dependent on myelin content, have been found to correlate with overall cognitive performance better than lesion metrics or atrophy.25 These correlations have been reported when whole brain MTR has been measured or when a region of interest–based approach to sample NAWM has been used. More recently, reductions of cortical MTR26 have also been found to correlate with measures of cognition in patients with benign MS (BMS). Using a voxel-based method, significant correlations were found between decrease of MTR value in specific cortical regions and PASAT performance in patients with primary progressive MS.27 In addition, global MTR changes of the NAWM at illness onset predicted impairment in executive function in patients with RRMS when cognitively examined several years later.28
Molecular diffusion in brain tissue can be measured in vivo using DT MRI. As WM tracts of the brain have an orientated microstructure, diffusion is easier along rather than across these tracts, a process known as anisotropy. Fractional anisotropy (FA), a measure derived from the diffusion tensor that reflects the degree of fiber alignment and integrity, and mean diffusivity (MD), which measures the average molecular motion independent of any tissue directionality, are the commonly used measures of diffusion. Diffusion-weighted MRI abnormalities in NAWM have been found to predict speed of information deficits. DT MRI quantities from the NAWM and GM were similar between patients with BMS and cognitive impairment and those with SPMS.29 Another DT MRI study30 showed that corpus callosum (CC) damage, in terms of both focal lesions and diffuse injury, was more pronounced in patients with BMS with cognitive impairment in comparison with those without. Using tract-based spatial statistics, 2 recent studies31,32 have found correlations between impaired attention, working memory, and speed of information processing and decreased FA in the CC and other tracts mainly connecting prefrontal cortical regions. Tract abnormalities overlapped only in part with lesion location highlighting the importance of lesion-independent NAWM abnormalities in cognition. The results of the previous studies support the notion of a relationship between damage to specific pathways and related cognitive domains.31,32 This is also strengthened by the demonstration of an association between the performance on the California Verbal Learning Test and damage to the uncinate fasciculus.33
1H-MRS adds chemical information to other quantitative MRI techniques. Decreases in N- acetylaspartate (NAA), a marker of neuronal and axonal viability, measured in frontal regions have been found to correlate with performance on tests of executive function,34 while decreased NAA in the pontine locus ceruleus correlates with attentional measures.35 It has also been recently reported that decreased NAA may be linked to carrier status for the HLA allele DRB1*1501 and this may, in turn, explain the greater illness severity and cognitive impairment in patients with this genotype.36 By contrast, the role of apolipoprotein genotype in MS remains to be determined and 2 different studies found no association between such a genotype and the presence of cognitive impairment or brain atrophy.37,38 Increases in myo-inositol (a glial and inflammatory marker) in the 3 years after a clinically isolated syndrome (CIS) has been found to predict poor executive function when patients were cognitively examined years later, suggesting that early and widespread inflammatory damage to the NAWM may play an important role in cognition.39
In summary, different aspects of MS-related pathology (inflammatory lesions and changes in normal-appearing brain tissue) involving the cortex and connecting WM tracts are relevant in determining cognitive impairment. Damage to WM tracts impairs cognitive functions that rely on rapid transfer of information (e.g., attention, information processing, and executive function), but the role of abnormalities in the cortex is increasingly recognized.
BRAIN CORTICAL REORGANIZATION
By measuring changes of concentration of what we can consider as a sort of physiologic contrast, that is deoxyhemoglobin, fMRI is contributing to defining the role of brain reorganization in limiting the cognitive impact of disease-related tissue damage in MS.40,41–42 Plasticity in MS occurs at a cellular level, by insertion of new sodium channels in demyelinated segment of axons,43 and at a system level, by an increased recruitment of parallel existing pathways or “latent” connections, as well as reorganization of local and distant sites, as supported by neurophysiologic evidence.44 Since fMRI does not measure neural activity directly, but relies on a cascade of physiologic events linking neural activity to the observed MRI signal changes, the interpretation of fMRI results obtained from patients should be done with caution, because several factors may have an impact on results. These include regional alterations of the BOLD response (e.g., increased perfusion due to the presence of inflammatory lesions) and differences in task performance between patients and controls.
