Abstract
Increasing empirical evidence indicates the number of released individuals (i.e. propagule pressure) and number of released species (i.e. colonization pressure) are key determinants of the number of species that successfully invade new habitats. In view of these relationships, and the possibility that ships transport whole communities of organisms, we collected 333 ballast water and sediment samples to investigate the relationship between propagule and colonization pressure for a variety of diverse taxonomic groups (diatoms, dinoflagellates and invertebrates). We also reviewed the scientific literature to compare the number of species transported by ships to those reported in nature. Here, we show that even though ships transport nearly entire local communities, a strong relationship between propagule and colonization pressure exists only for dinoflagellates. Our study provides evidence that colonization pressure of invertebrates and diatoms may fluctuate widely irrespective of propagule pressure. We suggest that the lack of correspondence is explained by reduced uptake of invertebrates into the transport vector and the sensitivity of invertebrates and diatoms to selective pressures during transportation. Selection during transportation is initially evident through decreases in propagule pressure, followed by decreased colonization pressure in the most sensitive taxa.
Keywords: ballast sediment, ballast water, diatoms, dinoflagellates, invertebrates, selective pressure
1. Introduction
Biological invasions by non-indigenous species (NIS) are pervasive and considered by many researchers as a leading threat to biodiversity [1]. While early studies sought to relate invasion success mainly to species traits and/or biotic and abiotic characteristics of the recipient habitat, recent empirical and statistical evidence suggests that propagule pressure (PP; i.e. number of individuals released) is of paramount importance [2–5]. The number of NIS in an ecosystem may, more generally, simply reflect the number of species introduced [4,6].
Based on random sampling theory [7], where larger sample sizes increase the probability of inclusion of rare species, a recent study used log series modelling to simulate the relationship between colonization pressure (CP; i.e. number of species released) and PP for situations when individuals are randomly entrained in and released from a transport vector [4]. When only a small proportion of individuals were released, no relationship was observed between the parameters; however, as the proportion of the total community released increased, both PP and CP increased, the former by a wider margin. Consequently, larger inocula result in a higher probability of establishment of NIS through a combination of higher CP and higher PP [4]. If supported by empirical data, this model could have important implications for management of NIS invasions. For example, managers could identify high-risk, species-rich events within a transport vector by measuring the total number of individuals rather than conducting difficult and time-consuming taxonomic surveys. While several studies have explored the relationship between abundance and species richness for different taxa in nature, results have been mixed [8–12]. To our knowledge, no similar studies exist for any transport vector, although the modelling study used a ballast water scenario when developing their conceptual model [4]. In theory, natural patchiness exhibited by plankton [13,14] could produce results counter to the model predictions [4] if uptake of biota into the transport vector was non-random. Further, even if a transport vector entrains species randomly, differential mortality or reproduction during transportation could skew the diversity and/or abundance of organisms released [15–17].
The shipping industry transports over 90 per cent of world trade [18] and represents a leading mechanism for spread of aquatic NIS globally [19–21]. Ballast water typically contains abundant and diverse aquatic life, including viable resting stages of many species within ballast tank sediment [15,16,22–25]. Over the past two decades, Canada and numerous additional countries around the world have implemented ballast water regulations to reduce the risk of new NIS introductions, though efficacy of these policies is mixed [23–33].
We tested the relationship between CP and PP by collecting and analysing ballast water and sediment samples for invertebrates, dinoflagellates and diatoms in ships visiting ports in Canada and the USA. We tested three hypotheses: (i) CP of a particular taxon is positively related to PP of that same taxon; (ii) non-indigenous PP and CP of a particular taxon is related to indigenous PP and CP of that same taxon; and (iii) PP and CP of any one taxon is related to the PP and the CP of other taxa. Additionally, we tested the model's assumption that taxa are entrained into and survive in ballast tanks randomly [4] by conducting a literature survey of the number of species reported in nature, and analysing natural water samples collected in European and Asian ports, and ballast water samples collected immediately after ballast was loaded at European or Asian ports into tanks of ships destined for Canada. We used these data to determine whether CP associated with ballast water of commercial ships is equivalent to that of natural communities.
While PP and CP are properly defined as measures of NIS released in a particular location, we use PP and CP to denote total abundance and species richness transported in ships' tanks, and NIS PP and NIS CP to denote abundance and richness of non-indigenous taxa discharged into recipient ports. This distinction was made to take into account the fact that shipping networks are truly global, while the designation of species as non-indigenous to the recipient habitat is trip-specific, enabling examination of patterns independent of ballast discharge location.
2. Material and methods
(a). Sampling, density counts and identification
Three hundred and thirty-three ballast water and ballast sediment samples were collected between May 2007 and August 2009 inclusive, from ships arriving at Pacific and Atlantic ports in Canada, and Laurentian Great Lakes' ports. Ships sampled serviced both international and domestic routes, with some ships having performed mid-ocean ballast water exchange (hereafter referred to as exchanged ships) and others not (hereafter, unexchanged ships). Unexchanged ships arrived from American or Canadian ports north of Cape Blanco, Oregon, on the Pacific coast and north of Cape Cod, MA, USA on the Atlantic coast, while exchanged ships arrived from any other global port, in accordance with local laws [32,33].
