Abstract
Compared with other species, exchange among non-kin is a hallmark of human sociality in both the breadth of individuals and total resources involved. One hypothesis is that extensive exchange evolved to buffer the risks associated with hominid dietary specialization on calorie dense, large packages, especially from hunting. ‘Lucky’ individuals share food with ‘unlucky’ individuals with the expectation of reciprocity when roles are reversed. Cross-cultural data provide prima facie evidence of pair-wise reciprocity and an almost universal association of high-variance (HV) resources with greater exchange. However, such evidence is not definitive; an alternative hypothesis is that food sharing is really ‘tolerated theft’, in which individuals possessing more food allow others to steal from them, owing to the threat of violence from hungry individuals. Pair-wise correlations may reflect proximity providing greater opportunities for mutual theft of food. We report a laboratory experiment of foraging and food consumption in a virtual world, designed to test the risk-reduction hypothesis by determining whether people form reciprocal relationships in response to variance of resource acquisition, even when there is no external enforcement of any transfer agreements that might emerge. Individuals can forage in a high-mean, HV patch or a low-mean, low-variance (LV) patch. The key feature of the experimental design is that individuals can transfer resources to others. We find that sharing hardly occurs after LV foraging, but among HV foragers sharing increases dramatically over time. The results provide strong support for the hypothesis that people are pre-disposed to evaluate gains from exchange and respond to unsynchronized variance in resource availability through endogenous reciprocal trading relationships.
Keywords: cooperation, exchange, hunter–gatherers, risk, evolution
1. Introduction and the hypotheses to be tested
Compared with other species, exchange among non-kin is a hallmark of human sociality in both the breadth of individuals and total resources involved. Anthropologists, economists and biologists have not reached consensus regarding why exchange is so pervasive in human groups. Hill et al. [1] have argued that extensive exchange, facilitated by home-based resource sharing, is a universal pattern among hunter–gatherers. This phenomenon is as ancient as the genus, Homo, and marks an established importance for hunted foods in the diet [2]. Virtually, all extant hunter–gatherers share food regularly—over 90 per cent of Ache hunter–gatherer families' food is shared by members of other nuclear families [3].
One explanation derives from a larger theory of human evolution, arguing that food sharing co-evolved with complementary traits, such as large brains, a long period of juvenile dependency to learn skill-intensive foraging strategies, a long lifespan and extensive food sharing among unrelated individuals [4,5]. In this theory, the link between diet and food sharing derives principally from the riskiness of large-package food harvesting. Hunting exhibits highly variable daily returns. Thus, Ache foragers acquire meat only on 43.5 per cent of days that they hunt [6]; success rates are slightly lower among Hiwi [7] and considerably lower for Hadza foragers [8], resulting in some days with superabundant food and many days with little or none. According to the theory, the challenge posed by inter-temporal variance in large-package food availability was addressed with an ‘insurance’ mechanism: successful foragers of high-variance (HV) resources share food with unsuccessful foragers of these resources with the expectation of a reciprocal flow in the other direction when the situation is reversed. By smoothing the intake rate variance, individuals maximize the daily nutritional benefit from pursuing large food packages.
An alternative views food sharing as ‘tolerated theft’ [9]: large food packages create variance among individuals in their available food, with the ‘lucky’ individuals possessing larger and the unlucky ones smaller quantities or none. Possessors tolerate theft from their supply by individuals with less, because the cost to the possessor of defending the surplus exceeds its value, and the value of the food to the hungry individual makes fighting for it worthwhile. Hence, exchange is not reciprocal, but based on a momentary variance of who is hungry and who has abundance. Also it is argued that men, not women, target HV resources precisely, because possession of the large packages attracts attention enabling men who tolerate theft to signal their quality, resulting in greater access to mates.
The two theories are linked to distinct models of human evolution. The first theory views sharing as the efficient way to maintain a steady food supply when HV packages provide the highest profitability per time spent foraging. Men hunt and share food in order to provide food for their wives and children. Women also target moderately HV calorically dense plant resources (explained by the incompatibility of childcare with mobile hunting), sharing them as well, but to a less-pressing extent since the variance is lower. The second theory specifically argues that men sub-optimize familial food provisioning in favour of targeting unreliable animal resources, which when stolen, allow men to ‘show off’ their quality to others and gain additional mating benefits [10].