Using different cognitive paradigms, fMRI changes have been disclosed in all the major disease clinical phenotypes of the disease,40–42 including patients at presentation with CIS suggestive of MS40 and those with BMS.42 Such abnormalities are mainly characterized by an increased recruitment of areas normally activated by healthy individuals when performing a given task and by the bilateral activation of these areas in cognitively preserved patients.45 On the contrary, cognitively impaired patients show significantly lower recruitment of cognitively related areas in comparison with healthy controls.45 Recently, reduced activity at rest within the anterior regions of the default-mode network has been also shown to occur in patients with progressive MS and cognitive impairment.46
Whether an increased activation of selected brain areas in patients at the early stages of the disease represents an adaptive mechanism to the underlying structural pathology or, conversely, reflects impending neural failure deserves further investigations. Indeed, evidence in patients with neurodegenerative conditions, such as Alzheimer disease, supports the notion that an increased hippocampal activation might characterize subjects at risk for the disease or who have already entered its initial stage. This over-recruitment is then followed by a pseudonormalization of activation when cognitive decline occurs.47
The correlation found by the majority of the studies between the extent of fMRI activations and structural MRI measures of disease burden, in terms of macroscopic lesions48 and damage to the NAWM and GM,40 suggests that these fMRI abnormalities might play, at least in same stages of the disease, an adaptive role in limiting the clinical consequences of widespread disease-related damage. This is also supported by the observation that, in patients with early MS, an increased activation of the dorsolateral prefrontal cortex over 1 year is associated with improved individual working memory and processing speed.49
More recently, by combining measures of abnormal structural and functional connectivity, a selectivity of the adaptive response toward damage of strategic WM fiber bundles has been demonstrated.42,50 Patients with RRMS with a low FA of the superior longitudinal fasciculus (SLF) experienced a more bilateral cortical activation during PASAT performance than healthy controls and patients with high FA values of the SLF.50 In patients with BMS, a correlation between diffusivity changes of the CC and an abnormal interhemispheric effective connectivity during the performance of attention-related tasks was shown,42 thus suggesting that a disconnection of brain areas may play a role in the pathophysiology of cognitive impairment in MS.
TREATMENT OPTIONS AND MRI
Given the significant effect that deficits in cognitive functioning have on quality of life in patients with MS, the alleviation of such deficits should be a major goal of MS research and practice. Potential treatment strategy approaches include disease-modifying treatments, symptomatic agents, and cognitive rehabilitation. Disease-modifying therapy studies are designed to alter the course of the disease process, where cognition tends to be a secondary outcome, if one at all. The results are mixed at best with some beneficial effects on cognition in studies with interferon (IFN)-β.51,52 MRI and cognitive outcomes have been combined to assess the efficacy of disease-modifying treatments in only a few studies. In a pivotal trial, patients treated with high-dose IFN-β-1b showed significant improvement in Wechsler Memory Scale Visual Reproduction–Delayed Recall scores between years 2 and 4 and stable MRI lesion load, whereas the low-dose and placebo groups showed an increase of T2 lesion burden and no changes in their cognitive performance.53 An open-label study in patients with RRMS treated with IFN-β-1b found that, after 2 years of treatment, the cognitive status remained stable or improved in the majority of the patients. However, no relation was found between cognitive outcome and MRI data.54 More recently, the 5-year follow-up of the Betaferon/Betaseron in newly emerging MS for initial treatment (BENEFIT) trial showed that early treatment with IFN-β-1b was associated with a reduction in the number of new brain MRI active lesions and had a favorable effect on the performance of PASAT.55
Symptomatic agents for improving cognition in MS include acetylcholinesterase inhibitors (AChEI), such as donepezil, rivastigmine, and galantamine, which have been extensively tested in Alzheimer disease. While rivastigmine proved to be ineffective in treating memory and cognitive dysfunction in 30 patients with MS,56 donepezil improved learning and memory in patients with MS with initial cognitive difficulties in a single-center clinical trial.57 A large multicenter trial in the United States on donepezil in MS was recently completed and the results are anticipated in the near future. A few studies applied fMRI to assess AChEI efficacy. Parry et al.58 showed a normalization of the Stroop-related pattern of activation in patients with RRMS a few hours after the administration of rivastigmine. More recently, by studying a larger sample of patients with a higher chronic administration of rivastigmine, Cader et al.59 showed that rivastigmine enhances the prefrontal function and alters the functional connectivity associated with cognition.