We collected 62 exchanged and 24 unexchanged ballast water samples in the Pacific region, 46 exchanged and 21 unexchanged samples in the Atlantic region, and 21 exchanged samples in the Great Lakes (electronic supplementary material, appendix S1). Sample sizes for ballast sediment were 52 exchanged and 21 unexchanged in the Pacific region, 46 exchanged and 21 unexchanged in the Atlantic region, and 19 exchanged in the Great Lakes region (electronic supplementary material, appendix S1). We attempted to collect a single ballast water or ballast sediment sample from each ship to ensure independence of samples. On 32 occasions, samples were collected from two tanks on the same ship which had different ballast histories, thus, sample independence was maintained. Additionally, nine natural water samples were collected in European and Asian ports and 12 ballast water samples were collected immediately after ballast was loaded at European or Asian ports into tanks of ships destined for Canada; the European and Asian ports sampled were the ports of origin for most ships sampled at arrival in Canada and/or the Great Lakes.
As a part of the Canadian Aquatic Invasive Species Network, samples collected during this study were divided and processed simultaneously by multiple academic and government laboratories, each with taxon-specific expertise. Methods for sample collection, enumeration and identification may be found in the scientific literature [15,16,23–25,34,35]. Port samples were collected and processed following the same methods as for ballast water.
(b). Literature review
We used Thomson Reuters' Web of Knowledge 5.3 Scientific Citation Index Expanded (SCI-EXPANDED), in July 2011, to search for reports of plankton species richness at single sampling stations from any region in the world published between 1965 and 2011. We used the search terms ‘species’, ‘richness’ and ‘diversity’ singly and combined with ‘plankton’, ‘zooplankton’, ‘phytoplankton’, ‘invertebrates’, ‘dinoflagellates’ and ‘diatoms’. Search results were then refined by subject area, retaining only field topics broadly relevant to ecology, including ecology, biodiversity conservation, environmental sciences, marine freshwater biology, oceanography, freshwater biology, biology and zoology. Recovered publications were reviewed for location-specific data on species richness. As we were interested in species richness of local communities (e.g. a specific port or bay) which may interact with ships' ballasting operations, publications reporting total species diversity for large geographical areas (e.g. entire lake, sea or river) were excluded.
(c). Statistical analysis
Based on previous research, data for CP : PP relationship analyses were grouped into three categories: exchanged water samples, unexchanged water samples and sediment [15,23–25]. Abundance and species richness data for each taxonomic group were log-transformed (log(x + 1)) to meet assumptions of parametric tests, allowing for comparisons between taxa. Samples containing zero individuals were excluded from analysis since no relationship between CP and PP is expected when a low proportion of individuals are released [4]. A series of model II regression analyses were conducted for every taxon with PP as the predictor variable to determine if CP is related to PP. Regression slopes and intercepts of significant relationships were compared statistically [36]. Ten pairwise comparisons were conducted to discriminate differences in regression estimates among different ship categories, and among different taxa. Further, we used model II regression analyses to test whether NIS PP and CP were related to comparable values for indigenous species for each taxon. Finally, we conducted model II regression analyses to determine whether PP and CP for one taxon could be used to predict results of another.
Literature reports of species richness in nature and results from our port samples were grouped together for analysis of natural local community richness. Natural local community richness was compared with that from ships' samples using analyses of variance (ANOVA) and the t-test. Data for each taxonomic group were log-transformed (log(x + 1)) to meet assumptions of parametric tests. Two different ANOVAs were applied to compare natural local species richness of two taxa (invertebrates and diatoms) to that of ballast water samples collected at the beginning of voyages and to that of ballast water samples collected at the end of voyages. Furthermore, a t-test was applied to compare richness of dinoflagellates from ballast water to that of natural local communities. All statistical analyses were conducted using SYSTAT v. 11 (SYSTAT Software Inc., 2004). Significance levels for statistical comparisons were adjusted for multiple pairwise comparisons by Bonferroni-type correction with a family-wise error rate of 0.05.