The empirical literature on societies where hunting and gathering generate a significant percentage of the diet provides important, but arguably not definitive, evidence to test the theories. The evidence overwhelmingly shows that hunted foods are shared most, but gathered foods tend to be shared in relation to their package size. The principal points of contention concern (i) whether people form reciprocal food-sharing relations or whether food transfers are merely opportunistic tolerated theft, and (ii) does food sharing increase or decrease available food for consumption? At the individual level, the cross-cultural and chimpanzee evidence show pair-wise relationships such that within groups, food shared from individual A to individual B (or from family A to family B) is highly correlated with food shared from B to A [11,12]. However, it has been argued that this is not definitive proof of reciprocity, since such correlations may reflect exogenous proximity [13] providing greater opportunities for mutual theft of food [14]. Moreover, theoretical models suggest that reciprocity is more difficult to support as group size increases, because of greater scope for free-riding [15].
Knowing whether reciprocity emerges spontaneously in response to risk and permits specialization in HV foods has important implications for understanding the evolution of economic systems, and perhaps the last 2 million years of hominid evolution. The goal of our virtual world laboratory experiment was to test the risk-reduction model of food sharing, under controlled conditions in which there was no scope for tolerated theft or gains from signalling quality. Compared with field studies, the advantage of a laboratory experiment is its ability to implement sharper controls and narrow the interpretation of observations. In particular, we here control for theft. Hence, if we fail to observe reciprocal risk-sharing among non-kin in this ecology-controlled virtual terrarium, the burden of proof shifts against those who suppose reciprocal risk-sharing from hunting is a puissant factor of human relations in the naturally occurring world.
Individuals have the option of foraging in one of two patches in repeated rounds, one with high-mean, HV large packages that are rarely captured, and the other with low-mean, low-variance (LV) small packages that are captured with near certainty. Following each foraging bout, the eight individuals in each experimental group ‘consume’ resources, either in proximity to others that they have sought out and chosen to group with, or alone. Since consumption is bounded above on each round (i.e. only 18 units may be consumed in any one round, and large packages were valued at 36 units), the HV patch yields lower total returns over the course of the experiment unless profitable food-sharing relationships are established.
The key feature of the experiment is that individuals are permitted to transfer food that they have captured but not to take food from other individuals. Unlike naturalistic field data, theft is impossible in this context, so it can be ruled out as a possible explanation of observed transfers. Other important features of the design were that participants were not told that the returns from one patch were more variable than the other; they had to discover that through experience. During the consumption phase, the individuals could choose to consume alone or to form groups. No clues are given about the value of food sharing. This means that foraging decisions, proximity, grouping decisions and sharing decisions are all endogenous. Participants could send messages to one another through computer chat screens, but the experiment provided no formal means by which commitments could be enforced. Each individual was represented by a coloured avatar, so that subjects could remember past interactions with different players without knowing their true identity. The choice of eight individuals for each experiment was made to represent the number of families in a moderately large foraging band.
This experimental design allows us to test the following predictions derived from the risk-reduction theory of reciprocal sharing:
- (H1)
Sharing conventions will evolve such that individuals who pursue HV resources will share when they are successful, whereas those who pursue LV resources will share less or not at all.
- (H2)
Sharing will increase over time (due to learning and the evolution of conventions).
- (H3)
In response to acquisition variance, people will form groups such that group size will be positively associated with sharing.
- (H4)
Individuals pursuing HV resources will preferentially share with others who choose HV resources, with individuals successfully foraging HV patches transferring resources to the less successful—in effect becoming HV specialized.
- (H5)
People will form reciprocal relationships with specific sharing partners.
- (H6)
Those who engage in the HV strategy and transfer more resources will achieve higher earnings.