Despite the need for cognitive rehabilitation services as a standard of care, there is a paucity of research studies designed to investigate treatment approaches or their effectiveness in patients with MS.1 Most of the existing studies suffer from significant methodologic flaws including small sample size, short follow-up periods, and lack of specific outcome criteria to determine “improvement.” None of these studies included MRI as measure of outcome. A comprehensive evidence-based review of the existing cognitive rehabilitation intervention literature found only 16 reviewable articles.60 From these, there was not enough evidence to support practice recommendations for the treatment of attention, executive functions, and nonspecific cognitive rehabilitation approaches. There was enough evidence to recommend a “practice guideline” for specific cognitive intervention (i.e., modified memory story technique) for impairments in learning and memory, based on one Class I and supporting Class II studies. Targeted memory-enhancing techniques (including self-generated learning, spaced learning, and spaced retrieval) have been shown to significantly improve learning and memory in MS, while the simple repetition of information has not.1
At least part of the aforementioned limitations of studies assessing the efficacy of cognitive rehabilitation in MS might be overcome by the use of MRI. Structural MRI techniques, including T2, T1, DIR, and DT MRI, might be useful to provide a baseline assessment of the extent of tissue damage before treatment, thus allowing us to stratify patients and to identify those who might benefit most from treatment (most likely those with a milder tissue injury). Once “beneficial” cortical reorganization changes will be defined, fMRI might provide outcome measures, which could result in a reduction of the size of the sample and the length of the follow-up needed to perform explorative studies with enough power. Clearly, ad hoc studies are required to prove that fMRI can provide useful outcome measures for therapeutic interventions in MS. Preliminary to this, the assessment of the reproducibility of fMRI measures over multiple time points for individual subjects as well as the setup of fMRI experiments in the context of a multicenter study are needed, but they are likely to represent a challenging task.
DISCUSSION
The application of conventional and quantitative MRI techniques has contributed to improve the understanding of the mechanisms responsible for the development of cognitive deficits in patients with MS. Available data suggest that focal WM lesions do play a role, but the overall effect of T2-visible lesions on MS-related cognitive impairment is limited. The location of lesions in critical brain areas appears to be important and, in this context, the improved capability to detect cortical lesions is likely to provide additional pieces of information central to this issue. Irreversible tissue loss, measured in terms of global and regional atrophy, is robustly associated with cognitive deficits. The impact of WM damage on cognition may be mediated by a disruption of crucial tracts or interference with specific functional nodes. In addition to all these aspects, other components of MS pathology, such as diffuse damage to the NAWM and GM and the strength of functional connections between distant functional areas, are likely to play a role in determining patients' cognitive profile. Indeed, the application of fMRI demonstrated different patterns of cortical activations according to the disease phenotype, which are likely to have an adaptive role, at least in some stages of the disease.
ACKNOWLEDGMENT
This article reports the conclusions of the Thirteenth Advanced Course on Magnetic Resonance Techniques in Multiple Sclerosis, Milan, Italy, September 24–25, 2009.
