Abstract
Manipulative studies have demonstrated that ocean acidification (OA) is a threat to coral reefs, yet no experiments have employed diurnal variations in pCO2 that are ecologically relevant to many shallow reefs. Two experiments were conducted to test the response of coral recruits (less than 6 days old) to diurnally oscillating pCO2; one exposing recruits for 3 days to ambient (440 µatm), high (663 µatm) and diurnally oscillating pCO2 on a natural phase (420–596 µatm), and another exposing recruits for 6 days to ambient (456 µatm), high (837 µatm) and diurnally oscillating pCO2 on either a natural or a reverse phase (448–845 µatm). In experiment I, recruits exposed to natural-phased diurnally oscillating pCO2 grew 6–19% larger than those in ambient or high pCO2. In experiment II, recruits in both high and natural-phased diurnally oscillating pCO2 grew 16 per cent larger than those at ambient pCO2, and this was accompanied by 13–18% higher survivorship; the stimulatory effect on growth of oscillatory pCO2 was diminished by administering high pCO2 during the day (i.e. reverse-phased). These results demonstrate that coral recruits can benefit from ecologically relevant fluctuations in pCO2 and we hypothesize that the mechanism underlying this response is highly pCO2-mediated, night-time storage of dissolved inorganic carbon that fuels daytime calcification.
Keywords: ocean acidification, calcification, diurnal, survivorship
1. Introduction
Ocean acidification (OA) arising from the dissolution of atmospheric CO2 in seawater is thought to be one of the most serious threats facing marine ecosystems [1–3], because most calcifying organisms deposit less CaCO3 at high pCO2 [4–6]. Some of the most striking examples of these effects are exhibited by scleractinian corals [7], yet despite the negative implications of these trends for coral reef ecosystems [8], progress has been slow in elucidating the mechanisms underlying the response of corals to high pCO2 [9–11].
Most evidence describing the effect of OA on corals has come from the exposure of adult colonies to seawater manipulated to create dissolved inorganic carbon (DIC) chemistries simulating the consequences of high pCO2 [8]. Most recently, attention has turned to early life stages of corals [12], because these may be more susceptible than adults to environmental challenges [13], and they play important roles in recruitment and population growth [14]. Early results from this effort show that larvae and newly recruiting corals can be affected negatively by OA [15–19]. An important advantage of studying early life stages is that they support an evaluation of the responses of environmentally naive organisms to novel conditions, thereby allowing the impacts of immediate conditions to be tested without the complexity of time-integrated effects that apply to studies of adults. The experimental benefits of studying early life stages of corals are strong for calcification, for which a contrast of pelagic larvae and benthic recruits supports a contrast of uncalcified and calcified tissue.
To date, all experimental studies of the response of corals to OA have been accomplished through manipulations of pH or pCO2 in seawater, thereby creating steady levels of perturbed conditions [4]. While such studies have advanced understanding of the responses of corals to OA, they have overlooked natural variation in seawater pCO2. The best-known example of this effect involves diurnal oscillations in pCO2 and pH in seawater flowing over shallow reefs [20–23], which can create changes between day and night of greater than 600 µatm pCO2 and pH 0.5 [22]. The magnitude of these oscillations is influenced first, by the benthic community structure, which drives DIC chemistry through metabolism and, second, by reef bathymetry, which determines the spatial extent of shallow habitats and the flux of seawater across them [22].
Oscillatory levels of physical and chemical conditions result in biological outcomes that can differ from those arising under stable conditions [24,25] and are more than the sum of exposure times to each extreme. For example, exposure of the corals Pocillopora damicornis and Porites rus to diurnally varying temperatures reduces their Symbiodinium content and dark-adapted quantum yield of PSII more than exposure to steady temperature perturbations similar in magnitude to those at the extremes of the diurnal treatment [26]. Similarly, exposure in tidal pools of Acropora hyacinthus to diurnally varying temperature increases their resistance to elevated thermal stress compared with corals in more homogeneous conditions [25]. Together, these results suggest that corals might respond to ecologically relevant natural oscillations in pCO2 in ways differing from those recorded under steady-state conditions.
The objective of this study was to test the response of newly settled coral recruits to diurnal oscillations in pCO2. To achieve this objective, we settled brooded larvae of Seriatopora caliendrum onto artificial substrata and exposed the recruits to factorial combinations of high and ambient pCO2 in two series of experiments. Our treatments included diurnal oscillations of pCO2, in which oscillatory exposure to high pCO2 was administered both at night (the natural phase) and day (a reverse phase) to gain insights into the interactive consequences of elevated pCO2 and daylight. The reversed-phase experiment was designed as the study progressed in order to explore processes potentially underlying the patterns that were emerging from the initial experiment.
2. Methods
Larvae were obtained from the brooding coral S. caliendrum, which is a common branching coral on reefs along the southern coast of Taiwan and throughout the Indo-Pacific [27,28]. Colonies of S. caliendrum (approx. 20 cm in diameter) were collected from 5 to 7 m deep on Hobihu Reef, Nanwan Bay, in March and June of 2010, and transported to the National Museum of Marine Biology and Aquarium, where they were placed into individual flow-through seawater tanks and exposed to partially shaded sunlight at an intensity of 131 ± 13 µmol quanta m−2 s−1 (experiment I) or 78 ± 3 µmol quanta m−2 s−1 (experiment II; mean ± s.e.m.). Overflow water from each tank passed through mesh-lined (110 µm) cups that captured larvae, which were released during the night [29]. Larvae were collected at 08.00, pooled across colonies and retained in a 1 l beaker until processed for the experiment.
Following collection, approximately 500 larvae were placed into plastic containers (12 × 24 × 12 cm) at approximately 11.00, each of which was fitted with mesh windows (110 µm) to allow the passage of seawater, and floated in the same tanks containing the adult colonies. Seriatopora caliendrum larvae were settled onto clean pre-weighed glass microscope coverslips (22 × 22 mm2, approximately 150 µm thick). The coverslips allowed the size of the settled coral recruits to be assessed gravimetrically (±0.0001 g, Mettler-Toledo AX205). Six coverslips were placed in each container and the larvae left to settle for 24 h under a natural light–dark cycle. The following day greater than 90 per cent of larvae had settled on the coverslips, with up to 40 on each coverslip. The swimming larvae remaining were discarded, and coverslips with coral recruits (n = 18: experiment I; n = 36: experiment II) were assigned randomly to the pCO2 treatments.