Although this study was not specifically designed to test predictions from the tolerated theft theory, our design does allow us to test the following hypotheses regarding male–female differences, proposed by proponents of that model: males will
- (H7)
pursue HV resources more than females.
- (H8)
share more than females.
- (H9)
sacrifice earnings by pursuing HV resources and sharing.
2. Results
Table 1 reports the results testing hypotheses (H1)–(H4), (H7) and (H8) using general estimating equations (GEEs) controlling for repeated measures with random effects for participant, period and session. Theoretically predicted effects are marked in italics. Adaptive hypotheses regarding variance and food sharing predict interactions: variance affects sharing conditional on success (H1); any difference between LV and HV strategies will increase over time, as conventions evolve (H2). The effect of period and associated interaction terms (period by amount captured and variance by period by amount captured) provide strong support for (H1) and (H2), jointly. Group size is also positively associated with sharing, as predicted by (H3). Males pursued HV resources more frequently (60% frequency for males versus 50% for females, p = 0.018), and there is a significant three- and four-way interaction among sex, period, patch variance and success, marked in italics.
Table 1.
Total transferred per period by participants (GEE; n = 1912 observations on 96 participants).
| parameter | B | significance |
|---|---|---|
| intercept | −1.134 | 0.001 |
| period | −0.236 | 0.007 |
| low variance (LV) | −0.378 | 0.324 |
| high variance (HV) | 0 | — |
| group size | 0.140 | 0.007 |
| female | 0.058 | 0.812 |
| male | 0 | — |
| amount captured (AC) | 0.097 | 0.005 |
| LV × AC | −0.022 | 0.741 |
| HV × AC | 0 | — |
| period × AC | 0.029 | 0.000 |
| female × AC | 0.020 | 0.769 |
| male × AC | 0 | — |
| LV × period × AC | −0.033 | 0.000 |
| HV × period × AC | 0 | — |
| female × period × AC | −0.015 | 0.003 |
| male × period × AC | 0 | — |
| LV × female × period × AC | 0.010 | 0.005 |
| LV × male × period × AC | 0 | — |
| HV × female × period × AC | 0 | — |
| HV × male × period × AC | 0 | — |
| period squared | −0.013 | 0.004 |
| LV × period squared | 0.005 | 0.003 |
| HV × period squared | 0 | — |
| (scale) | 19.03 | — |
The meaning and size of these results are summarized in figure 1 by extracting the predicted amount of resource sharing in successful foraging bouts from the regression model as a function of sex and period (evaluated at average group size, 3.5, an average of 8 units acquired from the LV patch, and 40 units when successful from the HV patch). Transparently, from figure 1, sharing hardly occurs at all in LV, with a mean less than 0.5 units transferred. In the HV patch, sharing increases dramatically over time—more for males than females. By round 20, there is about a 50-fold difference in sharing between the HV and LV patches.
Figure 1.
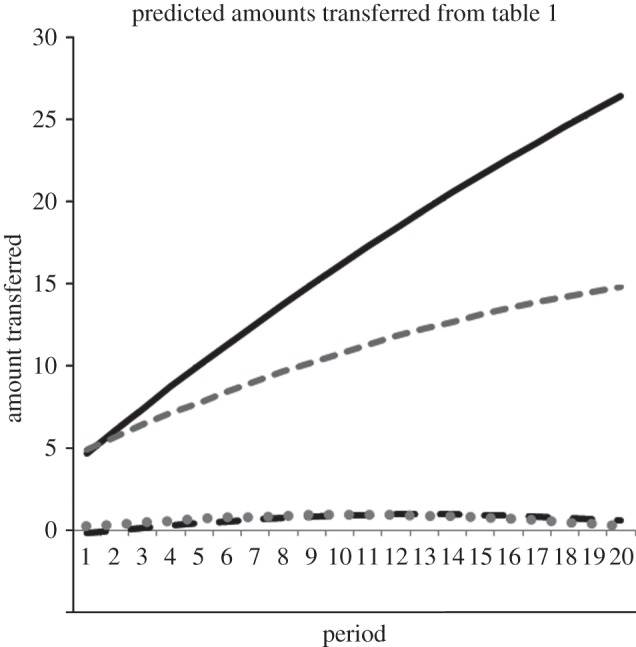
The predicted amount of resource sharing in successful foraging bouts is extracted from the regression model and plotted as a function of sex, foraging patch and period (evaluated at average group size, 3.5, an average of 8 units acquired from the LV (black dashed line, male; grey dotted line, female) patch, and 40 units when successful from the HV (black solid line, male; grey dashed line, female) patch).