3. Results
(a). Relationship between propagule and colonization pressure
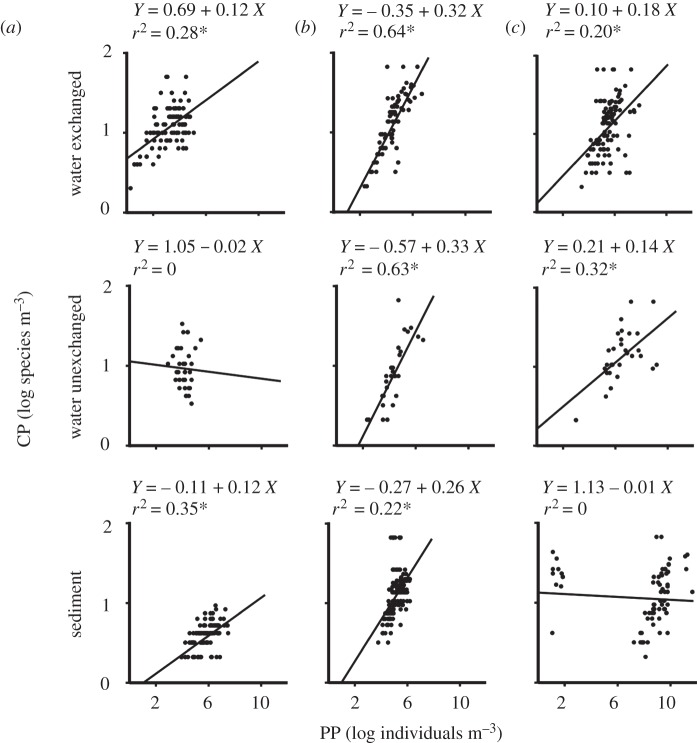
CP was positively related to PP for all three taxa examined in water of exchanged ships, though there was no such relationship for invertebrates in water of unexchanged ships or for diatoms in sediment (figure 1). The relationship between CP and PP was strongest for dinoflagellates in both exchanged and unexchanged water samples (figure 1). The regression line slope for the CP : PP relationship was significantly steeper for dinoflagellates than for invertebrates or diatoms in all cases, suggesting that incremental additions of new individuals to ballast tanks preferentially favours inclusion of new dinoflagellate species, or that they were less sensitive to biotic and abiotic conditions during transport (table 1 and figure 1). The CP : PP slope did not differ between invertebrates and diatoms in exchanged water samples, although the intercept was higher for the former group (table 1 and figure 1). There was no significant difference in the CP : PP slope for invertebrates in samples from exchanged water or sediment samples, although the intercept was higher for the former (table 1 and figure 1). For dinoflagellates, CP : PP slopes for both exchanged and unexchanged water samples differed from that of sediment, although the former two did not differ from each other. Estimated intercepts for dinoflagellates were significantly different between all three ship categories (table 1 and figure 1). The significantly higher intercept for exchanged when compared with unexchanged water samples, while having the same CP : PP slope, indicates that additional species of dinoflagellates were probably taken into tanks during mid-ocean ballast water exchange (table 1 and figure 1). Diatoms in exchanged water exhibited a significantly steeper CP : PP slope than those in unexchanged water, though their intercepts were similar (table 1 and figure 1), suggesting that mid-ocean ballast water exchange reduces PP but not CP of diatoms.
Figure 1.
Scatterplots and fitted regression lines with propagule pressure (PP) as the independent variable and colonization pressure (CP) as the dependent variable for (a) invertebrates, (b) dinoflagellates and (c) diatoms in exchanged and unexchanged water, and sediment samples. All data are log-transformed. Asterisks denote significance at 0.05.
Table 1.
Results of t-tests comparing the estimated regression coefficients (slope and intercept) of analyses where propagule pressure (PP) was a predictor variable for colonization pressure (CP). (Tests were conducted between different taxa (invertebrates, dinoflagellates and diatoms) for different ship categories (exchanged water, unexchanged water and sediment samples), and between different ship categories for different taxa. Significant p-values are presented in bold.)