In experiment I, treatments consisted of steady ambient pCO2, steady high pCO2 and diurnally oscillating pCO2 on a natural phase (table 1); this design was augmented in experiment II by including a diurnally oscillating pCO2 on a reverse phase. The oscillatory treatments were created by moving the plastic container containing the coverslips (and attached recruits) between high and ambient pCO2 with the transfers accomplished at dawn and dusk (07.00 and 19.00 under the laboratory conditions) without exposing the recruits to air. Containers in ambient and high pCO2 were also removed from treatment tanks in a similar fashion to control for movement in and out of tanks. In the diurnally oscillating pCO2 on a natural phase, high pCO2 was administered at night, and ambient pCO2 during the day, but in the diurnally oscillating pCO2 on a reverse phase, this sequence was reversed. The diurnally oscillating pCO2 on a natural phase mimicked the cycle of pCO2 in seawater passing over shallow reefs [23], and the diurnally oscillating pCO2 on a reverse phase was used to test coral calcification under novel conditions.
Table 1.
Seawater DIC chemistry in experiment I (March, 3 days duration) and experiment II (June, 6 days duration) based on samples collected daily in experiment 1, and every other day in experiment II. Reverse-phase diurnally oscillating pCO2 in experiment II was identical to the natural phase. Values are means ± s.e.m., n = 3 in experiment I and n = 4 in experiment II.
| experiment | pCO2 treatment | TA (µmol kgSW−1) | pHT | 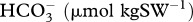 |
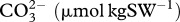 |
ΩA | pCO2 (µatm) |
|---|---|---|---|---|---|---|---|
| experiment I | ambient | 2160 ± 16 | 8.00 ± 0.05 | 1712.7 ± 55 | 180.4 ± 17.4 | 2.9 ± 0.3 | 456.0 ± 71.3 |
| natural-phase ambient | 2193 ± 64 | 8.02 ± 0.03 | 1710.7 ± 35 | 195.1 ± 16.4 | 3.1 ± 0.3 | 420.4 ± 38.0 | |
| high | 2178 ± 26 | 7.88 ± 0.02 | 1813.8 ± 31 | 147.5 ± 7.8 | 2.4 ± 0.1 | 623.6 ± 47.2 | |
| natural-phase high | 2234 ± 15 | 7.90 ± 0.04 | 1843.5 ± 33 | 158.8 ± 11.5 | 2.5 ± 0.2 | 596.8 ± 57.3 | |
| experiment II | ambient | 2187 ± 17 | 7.98 ± 0.01 | 1771 ± 17 | 169.8 ± 3.4 | 2.7 ± 0.1 | 464.0 ± 14.1 |
| natural-phase ambient | 2211 ± 16 | 8.00 ± 0.01 | 1774 ± 11 | 178.6 ± 3.9 | 2.9 ± 0.1 | 448.2 ± 10.6 | |
| high | 2213 ± 19 | 7.77 ± 0.00 | 1932 ± 16 | 115.0 ± 1.6 | 1.9 ± 0.0 | 830.3 ± 11.2 | |
| natural-phase high | 2187 ± 15 | 7.76 ± 0.00 | 1915 ± 13 | 111.4 ± 1.4 | 1.8 ± 0.0 | 845.0 ± 8.0 |
The pCO2 treatments were created in eight 30 l aquaria, with two maintained at ambient pCO2, two at high pCO2 and two pairs of tanks retained at ambient and high pCO2 and used to create the oscillatory pCO2 treatment. All tanks were independently heated (Taikong Corporation), chilled (Aquatech Ac11) and mixed (Rio 1110 pumps) to generate temperatures of 25.4 ± 0.4°C in March (experiment I, n = 128) and 25.1 ± 0.2°C in June (mean ± s.e.m., experiment II n = 136). Ambient seawater temperature was 25.5 ± 0.2°C in March, and although it was warmer in June (27.8 ± 0.2°C), the same incubation temperature was selected to strengthen the direct contrast between the two experiments. The tanks were filled with filtered seawater (1 µm), and the seawater was changed partially (10–15% volume) every night. All tanks were illuminated from 07.00 to 19.00 with lamps fitted with a metal halide bulb (Philllips 150 W 10 000 k) and two 39 W fluorescent bulbs (Phillips T5 460 nm) that provided light at an intensity of 179 ± 2 µmol quanta m−2 s−1 (mean ± s.e.m., n = 64) in experiment I, and 305 ± 20 µmol quanta in experiment II (n = 120; measured with an LI-192 sensor, LI-COR Biosciences, Lincoln, Nebraska).
(a). Experimental pCO2 manipulation
The DIC content of seawater was manipulated by bubbling premixed gas of a known pCO2 or by bubbling unmodified air for the ambient treatment. To mix the gas for the high pCO2 treatments, a system employing a variable-timed solenoid valve was used, which controlled the flow of air and CO2 into a mixing chamber to reach the target pCO2 of 650 µatm in experiment I. This target value was selected to provide a conservative estimate for the atmospheric pCO2 by 2100 following the business as usual emission scenario A1 [30]. In experiment II, pCO2 levels were increased to 800 µatm to test the effects of diurnally oscillating pCO2 levels that exceeded what is found at Hobihu reef. The solenoid valve was connected to an infrared gas analyser (S151, Qubit Systems, Ontario Canada), which monitored the output gas and provided dynamic control of the duty cycle of the solenoid, thereby providing a consistent concentration of mixed gas to the treatment tanks. Refer to the electronic supplementary material for seawater chemistry and field sampling methods.
(b). Growth
Upon completion of the experiments, coverslips with coral recruits were placed in bleach (6% NaOCl) for 8 h to dissolve the tissue on the small corals and leave behind the CaCO3 skeleton. Coverslips were then rinsed with deionized water to remove the bleach and air-dried for 24 h at approximately 27°C. Calcification was measured using the summed weight of the CaCO3 deposited by recruits on each coverslip and also as the planar area of the basal plate of each recruit. Coverslips without recruits but subjected to identical treatments served as procedural controls, and these did not change in weight in either experiment (t-test, experiment I, t = 2.80, d.f. = 11, p = 0.99; experiment II, t = 0.48, d.f. = 3, p = 0.64). In experiment I, the change in weight of each coverslip was divided by the number of corallites to provide a mean weight that was used as a statistical replicate. As some (approx. 5%) recruits died during the experiment, this technique slightly underestimated calcification. To remove this bias in experiment II, only recruits alive at the end of the experiment were used for growth measurements.