Footnotes
- AChEI
- acetylcholinesterase inhibitor
- BMS
- benign multiple sclerosis
- BRNB
- Brief Repeatable Neuropsychological Battery
- CC
- corpus callosum
- CIS
- clinically isolated syndrome
- DIR
- double inversion recovery
- DT
- diffusion tensor
- FA
- fractional anisotropy
- GM
- gray matter
- 1H-MRS
- proton MR spectroscopy
- IFN
- interferon
- MACFIMS
- Minimal Assessment of Cognitive Function in Multiple Sclerosis
- MD
- mean diffusivity
- MR
- magnetic resonance
- MS
- multiple sclerosis
- MSFC
- Multiple Sclerosis Functional Composite
- MT
- magnetization transfer
- MTR
- magnetization transfer ratio
- NA
- normal-appearing
- NAA
- N-acetylaspartate
- PASAT
- Paced Auditory Serial Addition Task
- RR
- relapsing-remitting
- SDMT
- Symbol Digit Modalities Test
- SLF
- superior longitudinal fasciculus
- SP
- secondary progressive
- WM
- white matter.
DISCLOSURE
Dr. Filippi serves on scientific advisory boards for Teva Pharmaceutical Industries Ltd. and Genmab A/S; has received funding for travel from Bayer Schering Pharma, Biogen-Dompé, Genmab A/S, Merck Serono, and Teva Pharmaceutical Industries Ltd.; serves on editorial boards of the American Journal of Neuroradiology, BMC Musculoskeletal Disorders, Clinical Neurology and Neurosurgery, Erciyes Medical Journal, Journal of Neuroimaging, Journal of Neurovirology, Lancet Neurology, Magnetic Resonance Imaging, Multiple Sclerosis, and Neurological Sciences; serves as a consultant to Bayer Schering Pharma, Biogen-Dompé, Genmab A/S, Merck Serono, Pepgen Corporation, and Teva Pharmaceutical Industries Ltd.; serves on speakers' bureaus for Bayer Schering Pharma, Biogen-Dompé, Genmab A/S, Merck Serono, and Teva Pharmaceutical Industries Ltd.; and receives research support from Bayer Schering Pharma, Biogen-Dompé, Genmab A/S, Merck Serono, Teva Pharmaceutical Industries Ltd., Fondazione Italiana Sclerosi Multipla, and Fondazione Mariani. Dr. Rocca serves as consultant to Bayer Schering Pharma and served on the speakers' bureau for Biogen-Dompé. Dr. Benedict serves on scientific advisory boards for Merck Serono, Biogen Idec, Bayer Schering Pharma, Novartis, and Pfizer Inc.; serves on the editorial boards of Multiple Sclerosis, Neuropsychology, and the International Journal of MS Care; receives royalties from Psychological Assessment Resources; has received speaker honoraria from Biogen Idec, Pfizer Inc., Abbott, Bayer Schering Pharma, Merck Serono, and Teva Pharmaceutical Industries Ltd.; and receives research support from Shire plc, Biogen Idec, the NIH (3R01DC004689-08S1 [coinvestigator]), and the National Multiple Sclerosis Society; and has given expert testimony in several personal injury trials. Dr. DeLuca serves on a scientific advisory board for Biogen Idec; serves as an Associate Editor for the Archives of Physical Medicine and Rehabilitation and on the editorial boards of Multiple Sclerosis, Rehabilitation Psychology, the Journal of Clinical and Experimental Neuropsychology, Neuro-psychoanalysis, and Neuropsychology Review; and receives research support from the NIH (NCMRR HD045798 [coinvestigator]) and the National Multiple Sclerosis Society. Dr. Geurts serves on scientific advisory boards for the Dutch MS Research Foundation and Merck Serono; serves on the editorial board of MS International; and has received research support from the Dutch MS Research Foundation and Biogen Idec. Dr. Rombouts reports no disclosures. Dr. Ron is a Guarantor of Brain; serves on scientific advisory boards for the Jules Thorn Charitable Trust and the Psychiatry Research Trust; and receives research support from the Multiple Sclerosis Society (UK) and the Henry Smith Charitable Trust. Dr. Comi serves on speakers' bureaus for Teva Pharmaceutical Industries Ltd., Sanofi-Aventis, Merck Serono, Bayer Schering Pharma, Biogen-Dompé, Boehringer Ingelheim, and Novartis; and has received speaker honoraria from Teva Pharmaceutical Industries Ltd., Sanofi-Aventis, Merck Serono, Serono Symposia International Foundation, Bayer Schering Pharma, Novartis, Biogen-Dompé, and Merz Pharmaceuticals GmbH.