| slope |
intercept |
||||||
|---|---|---|---|---|---|---|---|
| comparison | d.f. | t | p | d.f. | t | p | |
| ship category | |||||||
| exchanged water | invertebrates–dinoflagellates | 144 | 17.29 | <0.001 | 145 | 6.07 | <0.001 |
| invertebrates–diatoms | 179 | 0.73 | 0.466 | 180 | 12.03 | <0.001 | |
| dinoflagellates–diatoms | 179 | 16.57 | <0.001 | 180 | 5.08 | <0.001 | |
| unexchanged water | dinoflagellates–diatoms | 52 | 11.55 | <0.001 | 53 | 1.16 | 0.25 |
| sediment | invertebrates–dinoflagellates | 199 | 25.55 | <0.001 | 200 | 11.53 | <0.001 |
| taxa | |||||||
| invertebrates | exchanged water–sediment | 183 | 0.00 | 1.00 | 184 | 11.93 | <0.001 |
| dinoflagellates | exchanged water–unexchanged water | 97 | 0.74 | 0.46 | 98 | 3.41 | <0.001 |
| exchanged water–sediment | 199 | 8.11 | <0.001 | 200 | 3.97 | <0.001 | |
| unexchanged water–sediment | 154 | 5.14 | <0.001 | 155 | 0.37 | 0.71 | |
| diatoms | exchanged water–unexchanged water | 99 | 3.76 | <0.001 | 100 | 1.27 | 0.20 |
(b). Relationship between indigenous and non-indigenous species propagule and colonization pressures
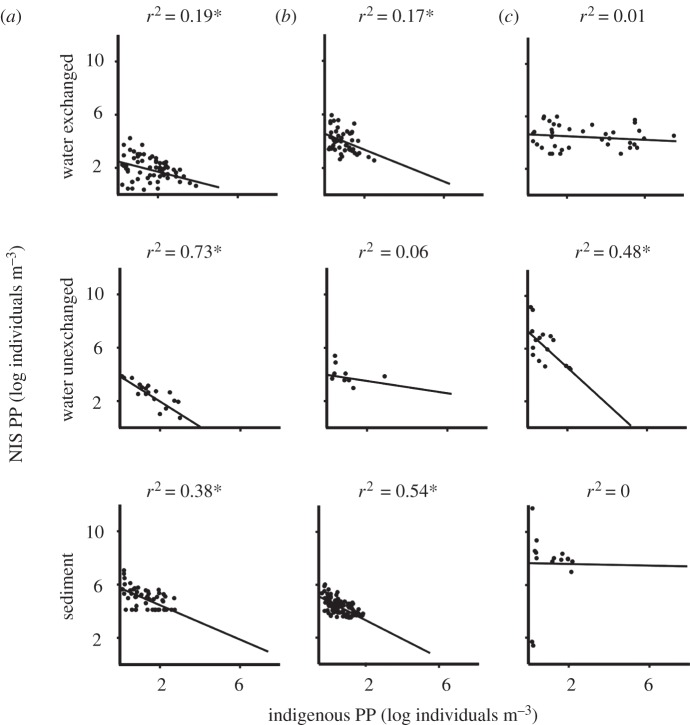
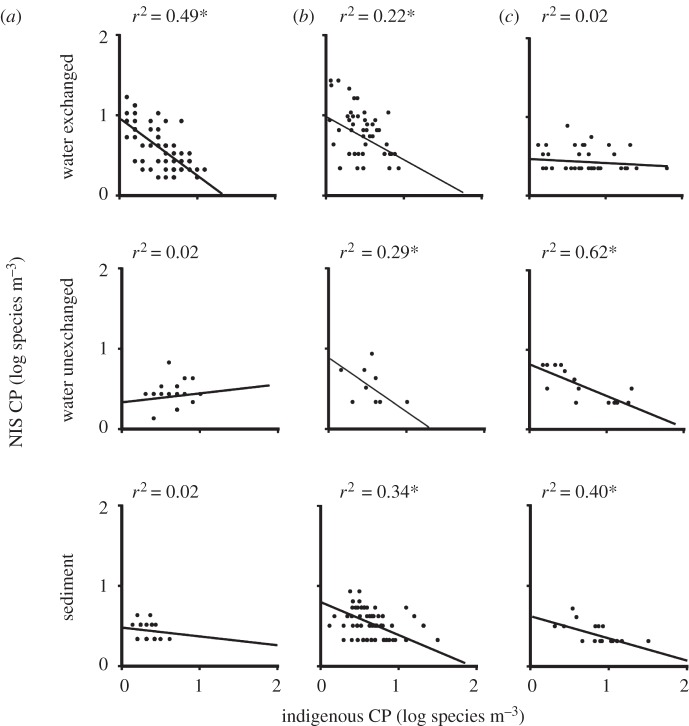
All significant relationships between indigenous and NIS PP were negative (figure 2), indicating that ships transporting a higher abundance of indigenous species have lower abundance of NIS, and vice versa. The strongest relationships were observed for invertebrates and diatoms in unexchanged water samples and for dinoflagellates in sediment (figure 2). All significant relationships between indigenous and NIS CP were also negative (figure 3), suggesting that ships transporting higher species richness of indigenous species have lower species richness of NIS, and vice versa. The relationship between indigenous and NIS CP was strongest for diatoms in unexchanged water and invertebrates in exchanged water samples (figure 3).
Figure 2.
Scatterplots and fitted regression lines with indigenous propagule pressure (PP) as the independent variable and non-indigenous propagule pressure (NIS PP) as the dependent variable for (a) invertebrates, (b) dinoflagellates and (c) diatoms in exchanged and unexchanged water, and sediment samples. All data are log-transformed. Asterisks denotes significance at 0.05.
Figure 3.
Scatterplots and fitted regression lines with indigenous colonization pressure (CP) as the independent variable and non-indigenous colonization pressure (NIS CP) as the dependent variable for (a) invertebrates, (b) dinoflagellates and (c) diatoms in exchanged and unexchanged water, and sediment samples. All data are log-transformed. Asterisks denotes significance at 0.05.
(c). Relationships between different taxa
PP of invertebrates, dinoflagellates and diatoms were significantly, though weakly, related to each other in water of exchanged ships (r2 between 0.04 and 0.10) although there was no relationship in unexchanged ships or in sediment. There also was no relationship of CP, NIS PP or NIS CP between any of the taxa examined.