To measure the area of the recruits, bleached and dried corallites were photographed (Nikon Coolpix 4500, 4.0 megapixel resolution) through a compound microscope (Zeiss Axiolab E, 40× magnification). Images were analysed using the Image J software (Wayne Rasband, v. 1.42q), with approximately 65 corallites in each treatment measured for experiment I, and approximately 33 in each treatment for experiment II. To obtain an unambiguous indication of area, only corallites that were not touching neighbouring corallites were selected for analysis, and each corallite was treated as a statistical replicate.
(c). Survivorship
To evaluate survivorship during experiment II, recruits were photographed (Canon 40D, 10 megapixel resolution) every 2 days throughout the 6-day experiment. Images were used to score the recruits as alive or dead based on the presence of tissue, which is easily discernable from photographs. Survivorship was not measured in experiment I owing to logistical constraints.
(d). Statistical analysis
The weight and area of corallites were analysed with a nested ANOVA in which pCO2 treatment was the independent variable and replicate tanks were nested within treatments. To satisfy the normality assumptions of ANOVA, calcification on the weight scale in experiment I was fourth power transformed [31], to correct for positive skewing that was created by a small number of unusually heavy corallites. All other data met the homogeneity of variances (Levene's test) and normality assumptions of ANOVA (Shapiro–Wilks test). When tank effects were not significant (p > 0.05, which was true for all analyses), they were removed from the statistical model when the probability for this effect was greater than 0.25 [32] and the analysis completed using corals as independent replicates. While this does not avoid pseudoreplication, the demonstration of statistically identical results in independent tanks makes it unlikely that results are an artefact of dependence among corals. Post hoc analyses of calcification data were completed using a Tukey's test. To test for differences between natural- and reversed-phase diurnal pCO2, a Bonferroni-adjusted one-tailed t-test (α = 0.05) was performed to test the hypotheses that any stimulatory effect of natural-phase diurnal pCO2 on calcification for both weight and area was diminished in the reverse phase.
To analyse survivorship in experiment II, a Kaplan–Meier (KM) product-limit analysis was used [33]. For this analysis, the probability of individual recruits surviving is assumed to be independent of all other recruits, and because KM analyses cannot accommodate nested experimental designs, replicate corallites were pooled within each treatment. Survival was analysed using the statistical program JMP (v. 9.0.2, 2010, SAS Institute Inc.) and a log-rank test was used to test for differential survival among treatments.
3. Results
(a). Treatment conditions
Experimental pCO2 conditions in experiment I (420–597 µatm pCO2, table 1) mimicked the conditions found at 6 m depth on Hobihu reef (365–515 µatm, electronic supplementary material, figure S1), where parent colonies were collected. In experiment II, the pCO2 range was expanded (448–845 µatm pCO2) to more closely match the experimental conditions to those occurring on shallow reefs [22].
(b). Growth
Seriatopora caliendrum larvae settled rapidly onto microscope coverslips at mean densities of 3.4 ± 1.5 recruits cm−2 (± s.e.m., n = 18) in experiment I, and at 6.3 ± 2.2 recruits cm−2 (± s.e.m., n = 36) in experiment II. Recruits began calcifying shortly after settlement, with septa and a basal plate visible within 24 h. In experiment I, single corallites weighed between 60 and 130 µg with areas between 1.1 and 2.8 mm2 after 3 days; in experiment II, corallites weighed between 110 and 210 µg with areas ranging 1.0–3.0 mm2 after 6 days. When standardized by time, the mean growth of recruits across all treatments was 26 per cent slower in experiment II compared with experiment I (figure 1, t = 5.86, d.f. = 25, p < 0.001).
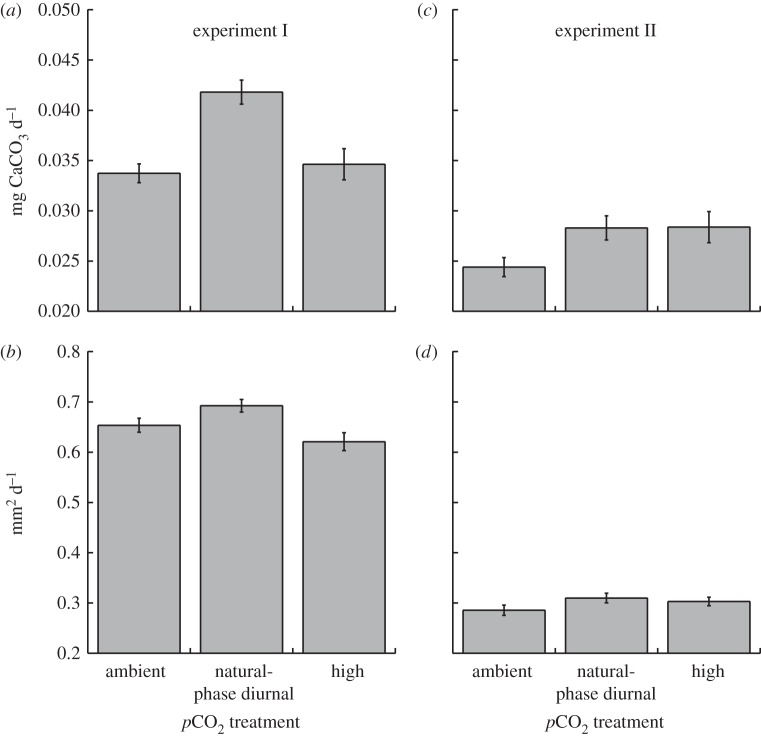
Figure 1.
Calcification of Seriatopora caliendrum recruits in ambient, high and diurnally oscillating pCO2 treatments during experiments I and II. Calcification was measured by (a,c) weight and (b,d) area. Mean ± s.e.m. displayed (weight n = 6–12, area n = 30–79).