REFERENCES
- 1. Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol 2008; 7: 1139– 1151 [DOI] [PubMed] [Google Scholar]
- 2. Rao SM, Leo GJ, Bernardin L, Unverzagt F. Cognitive dysfunction in multiple sclerosis: I: frequency, patterns, and prediction. Neurology 1991; 41: 685– 691 [DOI] [PubMed] [Google Scholar]
- 3. Bakshi R, Thompson AJ, Rocca MA, et al. MRI in multiple sclerosis: current status and future prospects. Lancet Neurol 2008; 7: 615– 625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Benedict RHB, Cookfair D, Gavett R, et al. Validity of the Minimal Assessment of Cognitive Function in Multiple Sclerosis (MACFIMS). J Int Neuropsychol Soc 2006; 12: 549– 558 [DOI] [PubMed] [Google Scholar]
- 5. DeLuca J, Johnson SK, Natelson BH. Information processing efficiency in chronic fatigue syndrome and multiple sclerosis. Arch Neurol 1993; 50: 301– 304 [DOI] [PubMed] [Google Scholar]
- 6. DeLuca J, Gaudino EA, Diamond BJ, Christodoulou C, Engel RA. Acquisition and storage deficits in multiple sclerosis. J Clin Exp Neuropsychol 1998; 20: 376– 390 [DOI] [PubMed] [Google Scholar]
- 7. Rao SM. A Manual for the Brief, Repeatable Battery of Neuropsychological Tests in Multiple Sclerosis. National Multiple Sclerosis Society; 1991. [Google Scholar]
- 8. Benedict RHB, Fischer JS, Archibald CJ, et al. Minimal neuropsychological assessment of MS patients: a consensus approach. Clin Neuropsychol 2002; 16: 381– 397 [DOI] [PubMed] [Google Scholar]
- 9. Drake A, Weinstock-Guttman B, Morrow S, Hojnacki D, Munschauer F, Benedict RHB. Psychometrics and normative data for the Multiple Sclerosis Functional Composite: replacing the PASAT with the Symbol Digit Modalities Test. Mult Scler 2010; 16: 228– 237 [DOI] [PubMed] [Google Scholar]
- 10. Parmenter BA, Weinstock-Guttman B, Garg N, Munschauer F, Benedict RHB. Screening for cognitive impairment in MS using the Symbol Digit Modalities Test. Mult Scler 2007; 13: 52– 57 [DOI] [PubMed] [Google Scholar]
- 11. Rovaris M, Comi G, Filippi M. MRI markers of destructive pathology in multiple sclerosis-related cognitive dysfunction. J Neurol Sci 2006; 245: 111– 116 [DOI] [PubMed] [Google Scholar]
- 12. Houtchens MK, Benedict RHB, Killiany R, et al. Thalamic atrophy and cognition in multiple sclerosis. Neurology 2007; 69: 113– 123 [DOI] [PubMed] [Google Scholar]
- 13. Zivadinov R, Sepcic J, Nasuelli D, et al. A longitudinal study of brain atrophy and cognitive disturbances in the early phase of relapsing-remitting multiple sclerosis. J Neurol Neurosurg Psychiatry 2001; 70: 773– 780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kutzelnigg A, Lucchinetti CF, Stadelmann C, et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain 2005; 128: 2705– 2712 [DOI] [PubMed] [Google Scholar]
- 15. Fisniku LK, Chard DT, Jackson JS, et al. Gray matter atrophy is related to long-term disability in multiple sclerosis. Ann Neurol 2008; 64: 247– 254 [DOI] [PubMed] [Google Scholar]
- 16. Amato MP, Portaccio E, Goretti B, et al. Association of neocortical volume changes with cognitive deterioration in relapsing-remitting multiple sclerosis. Arch Neurol 2007; 64: 1157– 1161 [DOI] [PubMed] [Google Scholar]