Table 2 reports a regression test of reciprocity using paired observations of how much participant A gave to participant B. Again, using GEE analysis, the amount transferred from A to B was regressed on the patch choice of the pair, amount captured, sex and the cumulative history of previous transfers from A to B and B to A. As predicted by H4, sharing is greatest when both A and B choose the HV patch, and when A is successful and B is unsuccessful. As predicted by H5, people form reciprocal partnerships; both the total amount previously given from A to B, and the amount previously given by B to A, predict the amount shared. All these effects are highly significant. Ceteris paribus, females both gave less and received less.
Table 2.
Total transferred from participant A to participant B per period (n = 13 330 observations).
| parameter | B | significance |
|---|---|---|
| intercept | 0.059 | 0.086 |
| period | 0.012 | 0.0001 |
| A: LV | −0.223 | 0.0001 |
| A: HV | 0.0001 | — |
| B: LV | −0.220 | 0.0001 |
| B: HV | 0.0001 | — |
| A: female | −0.147 | 0.0001 |
| A: male | 0 | — |
| B: female | −0.101 | 0.008 |
| B: male | 0.0001 | — |
| A: amount captured | 0.048 | 0.0001 |
| B: amount captured | 0.012 | 0.0001 |
| total previous from A to B | 0.017 | 0.002 |
| total previous from B to A | 0.015 | 0.001 |
| scale | 4.597 |
Finally, as predicted by (H6) and not by (H9), both total resources given to others, and choosing the HV strategy was associated with significantly higher earnings (figure 2).
Figure 2.
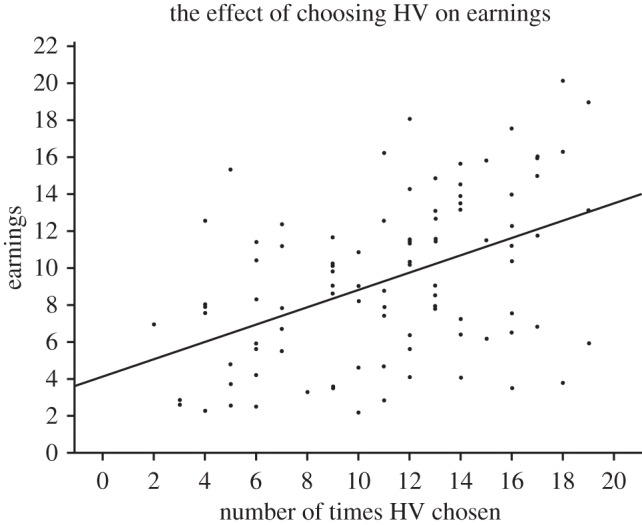
The number of times the HV strategy was chosen is plotted as a function of earnings.
3. Discussion and conclusions
The results provide strong support for the hypothesis that people are pre-disposed to evaluate the gains from exchange, and respond to unsynchronized variance in resource availability through endogenous reciprocal trading relationships. Inter-temporal exchange was virtually absent when LV is chosen. However, in response to the HV choice, the participants responded to the gains from trade, even in the first period, but as the experiment proceeded, exchange increased through time, as did mean group size, which was also endogenous. Remarkably, the statistical analysis shows that participants kept track of previous pair-wise transfers to and from specific individuals as previous transfers in both directions positively predicted the amount transferred in a given period.