In experiment I, the weight of S. caliendrum recruits was unaffected by the nested tank effect (p = 0.823), which was therefore dropped from the analysis; weight was affected significantly by pCO2 treatments (F2,17 = 5.155 p = 0.020). While the area of the recruits was unaffected by tank (p = 0.080), this effect could not be excluded from the analysis with the criterion applied (p > 0.25). Area was not affected by pCO2 treatments when tanks were retained in the analysis (F2,3 = 2.832 p = 0.204), but when the tank effect was dropped based on a more liberal criterion (i.e. p > 0.05), pCO2 treatments affected corallite area (F2,199 = 6.080, p = 0.003). For both weight and area, the highest growth occurred under diurnally oscillating pCO2 on a natural phase (figure 1). The increased growth under diurnally oscillating pCO2 on a natural phase amounted to a 17–19% increase by weight, and 6–10% larger area relative to recruits in the steady 456 and 624 µatm pCO2 conditions; growth on weight and area scales were similar between steady 456 and 624 µatm pCO2 (figure 1a,b).
In experiment II, the weight of corallites was again unaffected by the nested tank effect (p = 0.60), which was dropped from the analysis; weight was affected significantly by pCO2 treatments (F2,30 = 3.899, p = 0.032, figure 1c). Relative to steady ambient pCO2, weight was elevated 16 per cent under both diurnally oscillating pCO2 on a natural phase and high pCO2. The area of the corallites was unaffected by the tank effect (p = 0.494), which was dropped from the analysis; area was unaffected by the pCO2 treatments (F2,99 = 1.786, p = 0.173, figure 1d).
Coral recruits exposed to the diurnally oscillating pCO2 on a reverse phase in experiment II differed in area from those grown under diurnally oscillating pCO2 on the natural phase (t = 2.16, d.f. = 60, p = 0.018). There was an 11 per cent reduction in mean recruit area in the diurnally oscillating pCO2 on a reserve phase relative to recruits in the natural phase. The weight of recruits did not differ between pCO2 regimes oscillating on either a reverse or natural phase (t = 0.49, d.f. = 17, p = 0.315, figure 2).
Figure 2.
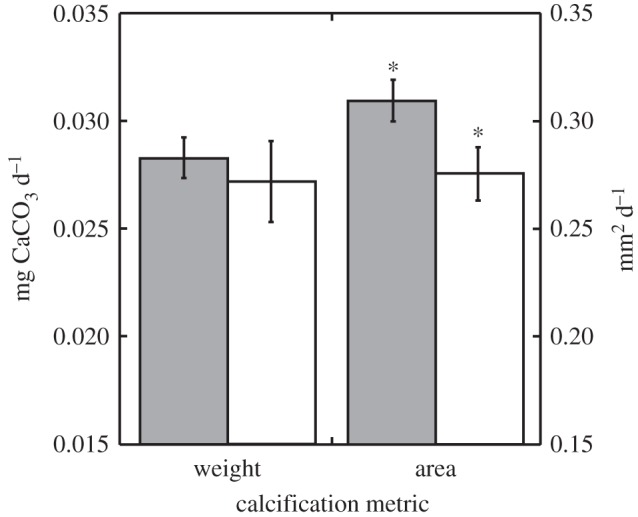
Calcification of S. caliendrum recruits in experiment II under natural-phase diurnal (grey bars) and reverse-phase diurnal pCO2 (white bars) treatments using weight (left ordinate) and area (right ordinate) scales. Significant paired t-tests between pCO2 treatments (p < 0.05) denoted by an asterisk. Mean ± s.e.m. displayed (weight: n = 32–36, area: n = 11–12).
(c). Survivorship
At the start of experiment II during which survivorship was measured, there were 331 recruits in the ambient pCO2 treatment, 138 in the high pCO2 treatment, 354 in the natural-phase diurnal pCO2 treatment and 402 in the reversed-phase diurnal pCO2 treatment. These recruits were scattered among a varying number of coverslips in each treatment, with a mean of 30.1 ± 1.7 (± s.e.m., n = 42) recruits per coverslip. At the end of the 6-day experiment, there were 239 (72%) survivors in ambient pCO2, 93 (67%) in the high pCO2, 301 (85%) in the natural-phase diurnal pCO2 and 221 (55%) in the reverse-phase diurnal pCO2 (figure 3). Survivorship differed significantly among treatments (χ2 = 107, d.f. = 3, p < 0.001) with the highest survival in the natural-phase diurnal pCO2 treatment.
Figure 3.
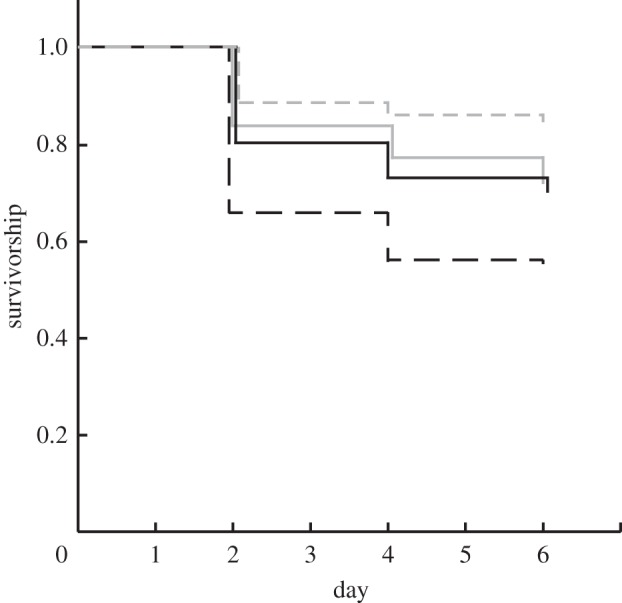
Survivorship of S. caliendrum recruits during experiment II as calculated by Kaplan–Meier product-limit analysis for ambient pCO2, high pCO2, diurnal pCO2 and reverse-diurnal pCO2 treatments. The numbers of recruits at the start of the experiment in each treatment are shown in the legend. Grey solid line, ambient pCO2, n = 331; black solid line, high pCO2, n=138; grey dashed line, natural-phase diurnal pCO2, n = 354; black dashed line, reverse-phase diurnal pCO2, n = 402.