(d). Species richness in nature and at initial uptake versus after transportation
Colonization pressure of dinoflagellates and diatoms associated with ballast water of commercial ships were equivalent to that of natural communities (table 2). However, in the case of invertebrates during initial uptake of ballast, but also during the voyage, some selection occurred resulting in reduced CP in ballast water compared with natural communities (table 2). Collectively, species richness of invertebrates in water was significantly higher in nature than at the beginning of a voyage, and both values were significantly higher than that recovered at the end of a voyage (F2,190 = 82.2, p < 0.001). Conversely, there was no difference in the number of dinoflagellate or diatom species reported in natural habitats when compared with ships' water samples (t = −1.1, p = 0.25; F2,161 = 2.8, p = 0.059, respectively). Zero, 10 and 1 per cent of ships' water samples and 21, 2 and 50 per cent of ships' sediment samples did not contain any species of invertebrates, dinoflagellates and diatoms, respectively. The absence of taxa may be owing to seasonality of plankton [27], but also owing to sample volume, very low abundances may not be detected.
Table 2.
Mean species richness (min−max) from nature, from ballast water sampled at initial uptake (start of voyage), and from ballast water sampled after transportation (end of voyage). (no., denotes the number of samples.)
| nature |
ships: initial uptake |
ship: end of voyage |
||||
|---|---|---|---|---|---|---|
| taxa | no. | mean species richness (min–max) | no. | mean species richness (min–max) | no. | mean species richness (min–max) |
| invertebrates | 38 | 49.9 (10–59) [37–40] | 12 | 23.5 (12–40) | 141 | 12.1 (0–53) |
| dinoflagellates | 7 | 15.3 (2–39) [39,41–43] | n.a. | n.a. | 114 | 10.8 (0–39) |
| diatoms | 16 | 17.8 (1–64) [39,41–43] | 8 | 12.7 (5–20) | 137 | 11.8 (0–38) |
4. Discussion
In situations where species introductions can be viewed as the result of a random sampling process, the number of species introduced should be positively related to the number of individuals introduced [4,7]. Using a large empirical dataset for the ballast transport vector, we found such a relationship between CP and PP for dinoflagellates, but a very weak to non-existent relationship for two additional taxa (invertebrates and diatoms). The generally absent to weak relationship between CP and PP suggests either that species are not taken up randomly by the transport vector or that selective pressures such as harsh environmental conditions [16,17,44,45] and/or biotic interactions [45,46] obscure the presence of this relationship. By comparing species richness in natural local communities to CP at initial uptake, or in the case of dinoflagellates at discharge, it appears likely that ships randomly entrain almost entire local communities of dinoflagellates and diatoms, but not invertebrates, into ballast tanks. Differences in uptake of phytoplankton and zooplankton may be owing to active avoidance by zooplankton, particularly for bigger species. Selection during ballast uptake and transport appear to be important factors in determining the pool of species available to colonize new areas.
Most of the CP : PP relationships in this study exhibit a relatively large range for CP and much narrower range for PP, indicating that selective pressures during transport mostly reduced PP. This finding is supported by the similarity between species richness of native local communities and CP before and after transport for diatoms and CP after transport for dinoflagellates. A previous study also showed reduction in PP and no reduction in CP for diatoms [17]. For diatoms, the relatively high decrease in PP, particularly for ships with relatively high CP, led to weakening of the CP : PP relationship. The opposite was observed for dinoflagellates, where the CP : PP relationship was very strong, suggesting little or no influence of transport on either CP or PP in water, although small reductions in PP were observed for sediment. The CP : PP relationship for invertebrates was more complicated. As, on average, less than 50 per cent of species in nature were taken into the transport vector, and we observed a significant decrease in CP after transport, we assume that PP also must have decreased. Collectively, the results for different taxa indicate that selection during transport initially reduces PP, followed by loss of CP for more sensitive taxonomic groups. While this is, to our knowledge, the first study to examine the role of environmental factors on the relationship between density and species richness in a transport vector, the CP : PP relationship was dependent on water depth for aquatic gastropods and nematodes [8,10]. A strong environmental influence on the community composition of lizard species was also documented with higher temperatures creating more species-rich communities without changes in abundance of individuals, and weakening the relationship between abundance and species richness [11,12].
The abundance and diversity of taxa within a vector ultimately depend on the community composition in the source region. The inverse relationship observed between indigenous and NIS supports the intuitive idea that a transport vector taking taxa farther from the point of discharge will transport a proportionately higher abundance and diversity of NIS and consequently pose a higher invasion risk. However, the same negative relationship between indigenous and NIS PP and indigenous and NIS CP was exhibited by the transport vector operating within a single biogeographic area (i.e. unexchanged ships). The negative relationship between diversity of indigenous and NIS within a biogeographic region is consistent with, but not proof of, biotic resistance (sensu [47]). Given that the strongest relationships were observed for diatoms, for which even native marine biodiversity in Canada is understudied [48], this counterintuitive finding might also result from numerous undetected invasions and/or cryptic species (i.e. status as native or NIS unknown [49,50]). Additionally, it is unlikely that a literature review will provide an exhaustive description of the full diversity of any area.