Compared with the effects of variance, the sex effects are not large (figure 1). Nevertheless, the sex differences were quite patterned: not only did females choose the HV patch less often (50% versus 60% for males), they also gave and received less in pair-wise interactions although sex could not be visually identified. Although choosing the HV patch was associated with higher earnings, there was no statistical difference in the earnings of males and females. Given the natural history of hunter–gatherer foraging, with males emphasizing hunting and females gathering, males may have targeted HV options more frequently over human evolutionary time, resulting in selection for stronger cognitive tendencies to establish reciprocal relationships with many partners for the purpose of reducing daily intake variance.
These findings address weaknesses in field studies. Given that participants had no means to take resources from others, and only could freely give them, it is impossible that tolerated theft explains the transfers. Of course, because theft is not possible in our experiment, we cannot rule out ‘tolerated theft’ as being able to explain comparable observations in the naturally occurring world. But that is not our project, for introducing theft into our virtual world creates the same interpretation problems that are present in field study data: is reciprocity at work or is an exogenous proximity providing greater opportunity for mutual theft? By controlling only the physical characteristics of the foraging environment, our data reproduce the major findings of field studies, and thus we can conclude that the environment induces socioeconomic patterns of behaviour that mimic results in the field—a remarkably unlikely finding if people do not intrinsically employ the same problem-solving techniques. Although participants could not take resources from others, there was no enforcement of reciprocal contracts, so there were ample opportunities for defection. Yet, we observed significant pair-wise reciprocity that increased over the course of the experiment. Moreover, our participants do it under anonymity.
Second, the results are not confounded by exogenous kinship and residential proximity. Participants could consume their resources wherever they chose, and had no history of previous interactions. Strong reciprocal relationships emerged as a means to betterment. Combining field research with experimental research provides strong support for the theory that variance associated with the evolved human diet promoted the evolution of exchange in our species. Consistent with this model, the participants draw on social exchange adaptations to fashion a solution to a generic problem of risky and unstable return rates. The results also suggest that the current disinterest in pair-wise reciprocity in favour of alternative concepts, such as ‘strong reciprocity’, may represent a premature rejection of the most important developmental force in human cooperation; in spite of sex differences, welfare improves through sharing and people do not appear to throw away resources for display.
These findings, coupled with the extensive anthropological field data, also have implications for economic theory. Trade and specialization are viewed as having co-evolved because trade enables gains from exchange to be leveraged by specialization;1 but it may be that humans have engaged in extensive between-family trade for perhaps 2 million years without any significant deepening of specialization. Moreover, this would also mean that important features of human social psychology regarding reciprocity evolved and long persisted without jump-starting growth. Consistent with this perspective, economic historians agree that the foundation for the dramatic subsequent take-off in per capita wealth emerged ‘suddenly’ by human pre-historical standards in the eighteenth century in northwestern Europe, long after institutions of trade, specialization and property had been established [17]. Neither property rights nor contract enforcement of promises appears sufficient to explain the take-off in per capita wealth.
One fundamental problem that still requires resolution in the evolution of exchange is the co-emergence of individual property rights. One view in the philosophical tradition of Hobbes and Bentham is that only the state can deliberately create rights to property. In contrast, we hypothesize that even in hunter–gatherer environments where there is no monopoly on the use of violence or enforcement, there is a respect for property [18]; however, much of that property is rapidly transferred to others due to reciprocal variance reduction. In many traditional small-scale societies that practice a mix of foraging and horticulture, there is no ownership of land, nor state sanctioning of land ownership and warfare is common. Nevertheless, individual fields are generally and mutually respected through the principle of usufruct in which people reciprocally observe that it is in one's own interest to allow each other to reap the benefits of personally preparing land for cultivation. More generally, some form of respect for property rights may provide incentives for people to produce risky resources for exchange in hunter–gatherer societies. The nature of property in stateless societies is not well understood and can be addressed in future experiments.
Finally, it is possible, and perhaps even likely, that food transfers in foraging societies now and in human evolutionary history were determined by a number of forces simultaneously. A consistent finding is that kinship plays an important role in food transfers, especially asymmetrical ones in which one party consistently gives more than the other [7,19]. Trade across different currencies; food for prestige, for sex and for better treatment of children have also been proposed to explain asymmetric food transfers [3,20–22]. Tolerated theft, pressure from hungry individuals and signalling behaviour could exist alongside reciprocal relationships. This experiment serves to show that reciprocal relationships form conditional on unsynchronized risk in resource acquisition, in the absence of those other motivations.