4. Discussion
Our experiments show for the first time that diurnally oscillating pCO2 can increase calcification and survival in coral recruits that are less than or equal to 6 days old. Despite the growing literature addressing the response of corals to OA [7,8,12], to our knowledge, only one previous study has employed a measure of fitness as a response variable for corals exposed to high pCO2 [34]; this earlier work demonstrated the survivorship of Porites panamensis larvae was unaffected by 861–950 µatm pCO2. Additionally, the impacts on corals of diurnally oscillating pCO2 with a natural-phase relationship—as occurs routinely on shallow reefs [20–23]—has not previously been considered. Instead, studies of the effects of OA on corals have employed steady DIC regimes mimicking those expected in open oceans under increased atmospheric pCO2 [8], and only recently have the limitations of these scenarios for the conditions in near-shore habitats been recognized [20]. Null or stimulatory effects of high pCO2 on coral calcification, similar to those reported here, have been reported for at least three species of corals studied as adult colonies. Anthony et al. [35] reported calcification of adult Porites lobata increased approximately 23 per cent under 520–700 ppm pCO2 at 28–29°C, Jury et al. [10] reported calcification in Madracis auretenra remained unaffected or increased up to 21 per cent when exposed to 876, 1406, 1480 ppm pCO2 at 27–28°C and Edmunds [36] reported area-normalized calcification in massive Porites spp. was unaffected by 815 µatm pCO2. In the present study, we show that newly settled coral recruits grown for 3–6 days in diurnally oscillating pCO2 with a natural-phase relationship are fitter than their counterparts in static treatments of either ambient or elevated pCO2.
Most scleractinian corals that have been studied respond to elevated pCO2 with decreased calcification [8], although a few exceptions to this trend have been found [10,35,36]. The present results contribute to these exceptions, with calcification of newly settled S. caliendrum recruits elevated by weight (relative to ambient pCO2) in both diurnally oscillating pCO2 on a natural phase, and high pCO2 treatments. The stimulatory effects on calcification of diurnally oscillating pCO2 on the natural phase were more pronounced in experiment I compared with experiment II, where calcification decreased in all pCO2 treatments. The decreased calcification in experiment II was probably caused by the approximately 2.7°C decrease in temperature between ambient conditions (27.8°C, when the research was conducted) and those chosen (i.e. 25.1°C) to facilitate a contrast with experiment I. Furthermore, stimulatory effects on calcification of pCO2 as high as 845 µatm have not been observed in coral recruits, and instead the calcification of coral recruits (less than 50 days old and based on seven species) has been reported to decrease up to 84 per cent when exposed to pCO2 ranging from 560 to 3585 µatm [12]. Recruits of Porites astreoides, Favia fragum and P. panamensis departed somewhat from this trend, with calcification in P. astreoides and F. fragum remaining constant until pCO2 was greater than approximately 800–850 µatm (calculated from de Putron et al. [18]), and calcification in P. panamensis declining only 3 per cent at a pCO2 of 926 µatm [34]. It is important to note that corallite size determined from planar area or skeletal mass (as used in the present study and several others [16,18,34]) do not reflect the presence of biomass, which exerts a strong biological control on the process of mineralization [37] that can be altered to beneficial effect under OA conditions [36]. Thus, most contemporary studies of the effects of OA on coral recruits are limited in their capacity to ascribe process to the experimental outcomes and, likewise, here we are unable to determine the extent to which biomass contributed to the increased calcification in diurnally oscillating pCO2 on a natural phase, and high pCO2.
While studies of the effects of OA on coral calcification are beginning to be published in large numbers, little has been done to determine the effects on fitness traits such as survivorship, fecundity and fertilization. There is evidence, however, that fertilization success in broadcast spawning corals declines with increased pCO2, especially at low-sperm concentrations [38], although survival of coral recruits has been found unresponsive to high pCO2 [34]. In the present study, there were striking differences in the survival of S. caliendrum recruits, with the highest 6-day survivorship (85%) occurring in conditions which most closely mimic those on shallow reefs (diurnally oscillating pCO2 on a natural phase). Survivorship decreased to 67–72% in static 464 and 830 µatm pCO2, and was only 55 per cent in the diurnally oscillating pCO2 on a reverse phase. For very young corals (i.e. colonies less than 2 cm), in situ survival is size-dependent [39], but this effect is unlikely to account for the present survivorship results, which were associated with only minor differences in the size of recruits in experiment II. Since our treatments did not result in a strong effect on the size of recruits, we infer that the treatment conditions acted directly on physiological processes closely related to organismic success. These processes may include the use of phenotypic plasticity, which presumably served to translate differential mass accretion into corallites of similar size. Increased light-enhanced dark respiration caused by high pCO2, similar to that recorded in adult Acropora formosa [40], could represent one physiological process affecting the survival of recruits by favouring the creation of an energetic deficiency.
While it was beyond the scope of this study to identify the mechanism underlying the stimulatory effects on calcification and survival of diurnally oscillating pCO2 on a natural phase, our results are consistent with at least two testable hypotheses. These focus on the extent to which calcification over 24 h is stimulated by low pCO2 during daylight, or high pCO2 at night. In the first hypothesis, low daytime pCO2 (with high night-time pCO2) could stimulate calcification directly by providing access to high concentrations of 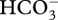 (assuming this form of DIC is used in coral calcification [41]), or indirectly by promoting photosynthesis of Symbiodium through the release of protons, which occurs during calcification [42]. We do not believe, however, that these mechanisms are in strong agreement with our results—even though there is a rich research history coupling high rates of photosynthesis and calcification in reef corals [11]—because both predict that calcification under natural-phase pCO2 oscillations should equal that under ambient (i.e. low) pCO2. Our findings do not support this outcome, and instead we suggest they support a second hypothesis, that high night-time pCO2 leads to enhanced daytime calcification at a time when calcification (and perhaps photosynthesis) might otherwise become DIC limited. According to this hypothesis, nocturnal increases in seawater pCO2 favours the accumulation of DIC (
(assuming this form of DIC is used in coral calcification [41]), or indirectly by promoting photosynthesis of Symbiodium through the release of protons, which occurs during calcification [42]. We do not believe, however, that these mechanisms are in strong agreement with our results—even though there is a rich research history coupling high rates of photosynthesis and calcification in reef corals [11]—because both predict that calcification under natural-phase pCO2 oscillations should equal that under ambient (i.e. low) pCO2. Our findings do not support this outcome, and instead we suggest they support a second hypothesis, that high night-time pCO2 leads to enhanced daytime calcification at a time when calcification (and perhaps photosynthesis) might otherwise become DIC limited. According to this hypothesis, nocturnal increases in seawater pCO2 favours the accumulation of DIC (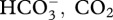 ) in the coral tissue at night through increased flux of DIC [43]. In turn, this leads to the creation of an intracellular DIC pool that could support calcification during the early part of the following day. In the present study, coral recruits in diurnally oscillating pCO2 on a natural phase were exposed to elevated DIC mostly in the form of
) in the coral tissue at night through increased flux of DIC [43]. In turn, this leads to the creation of an intracellular DIC pool that could support calcification during the early part of the following day. In the present study, coral recruits in diurnally oscillating pCO2 on a natural phase were exposed to elevated DIC mostly in the form of 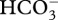 at night (table 1), which is thought to be used as the primary DIC source for calcification and photosynthesis in corals during the day [10,41,44–45]. During night, HCO3− might then be sequestered in the coral tissue.