Contrary to the model prediction that increases in PP should be followed by increases in CP and vice versa [4], our study provides evidence that CP of invertebrates and diatoms may fluctuate widely irrespective of PP. This finding suggests that a decrease in PP during the transportation phase is not necessarily connected with a decrease in CP, leaving open the question if reduced PP, but no change in CP, through vector management would directly (linearly) impact invasion risk? The PP model suggests that the probability of successful invasion is reduced by reducing the number of inoculation events or by reducing the number of propagules released per event, owing to increasing environmental and demographic stochasticity, respectively [3–5]. Even though our data suggest that CP may not diminish markedly with a reduction in propagule delivery, the expected increase in demographic limitations owing to reduced PP is expected to reduce overall invasion success. To further estimate invasion success, abiotic parameters of donor and recipient habitats should be considered. Although PP and CP may be high, a species' success is unlikely if the donor and recipient habitats are very different in their abiotic parameters; marine species probably will not establish in freshwater bodies even if introduction effort is high. We anticipate that additional efforts to explore the CP : PP relationship could greatly increase understanding of the invasion process, which may, in turn, be used to reduce risks of new invasions globally.
The results of this study show that even though some transport vectors entrain nearly entire local communities, the relationship between CP and PP is taxon-specific and strongest for dinoflagellates. We suggest that the CP : PP relationship is weakened or even lost for some taxa owing to selective uptake by the transport vector (invertebrates) and/or survival during transport (invertebrates and diatoms). Selection during transportation is initially evident through decreases in PP, followed by decreased CP in the most sensitive taxa. Selective pressures during uptake and transport of taxa by a vector are important factors in the invasion process.
Acknowledgements
We thank participating shipping companies, crews, port authorities and the Shipping Federation of Canada for facilitating access to ships. We are grateful to our sampling teams: C. van Overdijk, J.-Y. Couture, M. Huot, C. Owens, O. Lacasse, K. MacIntosh, D. Humphrey, S. Ballard, M. Whitehead, P. Lolic, G. Leung, F. Choi and P. Luk. Many thanks to J. Young and Dr A. Drake and Dr W. Currie for statistical advice, and to Dr G. Klein for diatom identification and enumeration. This research was supported by the Natural Sciences and Engineering Research Council's (NSERC) Canadian Aquatic Invasive Species Network, Transport Canada, Fisheries and Oceans Canada and NSERC Discovery grants to S.A.B. and H.J.M., and by a NSERC Discovery Accelerator Supplement to H.J.M.
References
- 1.McGeoch M. A., Butchart S. H. M., Spear D., Marais E., Kleynhans E. J., Symes A., Chanson J., Hoffman M. 2010. Global indicators of biological invasion: species numbers, biodiversity impact and policy responses. Divers. Distrib. 16, 95–108 10.1111/j.1472-4642.2009.00633.x (doi:10.1111/j.1472-4642.2009.00633.x) [DOI] [Google Scholar]
- 2.Von Holle B., Simberloff D. 2005. Ecological resistance to biological invasion overwhelmed by propagule pressure. Ecology 86, 3212–3218 10.1890/05-0427 (doi:10.1890/05-0427) [DOI] [Google Scholar]
- 3.Colautti R. I., Grigorovich I. A., MacIsaac H. J. 2006. Propagule pressure: a null model for biological invasions. Biol. Invasion 8, 1023–1037 10.1007/s10530-005-3735-y (doi:10.1007/s10530-005-3735-y) [DOI] [Google Scholar]
- 4.Lockwood J. L., Cassey P., Blackburn T. 2009. The more you introduce the more you get: the role of colonization pressure and propagule pressure in invasion ecology. Divers. Distrib. 15, 904–910 10.1111/j.1472-4642.2009.00594.x (doi:10.1111/j.1472-4642.2009.00594.x) [DOI] [Google Scholar]