- 17. Morgen K, Sammer G, Courtney SM, et al. Evidence for a direct association between cortical atrophy and cognitive impairment in relapsing-remitting MS. Neuroimage 2006; 30: 891– 898 [DOI] [PubMed] [Google Scholar]
- 18. Fisher E, Lee JC, Nakamura K, Rudick RA. Gray matter atrophy in multiple sclerosis: a longitudinal study. Ann Neurol 2008; 64: 255– 265 [DOI] [PubMed] [Google Scholar]
- 19. Peterson JW, Bo L, Mork S, Chang A, Trapp BD. Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann Neurol 2001; 50: 389– 400 [DOI] [PubMed] [Google Scholar]
- 20. Geurts JJ, Pouwels PJ, Uitdehaag BM, Polman CH, Barkhof F, Castelijns JA. Intracortical lesions in multiple sclerosis: improved detection with 3D double inversion-recovery MR imaging. Radiology 2005; 236: 254– 260 [DOI] [PubMed] [Google Scholar]
- 21. Roosendaal SD, Moraal B, Pouwels PJ, et al. Accumulation of cortical lesions in MS: relation with cognitive impairment. Mult Scler 2009; 15: 708– 714 [DOI] [PubMed] [Google Scholar]
- 22. Calabrese M, Agosta F, Rinaldi F, et al. Cortical lesions and atrophy associated with cognitive impairment in relapsing-remitting multiple sclerosis. Arch Neurol 2009; 66: 1144– 1150 [DOI] [PubMed] [Google Scholar]
- 23. Calabrese M, Rinaldi F, Mattisi I, et al. Widespread cortical thinning characterizes patients with MS with mild cognitive impairment. Neurology 2010; 74: 321– 328 [DOI] [PubMed] [Google Scholar]
- 24. Sicotte NL, Kern KC, Giesser BS, et al. Regional hippocampal atrophy in multiple sclerosis. Brain 2008; 131: 1134– 1141 [DOI] [PubMed] [Google Scholar]
- 25. Filippi M, Tortorella C, Rovaris M, et al. Changes in the normal appearing brain tissue and cognitive impairment in multiple sclerosis. J Neurol Neurosurg Psychiatry 2000; 68: 157– 161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Amato MP, Portaccio E, Stromillo ML, et al. Cognitive assessment and quantitative magnetic resonance metrics can help to identify benign multiple sclerosis. Neurology 2008; 71: 632– 638 [DOI] [PubMed] [Google Scholar]
- 27. Khaleeli Z, Cercignani M, Audoin B, Ciccarelli O, Miller DH, Thompson AJ. Localized grey matter damage in early primary progressive multiple sclerosis contributes to disability. Neuroimage 2007; 37: 253– 261 [DOI] [PubMed] [Google Scholar]
- 28. Summers M, Fisniku L, Anderson V, Miller D, Cipolotti L, Ron M. Cognitive impairment in relapsing-remitting multiple sclerosis can be predicted by imaging performed several years earlier. Mult Scler 2008; 14: 197– 204 [DOI] [PubMed] [Google Scholar]
- 29. Rovaris M, Riccitelli G, Judica E, et al. Cognitive impairment and structural brain damage in benign multiple sclerosis. Neurology 2008; 71: 1521– 1526 [DOI] [PubMed] [Google Scholar]
- 30. Mesaros S, Rocca MA, Riccitelli G, et al. Corpus callosum damage and cognitive dysfunction in benign MS. Hum Brain Mapp 2009; 30: 2656– 2666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Roosendaal SD, Geurts JJ, Vrenken H, et al. Regional DTI differences in multiple sclerosis patients. Neuroimage 2009; 44: 1397– 1403 [DOI] [PubMed] [Google Scholar]