at night (table 1), which is thought to be used as the primary DIC source for calcification and photosynthesis in corals during the day [10,41,44–45]. During night, HCO3− might then be sequestered in the coral tissue.
In the coral Stylophora pistillata, the DIC pool equilibrates with surrounding seawater after approximately 3 h of calcification [11] (adapted from Furla et al. [43]), indicating active DIC storage is possible. Short-term storage of DIC would benefit corals if calcification becomes DIC-limited during periods of light-enhanced calcification [10,41,45], and potentially could sustain calcification until it became depleted. One means by which corals could store DIC is through storage in the cytosol, with the effect mediated by carbonic anhydrase (CA) in the oral ectoderm, which transports DIC from seawater into coral tissues [46]. Transport into coral tissue could be achieved by converting 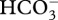 to CO2 for passive transport across cell membranes, and then converting it back to
to CO2 for passive transport across cell membranes, and then converting it back to 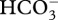 in the ectoderm to be later transferred throughout the coral for photosynthesis or calcification [46]. The hypothesized role of night-time DIC storage to explain stimulation of calcification in diurnally oscillating pCO2 on a natural phase also has the potential to account for the erosion of this effect in diurnally oscillating pCO2 on a reverse phase. Corals in this reverse-phase treatment calcified less than those in the natural-phase treatment, and this supports the ‘DIC storage hypothesis’ as these corals were not subjected to increased DIC at night, instead being subjected to ambient DIC.
in the ectoderm to be later transferred throughout the coral for photosynthesis or calcification [46]. The hypothesized role of night-time DIC storage to explain stimulation of calcification in diurnally oscillating pCO2 on a natural phase also has the potential to account for the erosion of this effect in diurnally oscillating pCO2 on a reverse phase. Corals in this reverse-phase treatment calcified less than those in the natural-phase treatment, and this supports the ‘DIC storage hypothesis’ as these corals were not subjected to increased DIC at night, instead being subjected to ambient DIC.
Several other lines of evidence that allude to different mechanisms of daytime versus night-time calcification [43,47,48] may provide an alternative explanation for the physiological benefits described in the present study of diurnally oscillating pCO2 on coral calcification. To test the validity of the ‘DIC storage hypothesis’ versus alternative hypotheses (i.e. multiple calcification mechanisms and/or changes in photosynthesis), it might be productive to expose corals to combinations of labelled DIC in the form of either carbonate or bicarbonate over the course of several diurnal cycles and determine the incorporation of labelled DIC into host tissues and skeleton under ambient, high and natural-phased diurnally oscillating pCO2. Such an experiment would reveal if there was a diurnal preference for DIC species, as well as DIC storage, which could support enhanced calcification in night or early morning as proposed above. Alternatively, measuring the net productivity of coral recruit's Symbiodinium sp. could help determine what role changes in photosynthesis may contribute to the observed calcification trends. Identifying the extent to which newly recruited juvenile and adult corals are able to benefit from diurnally oscillating pCO2 will play an important role in understanding the complexities of coral responses to ocean acidification and climate change.
Acknowledgements
We thank H. M. Putnam, Y.-H. Chen, O. Chan and S. Zamudio for field and laboratory help, R. C. Carpenter for assistance with project development, and S. Comeau and S. R. Dudgeon for manuscript review. We also thank the two anonymous reviewers whose comments improved an earlier draft of this paper. This work was funded by the US National Science Foundation (grant OCE 08-44785) and, in part, by a Graduate Thesis Support grant from California State University, Northridge (CSUN). This is contribution no. 178 of the Marine Biology Program of CSUN.
References
- 1.Orr J. C., et al. 2005. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437, 681–686 10.1038/nature04095 (doi:10.1038/nature04095) [DOI] [PubMed] [Google Scholar]
- 2.Pelejero C., Calvo E., Hoegh-Guldberg O. 2005. Paleo-perspectives on ocean acidification. Trends Ecol. Evol. 25, 332–334 10.1016/j.tree.2010.02.002 (doi:10.1016/j.tree.2010.02.002) [DOI] [PubMed] [Google Scholar]
- 3.Kiessling W., Simpson C. 2011. On the potential for ocean acidification to be a general cause of ancient reef crises. Glob. Change Biol. 17, 56–67 10.1111/j.1365-2486.2010.02204.x (doi:10.1111/j.1365-2486.2010.02204.x) [DOI] [Google Scholar]
- 4.Kleypas J. A., Langdon C. 2006. Coral reefs and changing seawater chemistry. In Coral reefs and climate change: science and management. AGU Monograph Series, Coastal and Estuarine Studies, vol. 61 (eds Phinney J. T., Hoegh-Guldberg O., Kleypas J., Skirving W., Strong A.), pp. 73–110 Washington, DC: American Geophysical Union [Google Scholar]