4. Material and methods
We recruited 96 undergraduates, 50 male and 46 female, to participate in 12 eight-person sessions of a laboratory experiment at Chapman University. Eight participants at a time were taken into a computer laboratory, seated at visually isolated computer stations to preserve anonymity, and presented with the same virtual environment on their computer screens. They privately read a set of experimental instructions describing the environment and the capabilities of their avatars during which they could practice moving around the environment, capturing hexagons and moving hexagons to a pot for consumption (see the electronic supplementary material, for the instructions and sample screenshots). The experiment proper lasted for 53 min: 20 periods of 2.5 min each, plus three break periods of 1 min each (after periods 5, 10 and 15). After the experiment, participants were privately paid their show-up payment plus experimental earnings and then dismissed. The participants were recruited for a 90-minute experiment, but were not informed in advance of the total number of periods in order to mitigate potential end-game effects. The self-paced instructions were completed in approximately 5–10 min, and it took 10 min to privately pay the participants their earnings. Participants were paid $7 for showing up on-time, plus what they earned as a result of their decisions (mean = $9.37, s.d. = $4.38).
Each period was subdivided into three phases. In the first phase, participants had 10 s to walk to the left red grassy area or to right blue grassy area for the next phase of 90 s. A mini-map at the top of screen displayed a bird's eye view of where the participant was currently located in the larger area as the participants only had limited view of the area around them. During the second phase of 90 s the participant's task was to capture hexagons (food items with a caloric value). Each participant had their own private red and blue grassy areas for capturing hexagons so that each individual's experience with collecting food was independent of the others. The calories from successfully captured hexagons were processed at the conclusion of the third phase of 50 s.
If the participant chose the left red grassy area (HV strategy), the computer software randomly spawned three hexagons that independently moved around a red grassy area. Once avatar entered the red or blue area, the other side was unavailable to the participant (blacked out) until the next period of decision. The hexagons moved in independent straight lines to a randomly chosen location unknown to the participants. After the hexagon reached its destination, it immediately embarked on a path towards another randomly chosen location. If successfully captured with probability 0.15, a hexagon in the red grassy area yielded 36 calories which were displayed numerically on the avatar. If the participant was unsuccessful with probability 0.85, the hexagon eluded capture and was never seen again. Thus, each hexagon was an independent trial that followed a binomial distribution. With probability 0.853 = 0.6141, a participant would capture no prey, and with probability 0.3859 the participant would capture at least one 36-calorie hexagon.
In the right blue grassy area (LV strategy), the computer randomly spawned X small hexagons from the discrete uniform distribution f(x) = 0.25, for x = 7, 8, 9 and 10. Each small hexagon in the blue grassy area was worth one calorie and also moved in an independent straight line to a randomly chosen location unbeknownst to the participants. However, unlike the 36-calorie hexagons on the left, once a participant clicks on a blue grassy area hexagon, it was successfully captured with probability 1.
When the 90-second capturing phase ended, the software automatically marched the avatar back to the centre area of the screen at which point the participant retook control of the avatar's movement. At this point in the period, the left and right areas were both blacked out. Once back in the central area, the software prevented the participant from returning to the red and blue grassy areas until the start of the next period. If two avatars were in the same vicinity of the central area, they could chat via a speech bubble seen above their head. Anyone in the same viewable area could see the chat of anyone in the same area.
To convert the captured calories into earnings, a participant set up a pot, which was the same colour as the individual. Anyone who had seen a pot in a viewable area could see the pot displayed in the mini-map. A participant had to transfer his calories to his pot before the third phase (and period) ended. When period ended, the calories in the pot were converted into health units and added to the current health total for the individual. Each pot had a maximum capacity of 18 calories. If an avatar had more than 18 calories on his person, only 18 could be moved to the pot. The remaining calories stayed on the avatar or they could be moved to another pot or another avatar. Any calories remaining on an avatar disappeared when the period ended and were wasted. Avatars could only move their calories to pots and other avatars; they could not move food from other pots or avatars.