- 5.Simberloff D. 2009. The role of propagule pressure in biological invasions. Ann. Rev. Ecol. Evol. Syst. 40, 81–102 10.1146/annurev.ecolsys.110308.120304 (doi:10.1146/annurev.ecolsys.110308.120304) [DOI] [Google Scholar]
- 6.Chiron F., Shirley S., Kark S. 2009. Human-related processes drive the richness of exotic birds in Europe. Proc. R. Soc. B 276, 47–53 10.1098/rspb.2008.0994 (doi:10.1098/rspb.2008.0994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Preston F. W. 1948. The commonness, and rarity of species. Ecology 29, 254–283 10.2307/1930989 (doi:10.2307/1930989) [DOI] [Google Scholar]
- 8.McClain C. R. 2004. Connecting species richness, abundance and body size in deep-sea gastropods. Global Ecol. Biogeogr. 13, 327–334 10.1111/j.1466-822X.2004.00106.x (doi:10.1111/j.1466-822X.2004.00106.x) [DOI] [Google Scholar]
- 9.Bock C. E., Jomes Z. F., Bock J. H. 2007. Relationships between species richness, evenness, and abundance in a southwestern savanna. Ecology 88, 1322–1327 10.1890/06-0654 (doi:10.1890/06-0654) [DOI] [PubMed] [Google Scholar]
- 10.Fonseca G., Muthumbi A. W., Vanreusel A. 2007. Species richness of the genus Molgolaimus (Nematoda) from local to ocean scale along continental slopes. Mar. Ecol. 28, 446–459 10.1111/j.1439-0485.2007.00202.x (doi:10.1111/j.1439-0485.2007.00202.x) [DOI] [Google Scholar]
- 11.Buckley L. B., Jetz W. 2010. Lizard community structure along environmental gradients. J. Appl. Ecol. 79, 358–365 10.1111/j.1365-2656.2009.01612.x (doi:10.1111/j.1365-2656.2009.01612.x) [DOI] [PubMed] [Google Scholar]
- 12.Nimmo D. G., James S. G., Kelly L. T., Watson S. J., Bennett A. F. 2011. The decoupling of abundance and species richness in lizard communities. J. Appl. Ecol. 80, 650–656 10.1111/j.1365-2656.2010.01797.x (doi:10.1111/j.1365-2656.2010.01797.x) [DOI] [PubMed] [Google Scholar]
- 13.Strutton P. G., Mitchell J. G., Parslow J. S., Greene R. M. 1997. Phytoplankton patchiness: quantifying the biological contribution using Fast Repetition Rate Flourometry. J. Plankton Res. 19, 1265–1274 10.1093/plankt/19.9.1265 (doi:10.1093/plankt/19.9.1265) [DOI] [Google Scholar]
- 14.Folt C. L., Burns C. W. 1999. Biological drivers of zooplankton patchiness. Trends Ecol. Evol. 14, 300–305 10.1016/S0169-5347(99)01616-X (doi:10.1016/S0169-5347(99)01616-X) [DOI] [PubMed] [Google Scholar]
- 15.Briski E., Bailey S. A., Cristescu M. E., MacIsaac H. J. 2010. Efficacy of ‘saltwater flushing’ in protecting the Great Lakes from biological invasions by invertebrate eggs in ships’ ballast sediment. Freshwater Biol. 55, 2414–2424 10.1111/j.1365-2427.2010.02449.x (doi:10.1111/j.1365-2427.2010.02449.x) [DOI] [Google Scholar]
- 16.Klein G., MacIntosh K., Kaczmarska I., Ehrman J. M. 2010. Diatom survivorship in ballast water during trans-Pacific crossings. Biol. Invasion 12, 1031–1044 10.1007/s10530-009-9520-6 (doi:10.1007/s10530-009-9520-6) [DOI] [Google Scholar]
- 17.Simard N., Plourde S., Gilbert M., Gollasch S. 2011. Net efficacy of open ocean ballast water exchange on plankton communities. J. Plankton Res. 33, 1378–1395 10.1093/plankt/fbr038 (doi:10.1093/plankt/fbr038) [DOI] [Google Scholar]
- 18.Hulme P. E. 2009. Trade, transport and trouble: managing invasive species pathways in an era of globalization. J. Appl. Ecol. 46, 10–18 10.1111/j.1365-2664.2008.01600.x (doi:10.1111/j.1365-2664.2008.01600.x) [DOI] [Google Scholar]
- 19.Drake J. M., Lodge D. M. 2004. Global hot spots of biological invasions: evaluating options for ballast-water management. Proc R. Soc. Lond. B 271, 575–580 10.1098/rspb.2003.2629 (doi:10.1098/rspb.2003.2629) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tatem A. J., Hay S. I., Rogers D. J. 2006. Global traffic and disease vector dispersal. Proc Natl Acad. Sci. USA 103, 6242–6247 10.1073/pnas.0508391103 (doi:10.1073/pnas.0508391103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leuven R. S. E. W., van der Velde G., Baijens I., Snijders J., van der Zwart C., Lenders H. J. R., bij de Vaate A. 2009. The river Rhine: a global highway for dispersal of aquatic invasive species. Biol. Invasion 11, 1989–2008 10.1007/s10530-009-9491-7 (doi:10.1007/s10530-009-9491-7) [DOI] [Google Scholar]