- 32. Dineen RA, Vilisaar J, Hlinka J, et al. Disconnection as a mechanism for cognitive dysfunction in multiple sclerosis. Brain 2009; 132: 239– 249 [DOI] [PubMed] [Google Scholar]
- 33. Fink F, Eling P, Rischkau E, et al. The association between California Verbal Learning Test performance and fibre impairment in multiple sclerosis: evidence from diffusion tensor imaging. Mult Scler 2010; 16: 332– 341 [DOI] [PubMed] [Google Scholar]
- 34. Staffen W, Zauner H, Mair A, et al. Magnetic resonance spectroscopy of memory and frontal brain region in early multiple sclerosis. J Neuropsychiatry Clin Neurosci 2005; 17: 357– 363 [DOI] [PubMed] [Google Scholar]
- 35. Gadea M, Martinez-Bisbal MC, Marti-Bonmati L, et al. Spectroscopic axonal damage of the right locus coeruleus relates to selective attention impairment in early stage relapsing-remitting multiple sclerosis. Brain 2004; 127: 89– 98 [DOI] [PubMed] [Google Scholar]
- 36. Okuda DT, Srinivasan R, Oksenberg JR, et al. Genotype-Phenotype correlations in multiple sclerosis: HLA genes influence disease severity inferred by 1H MR spectroscopy and MRI measures. Brain 2009; 132: 250– 259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Portaccio E, Goretti B, Zipoli V, et al. APOE-epsilon4 is not associated with cognitive impairment in relapsing-remitting multiple sclerosis. Mult Scler 2009; 15: 1489– 1494 [DOI] [PubMed] [Google Scholar]
- 38. van der Walt A, Stankovich J, Bahlo M, et al. Apolipoprotein genotype does not influence MS severity, cognition, or brain atrophy. Neurology 2009; 73: 1018– 1025 [DOI] [PubMed] [Google Scholar]
- 39. Summers M, Swanton J, Fernando K, et al. Cognitive impairment in multiple sclerosis can be predicted by imaging early in the disease. J Neurol Neurosurg Psychiatry 2008; 79: 955– 958 [DOI] [PubMed] [Google Scholar]
- 40. Audoin B, Au Duong MV, Ranjeva JP, et al. Magnetic resonance study of the influence of tissue damage and cortical reorganization on PASAT performance at the earliest stage of multiple sclerosis. Hum Brain Mapp 2005; 24: 216– 228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mainero C, Caramia F, Pozzilli C, et al. fMRI evidence of brain reorganization during attention and memory tasks in multiple sclerosis. Neuroimage 2004; 21: 858– 867 [DOI] [PubMed] [Google Scholar]
- 42. Rocca MA, Valsasina P, Ceccarelli A, et al. Structural and functional MRI correlates of Stroop control in benign MS. Hum Brain Mapp 2009; 30: 276– 290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Waxman SG, Craner MJ, Black JA. Na+ channel expression along axons in multiple sclerosis and its models. Trends Pharmacol Sci 2004; 25: 584– 591 [DOI] [PubMed] [Google Scholar]
- 44. Tecchio F, Zito G, Zappasodi F, et al. Intra-cortical connectivity in multiple sclerosis: a neurophysiological approach. Brain 2008; 131: 1783– 1792 [DOI] [PubMed] [Google Scholar]
- 45. Penner IK, Rausch M, Kappos L, Opwis K, Radu EW. Analysis of impairment related functional architecture in MS patients during performance of different attention tasks. J Neurol 2003; 250: 461– 472 [DOI] [PubMed] [Google Scholar]
- 46. Rocca MA, Valsasina P, Absinta M, et al. Default-mode network dysfunction and cognitive impairment in progressive MS. Neurology 2010; 74: 1252– 1259 [DOI] [PubMed] [Google Scholar]