The calories in a pot were added to the health index of an individual when the first phase of a new period began. The health index at the beginning of the period was converted into cash earnings at the rate of one health point to one US cent. Each participant began the experiment with an initial health index of 30. The maximum health of an avatar was 100 and the minimum 0. At the end of the 10-second first phase (as avatars were preparing to enter the red or blue grassy area), the health index of every individual was decremented by eight health points due to metabolism. If the health index was less than eight, then the health index dropped to zero. In one half of the sessions, the current level of the health index was displayed on the avatar, and in the other half it was not. We did not find any significant effects of this difference in information.
If a participant chose the right blue grassy area (the LV strategy), his expected calorie yield was 8.5 per day. This was greater than the expected calorie yield of 6.95 (=18 × 0.3859) from the HV strategy without sharing. If two participants teamed up to share calories from HV strategy when one of them was not successful, their expected calorie yield was 11.21 [=18 × (1−0.61412)] which was greater than the LV strategy. With a metabolism of eight health units per day, a participant that chose LV every period would earn $7.05 on average and would end the experiment with a health index of 40. Two participants who shared returns from the HV strategy every period would on average add 3.21 health points per day and could reach a health index of 94.2 in 20 periods. A pair of HV participants who shared for the entire experiment would earn $12.74 on average. The health index of a loner HV participant would erode every period.
Acknowledgments
We gratefully acknowledge the financial support of Chapman University. We also thank Jeffrey Kirchner for software programming and Jennifer Cunningham for recruiting the participants.
Endnote
Crockett et al. [16] report that experimental participants in successful groups (about 75%) discover exchange and learn to specialize by voluntarily forming complementary pairs and engaging in sharing contracts that over time approach maximization of their individual and joint payoffs.
References
- 1.Hill K. R., et al. 2011. Co-residence patterns in hunter–gatherer societies show unique human social structure. Science 331, 1286–1289 10.1126/science.1199071 (doi:10.1126/science.1199071) [DOI] [PubMed] [Google Scholar]
- 2.Isaac G. 1978. The food-sharing behavior of protohuman hominids. Sci. Am. 238, 90–108 10.1038/scientificamerican0478-90 (doi:10.1038/scientificamerican0478-90) [DOI] [PubMed] [Google Scholar]
- 3.Kaplan H., Hill K. 1985. Food-sharing among Ache foragers: tests of explanatory hypotheses. Curr. Anthropol. 26, 223–245 10.1086/203251 (doi:10.1086/203251) [DOI] [Google Scholar]
- 4.Kaplan H., Hill K., Lancaster J. B., Hurtado A. M. 2000. A theory of human life history evolution: diet, intelligence, and longevity. Evol. Anthropol. 9, 156–185 (doi:10.1002/1520-6505(2000)9:4<156::AID-EVAN5>3.0.CO;2-7) [DOI] [Google Scholar]
- 5.Kaplan H. S. 1997. The evolution of the human life course. In Between Zeus and Salmon: the biodemography of aging (eds Wachter K., Finch C.), pp. 175–211 Washington, DC: National Academy of Sciences [Google Scholar]
- 6.Hill K., Hawkes K. 1983. Neotropical hunting among the Ache of Eastern Paraguay. In Adaptive responses of native Amazonians (eds Hames R., Vickers W.), pp. 139–188 New York, NY: Academic Press [Google Scholar]