- 5.Doney S. C., Fabry V. J., Feely R. A., Kleypas J. A. 2009. Ocean acidification: the other CO2 problem. Annu. Rev. Mar. Sci. 1, 169–192 10.1146/annurev.marine.010908.163834 (doi:10.1146/annurev.marine.010908.163834) [DOI] [PubMed] [Google Scholar]
- 6.Ries J. B., Cohen A. L., McCorkle D. C. 2009. Marine calcifiers exhibit mixed responses to CO2-induced ocean acidification. Geology 37, 1131–1134 10.1130/G30210A.1 (doi:10.1130/G30210A.1) [DOI] [Google Scholar]
- 7.Pandolfi J. M., Connolly S. R., Marshall D. J., Cohen A. L. 2011. Projecting coral reef futures under global warming and ocean acidification. Science 333, 418–422 10.1126/science.1204794 (doi:10.1126/science.1204794) [DOI] [PubMed] [Google Scholar]
- 8.Erez J., Reynaud S., Silverman J., Schneider K., Allemand D. 2011. Coral calcification under ocean acidification and global change. In Coral reefs: an ecosystem in transition (eds Dubinsky Z., Stambler N.), pp. 151–176 New York, NY: Springer [Google Scholar]
- 9.Cohen A. L., Holcomb M. 2009. Why corals care about ocean acidification: uncovering the mechanism. Oceanography 22, 118–127 10.5670/oceanog.2009.102 (doi:10.5670/oceanog.2009.102) [DOI] [Google Scholar]
- 10.Jury C. P., Whitehead R. F., Szmant A. M. 2010. Effects of variations in carbonate chemistry on the calcification rates of Madracis auretenra (=Madracis mirabilis sensu Wells, 1973): bicarbonate concentrations best predict calcification rates. Glob. Change Biol. 16, 1632–1644 10.1111/j.1365-2486.2009.02057.x (doi:10.1111/j.1365-2486.2009.02057.x) [DOI] [Google Scholar]
- 11.Allemand D., Tambutté É., Zoccola D., Tambutté S. 2011. Coral calcification, cells to reefs. In Coral reefs: an ecosystem in transition (eds Dubinsky Z., Stambler N.), pp. 119–150 New York, NY: Springer; 10.1007/978-94-007-0114-4_9 (doi:10.1007/978-94-007-0114-4_9) [DOI] [Google Scholar]
- 12.Albright R. 2011. Reviewing the effects of ocean acidification on sexual reproduction and early life history stages of reef-building corals. J. Mar. Biol. 2011, 473615. 10.1155/2011/473615 (doi:10.1155/2011/473615) [DOI] [Google Scholar]
- 13.Kurihara H. 2008. Effects of CO2-driven ocean acidification on the early developmental stages of invertebrates. Mar. Ecol. Prog. Ser. 373, 275–284 10.3354/meps07802 (doi:10.3354/meps07802) [DOI] [Google Scholar]
- 14.Hughes T. P., Jackson J. B. C. 1985. Population dynamics and life histories of foliaceous corals. Ecol. Monogr. 55, 141–166 10.2307/1942555 (doi:10.2307/1942555) [DOI] [Google Scholar]
- 15.Cohen A. L., McCorkle D. C., de Putron S. J., Gaetani G. A., Rose K. A. 2009. Morphological and compositional changes in the skeletons of new coral recruits reared in acidified seawater: insights into the biomineralization response to ocean acidification. Geochem. Geophys. Geosyst. 10, 1–12 10.1029/2009GC002411 (doi:10.1029/2009GC002411) [DOI] [Google Scholar]
- 16.Albright R., Langdon C. 2011. Ocean acidification impacts multiple early life history processes of the Caribbean coral Porites astreoides. Glob. Change Biol. 17, 2478–2487 10.1111/j.1365-2486.2011.02404.x (doi:10.1111/j.1365-2486.2011.02404.x) [DOI] [Google Scholar]
- 17.Suwa R., Nakamura M., Morita M., Shimada K., Iguchi A., Sakai K., Suzuki A. 2010. Effects of acidified seawater on early life stages of scleractinian corals (Genus Acropora). Fish. Sci. 76, 93–99 10.1007/s12562-009-0189-7 (doi:10.1007/s12562-009-0189-7) [DOI] [Google Scholar]
- 18.de Putron S. J., McCorkle D. C., Cohen A. L., Dillon A. B. 2011. The impact of seawater saturation state and bicarbonate ion concentration on calcification by new recruits of two Atlantic corals. Coral Reefs 30, 321–328 10.1007/s00338-010-0697-z (doi:10.1007/s00338-010-0697-z) [DOI] [Google Scholar]
- 19.Nakamura M., Ohki A., Suzuki A., Sakai K. 2011. Coral larvae under ocean acidification: survival, metabolism, and metamorphosis. PLoS ONE 6 e14521. 10.1371/journal.pone.0014521 (doi:10.1371/journal.pone.0014521) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andersson A. J., Mackenzie F. T. 2011. Ocean acidification: setting the record straight. Biogeosci. Discuss. 8, 6161–6190 10.5194/bgd-8-6161-2011 (doi:10.5194/bgd-8-6161-2011) [DOI] [Google Scholar]
- 21.Gattuso J. P., Payri C., Pichon M., Delesalle B., Frankignoulle M. 1997. Primary production, calcification and air-sea CO2 fluxes of a macro algal dominated coral reef community (Moorea, French Polynesia). J. Phycol. 33, 729–738 10.1111/j.0022-3646.1997.00729.x (doi:10.1111/j.0022-3646.1997.00729.x) [DOI] [Google Scholar]
- 22.Ohde S., Van Woesik R. 1999. Carbon dioxide flux and metabolic processes of a coral reef, Okinawa. Bull. Mar. Sci. 65, 559–576 [Google Scholar]
- 23.Bates N. R., Amat A., Andersson A. J. 2010. Feedbacks and responses of coral calcification on the Bermuda reef system to seasonal changes in biological processes and ocean acidification. Biogeosciences 7, 2509–2530 10.5194/bg-7-2509-2010 (doi:10.5194/bg-7-2509-2010) [DOI] [Google Scholar]
- 24.Coles S. 1975. A comparison of effects of elevated temperature versus temperature fluctuations on reef corals at Kahe Point. Oahu. Pac. Sci. 29, 15–18 [Google Scholar]
- 25.Oliver T. A., Palumbi S. R. 2011. Do fluctuating temperature environments elevate coral thermal tolerance? Coral Reefs 30, 429–440 10.1007/s00338-011-0721-y (doi:10.1007/s00338-011-0721-y) [DOI] [Google Scholar]