- 47. Sperling RA, Dickerson BC, Pihlajamaki M, et al. Functional alterations in memory networks in early Alzheimer's disease. Neuromolecular Med 2010; 12: 27– 43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bobholz JA, Rao SM, Lobeck L, et al. fMRI study of episodic memory in relapsing-remitting MS: correlation with T2 lesion volume. Neurology 2006; 67: 1640– 1645 [DOI] [PubMed] [Google Scholar]
- 49. Audoin B, Reuter F, Duong MV, et al. Efficiency of cognitive control recruitment in the very early stage of multiple sclerosis: a one-year fMRI follow-up study. Mult Scler 2008; 14: 786– 792 [DOI] [PubMed] [Google Scholar]
- 50. Bonzano L, Pardini M, Mancardi GL, Pizzorno M, Roccatagliata L. Structural connectivity influences brain activation during PVSAT in Multiple Sclerosis. Neuroimage 2009; 44: 9– 15 [DOI] [PubMed] [Google Scholar]
- 51. Galetta SL, Markowitz C, Lee AG. Immunomodulatory agents for the treatment of relapsing multiple sclerosis: a systematic review. Arch Intern Med 2002; 162: 2161– 2169 [DOI] [PubMed] [Google Scholar]
- 52. Patti F. Cognitive impairment in multiple sclerosis. Mult Scler 2009; 15: 2– 8 [DOI] [PubMed] [Google Scholar]
- 53. Pliskin NH, Hamer DP, Goldstein DS, et al. Improved delayed visual reproduction test performance in multiple sclerosis patients receiving interferon beta-1b. Neurology 1996; 47: 1463– 1468 [DOI] [PubMed] [Google Scholar]
- 54. Lanzillo R, Prinster A, Scarano V, et al. Neuropsychological assessment, quantitative MRI and ApoE gene polymorphisms in a series of MS patients treated with IFN beta-1b. J Neurol Sci 2006; 245: 141– 145 [DOI] [PubMed] [Google Scholar]
- 55. Kappos L, Freedman MS, Polman CH, et al. Long-term effect of early treatment with interferon beta-1b after a first clinical event suggestive of multiple sclerosis: 5-year active treatment extension of the phase 3 BENEFIT trial. Lancet Neurol 2009; 8: 987– 997 [DOI] [PubMed] [Google Scholar]
- 56. Shaygannejad V, Janghorbani M, Ashtari F, Zanjani HA, Zakizade N. Effects of rivastigmine on memory and cognition in multiple sclerosis. Can J Neurol Sci 2008; 35: 476– 481 [DOI] [PubMed] [Google Scholar]
- 57. Krupp LB, Christodoulou C, Melville P, Scherl WF, MacAllister WS, Elkins LE. Donepezil improved memory in multiple sclerosis in a randomized clinical trial. Neurology 2004; 63: 1579– 1585 [DOI] [PubMed] [Google Scholar]
- 58. Parry AM, Scott RB, Palace J, Smith S, Matthews PM. Potentially adaptive functional changes in cognitive processing for patients with multiple sclerosis and their acute modulation by rivastigmine. Brain 2003; 126: 2750– 2760 [DOI] [PubMed] [Google Scholar]
- 59. Cader S, Palace J, Matthews PM. Cholinergic agonism alters cognitive processing and enhances brain functional connectivity in patients with multiple sclerosis. J Psychopharmacol 2009; 23: 686– 696 [DOI] [PubMed] [Google Scholar]
- 60. O'Brien AR, Chiaravalloti N, Goverover Y, Deluca J. Evidenced-based cognitive rehabilitation for persons with multiple sclerosis: a review of the literature. Arch Phys Med Rehabil 2008; 89: 761– 769 [DOI] [PubMed] [Google Scholar]