- 7.Gurven M., Hill K., Kaplan H., Hurtado M., Lyles R. 2000. Food transfers among Hiwi foragers of Venezuela: tests of reciprocity. Hum. Ecol. 28, 171–218 10.1023/A:1007067919982 (doi:10.1023/A:1007067919982) [DOI] [Google Scholar]
- 8.Hawkes K., O'Connell J. F., Blurton Jones N. 2001. Hadza meat sharing. Evol. Hum. Behav. 22, 113–142 10.1016/S1090-5138(00)00066-0 (doi:10.1016/S1090-5138(00)00066-0) [DOI] [PubMed] [Google Scholar]
- 9.Blurton Jones N. 1987. Tolerated theft, suggestions about the ecology and evolution of sharing, hoarding, and scrounging. Soc. Sci. Info. 26, 31–54 10.1177/053901887026001002 (doi:10.1177/053901887026001002) [DOI] [Google Scholar]
- 10.Blurton Jones N., Marlowe F. W., Hawkes K., O'Connell J. F. 2000. Hunter–gatherer divorce rates and the paternal investment theory of human pair-bonding. In Human behavior and adaptation: an anthropological perspective (eds Cronk L., Chagnon N., Irons W.), pp. 237–260 Hawthorne, NY: Aldine de Gruyter [Google Scholar]
- 11.Gurven M. 2004. To give and to give not: the behavioral ecology of human food transfers. Behav. Brain Sci. 27, 543–583 [Google Scholar]
- 12.Hames R. 2000. Reciprocal altruism in Yanomamo food exchange. In Human behavior and adaptation: an anthropological perspective (eds Chagnon N., Cronk L., Irons E.), pp. 397–416 New York, NY: Aldine de Gruyter [Google Scholar]
- 13.Gurven M., Allen-Arave W., Hill K., Hurtado M. 2001. Reservation food sharing among the Ache of Paraguay. Hum. Nat. 12, 273–298 10.1007/s12110-001-1000-3 (doi:10.1007/s12110-001-1000-3) [DOI] [PubMed] [Google Scholar]
- 14.Winterhalder B. 1986. Diet choice, risk, and food sharing in a stochastic environment. J. Anthropol. Archaeol. 5, 369–392 10.1016/0278-4165(86)90017-6 (doi:10.1016/0278-4165(86)90017-6) [DOI] [Google Scholar]
- 15.Boyd R., Richerson P. J. 1988. The evolution of reciprocity in sizeable groups. J. Theor. Biol. 132, 337–356 10.1016/S0022-5193(88)80219-4 (doi:10.1016/S0022-5193(88)80219-4) [DOI] [PubMed] [Google Scholar]
- 16.Crockett S., Smith V., Wilson B. 2009. Exchange and specialisation as a discovery process. Econ. J. 119, 1162–1188 10.1111/j.1468-0297.2009.02254.x (doi:10.1111/j.1468-0297.2009.02254.x) [DOI] [Google Scholar]
- 17.McCloskey D. 2010. Bourgeois dignity: Why economics can't explain the modern world. Chicago, IL: The University of Chicago Press [Google Scholar]
- 18.Wilson B., Jaworski T., Schurter K., Smyth A. 2012 The ecological and civil mainsprings of property: an experimental economic history of whalers’ rules of capture. J. Law Econ. Organ. 10.1093/jleo/ewr024 (doi:10.1093/jleo/ewr024) [DOI] [Google Scholar]
- 19.Betzig L., Turke P. W. 1986. Food sharing on Ifaluk. Curr. Anthropol. 27, 397–400 10.1086/203457 (doi:10.1086/203457) [DOI] [Google Scholar]
- 20.Kaplan H., Hill K. 1985. Hunting ability and reproductive success among male Ache foragers. Curr. Anthropol. 26, 131–133 10.1086/203235 (doi:10.1086/203235) [DOI] [Google Scholar]
- 21.Patton J. 2005. Meat sharing for coalitional support. Evol. Hum. Behav. 26, 137–157 10.1016/j.evolhumbehav.2004.08.008 (doi:10.1016/j.evolhumbehav.2004.08.008) [DOI] [Google Scholar]
- 22.Sugiyama L., Chacon R. 2000. Effects of illness and injury on foraging among the Yora and Shiwar: pathology risk as adaptive problem. In Human behavior and adaptation: an anthropological perspective (eds Cronk L., Irons W., Chagnon N.), pp. 371–396 New York, NY: Aldine de Gruyter [Google Scholar]