- 26.Putnam H. M., Edmunds P. J., Fan T. Y. 2011. Effect of a fluctuating thermal regime on adult and larval reef corals. Invert. Biol. 129, 199–209 10.1111/j.1744-7410.2010.00199.x (doi:10.1111/j.1744-7410.2010.00199.x) [DOI] [Google Scholar]
- 27.Veron J. E. N. 2000. Corals of the world, vol. 2 Townsville, Australia: Australian Institute of Marine Science [Google Scholar]
- 28.Dai C. F., Horng S. 2009. Scleractinia fauna of Taiwan II. The robust group. Taipei, Taiwan: National Taiwan University [Google Scholar]
- 29.Fan T. Y., Lin K. H., Kuo F. W., Soong K., Liu L. L., Fang L. S. 2006. Diel patterns of larval release by 5 brooding scleractinian corals. Mar. Ecol. Prog. Ser. 321, 133–142 10.3354/meps321133 (doi:10.3354/meps321133) [DOI] [Google Scholar]
- 30.IPCC 2007. Climate Change 2007: the physical science basis: contribution of Working Group I to the fourth assessment report of the intergovernmental panel on climate change. New York, NY: Cambridge University Press; . [Google Scholar]
- 31.Zar J. H. 1999. Biostatistical analysis, pp. 663 Eaglewood Cliffs, NJ: Prentice-Hall [Google Scholar]
- 32.Underwood A. J. 1997. Experiments in ecology: their logical design and interpretation using analysis of variance. Cambridge, UK: Cambridge University Press [Google Scholar]
- 33.Machin D., Cheung Y. B., Parmar M. K. B. 2006. Survival analysis, a practical approach, 2nd edn. West Sussex, UK: Wiley [Google Scholar]
- 34.Anlauf H., D'Croz L., O'Dea A. 2011. A corrosive concoction: the combined effects of ocean warming and acidification on the early growth of a stony coral are multiplicative. J. Exp. Mar. 397, 13–20 10.1016/j.jembe.2010.11.009 (doi:10.1016/j.jembe.2010.11.009) [DOI] [Google Scholar]
- 35.Anthony K. R. N., Kline D. I., Diaz-Pulido G., Dove S., Hoegh-Guldberg O. 2008. Ocean acidification causes bleaching and productivity loss in coral reef builders. Proc. Natl Acad. Sci. USA 105, 17 442–17 446 10.1073/pnas.0804478105 (doi:10.1073/pnas.0804478105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edmunds P. J. 2011. Zoolanktivory ameliorates the effects of ocean acidification on the reef coral Porites spp. Limnol. Oceanogr. 56, 2402–2410 10.4319/lo.2011.56.6.2402 (doi:10.4319/lo.2011.56.6.2402) [DOI] [Google Scholar]
- 37.Hofmann G. E., Barry J. P., Edmunds P. J., Gates R. D., Hutchins D. A., Klinger T., Sewell M. A. 2010. The effect of ocean acidification on polar, temperate and tropical marine calcifying organisms: an organism to ecosystem perspective. Annu. Rev. Ecol. Evol. 41, 127–147 10.1146/annurev.ecolsys.110308.120227 (doi:10.1146/annurev.ecolsys.110308.120227) [DOI] [Google Scholar]
- 38.Albright R., Mason B., Miller M., Langdon C. 2010. Ocean acidification compromises recruitment success of the threatened Caribbean coral Acropora palmata. Proc. Natl Acad. Sci. USA 107, 20 400–20 404 10.1073/pnas.1007273107 (doi:10.1073/pnas.1007273107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Babcock R. C. 1991. Comparative demography of three species of scleractinian coral using age- and size-dependent classifications. Ecol. Monogr. 61, 225–244 10.2307/2937107 (doi:10.2307/2937107) [DOI] [Google Scholar]
- 40.Crawley A., Kline D., Dunn S., Anthony K., Dove S. 2010. The effects of ocean acidification on symbiont photorespiration and productivity in Acropora formosa. Glob. Change Biol. 15, 851–863 10.1111/j.1365-2486.2009.01943.x (doi:10.1111/j.1365-2486.2009.01943.x) [DOI] [Google Scholar]
- 41.Marubini F., Thake B. 1999. Bicarbonate addition promotes coral growth. Limnol. Oceanogr. 44, 716–720 10.4319/lo.1999.44.3.0716 (doi:10.4319/lo.1999.44.3.0716) [DOI] [Google Scholar]
- 42.McConnaghey T. A., Whelan J. F. 1997. Calcification generates protons for nutrient and bicarbonate uptake. Earth Sci. Rev. 967, 95–117 10.1016/S0012-8252(96)00036-0 (doi:10.1016/S0012-8252(96)00036-0) [DOI] [Google Scholar]
- 43.Furla P., Galgani I., Durand I., Allemand D. 2000. Sources and mechanisms of inorganic carbon transport for coral calcification and photosynthesis. J. Exp. Biol. 203, 3445–3457 [DOI] [PubMed] [Google Scholar]
- 44.Schneider K., Erez J. 2006. The effect of carbonate chemistry on calcification and photosynthesis in the hermatypic coral Acropora eurystoma. Limnol. Oceanogr. 51, 1284–1293 10.4319/lo.2006.51.3.1284 (doi:10.4319/lo.2006.51.3.1284) [DOI] [Google Scholar]
- 45.Herfort L., Thake B., Taubner I. 2008. Bicarbonate stimulation of calcification and photosynthesis in two hermatypic corals. J. Phycol. 44, 91–98 10.1111/j.1529-8817.2007.00445.x (doi:10.1111/j.1529-8817.2007.00445.x) [DOI] [PubMed] [Google Scholar]
- 46.Bertucci A., Tambutté S., Supuran C. T., Allemand D., Zoccola D. 2011. A new coral carbonic anhydrase in Stylophora pistillata. Mar. Biotechnol. 13, 992–1002 10.1007/s10126-011-9363-x (doi:10.1007/s10126-011-9363-x) [DOI] [PubMed] [Google Scholar]
- 47.Marshall A. T., Clode P. 2004. Calcification rate and the effect of temperature in a zooxanthellate and azooxanthellate scleractinian reef coral. Coral Reefs 23, 218–224 10.1007/s00338-004-0369-y (doi:10.1007/s00338-004-0369-y) [DOI] [Google Scholar]
- 48.Schneider K., Levy O., Dubinsky Z., Erez J. 2009. In situ diel cycles of photosynthesis and calcification in hermatypic corals. Limnol. Oceanogr. 54, 1995–2002 10.4319/lo.2009.54.6.1995 (doi:10.4319/lo.2009.54.6.1995) [DOI] [Google Scholar]