- 22.Gollasch S., et al. 2000. Fluctuations of zooplankton taxa in ballast water during short-term and long-term ocean-going voyages. Int. Rev. Hydrobiol. 85, 597–608 (doi:10.1002/1522-2632(200011)85:5/6<597::AID-IROH597>3.0.CO;2-4) [DOI] [Google Scholar]
- 23.Casas-Monroy O., Roy S., Rochon A. 2011. Ballast sediment-mediated transport of non-indigenous species of dinoflagellates on the East coast of Canada. Aquat. Invasion 6, 231–248 10.3391/ai.2011.6.3.01 (doi:10.3391/ai.2011.6.3.01) [DOI] [Google Scholar]
- 24.Villac M. C., Kaczmarska I. 2011. Estimating propagule pressure and viability of diatoms detected in ballast tank sediments of ships arriving at Canadian ports. Mar. Ecol. Progr. Ser. 425, 47–61 10.3354/meps08999 (doi:10.3354/meps08999) [DOI] [Google Scholar]
- 25.DiBacco C., Humphrey D. B., Nasmith L. E., Levings C. D. In press Ballast water transport of non-indigenous zooplankton to Canadian ports. ICES J. Mar. Sci. (doi:10.1093/icesjms/fsr133) [Google Scholar]
- 26.Gray D. K., Johengen T. H., Reid D. F., MacIsaac H. J. 2007. Efficacy of open-ocean ballast water exchange as a means of preventing invertebrate invasions between freshwater ports. Limnol. Oceanogr. 5, 2386–2397 10.4319/lo.2007.52.6.2386 (doi:10.4319/lo.2007.52.6.2386) [DOI] [Google Scholar]
- 27.McCollin T., Shanks A. M., Dunn J. 2007. The efficiency of regional ballast water exchange: changes in phytoplankton abundance and diversity. Harmful Algae 6, 531–546 10.1016/j.hal.2006.04.015 (doi:10.1016/j.hal.2006.04.015) [DOI] [Google Scholar]
- 28.David M., Gollasch S. 2008. EU shipping in the dawn of managing the ballast water issue. Mar. Pollut. Bull. 56, 1966–1972 10.1016/j.marpolbul.2008.09.027 (doi:10.1016/j.marpolbul.2008.09.027) [DOI] [PubMed] [Google Scholar]
- 29.McCollin T., Shanks A. M., Dunn J. 2008. Changes in zooplankton abundance and diversity after ballast water exchange in regional seas. Mar. Pollut. Bull. 56, 834–844 10.1016/j.marpolbul.2008.02.004 (doi:10.1016/j.marpolbul.2008.02.004) [DOI] [PubMed] [Google Scholar]
- 30.Bailey S. A., Deneau M. G., Jean L., Wiley C. J., Leung B., MacIsaac H. J. 2011. Evaluating efficacy of an environmental policy to prevent biological invasions. Environ. Sci. Technol. 45, 2554–2561 10.1021/es102655j (doi:10.1021/es102655j) [DOI] [PubMed] [Google Scholar]
- 31.IMO (International Maritime Organization) 2004. International Convention for the Control and Management of Ships’ Ballast Water and Sediments; as adopted by consensus at a Diplomatic Conference at IMO, London, UK, 13 February 2004.
- 32.Government of Canada 2006. Ballast water control and management regulations. Canada Gazette 140 (13). See http://gazette.gc.ca/archives/p2/2006/2006-06-28/html/sor-dors129-eng.html [Google Scholar]
- 33.SLSDC (Saint Lawrence Seaway Development Corporation) 2008. Seaway Regulations and Rules: Ballast Water. Code of Federal Regulations 33-CFR Part 401.
- 34.Briski E., Cristescu M. E., Bailey S. A., MacIsaac H. J. 2011. Use of DNA barcoding to detect invertebrate invasive species from diapausing eggs . Biol. Invasion 13, 1325–1340 10.1007/s10530-010-9892-7 (doi:10.1007/s10530-010-9892-7) [DOI] [Google Scholar]
- 35.Roy S., Parenteau M., Casas-Monroy O., Rochon A. In press Coastal ship traffic: a significant introduction vector for potentially harmful dinoflagellates in Eastern Canada. Can. J. Fish. Aquat. Sci . (doi:10.1139/F2012-008) [Google Scholar]
- 36.Zar J. H. 1999. Comparing simple linear regression equations. In Biostatistical analyses (ed. Zar J. H.), pp. 360–376 Upper Saddle River, NJ: Prentice-Hall Inc [Google Scholar]
- 37.Lindley J. A., Batten S. D. 2002. Long-term variability in the diversity of North Sea zooplankton. J. Mar. Biol. Assoc. UK 82, 31–40 [Google Scholar]
- 38.Hunt B. P. V., Hoise G. W. 2003. The continuous plankton recorder in the Southern Ocean: a comparative analysis of zooplankton communities sampled by the CPR and vertical net hauls along 140° E. J. Plankton Res. 12, 1561–1579 10.1093/plankt/fbg108 (doi:10.1093/plankt/fbg108) [DOI] [Google Scholar]
- 39.Dermott R., Johannsson O., Munawar M., Bonnell R., Bowen K., Burley M., Fitzpatrick M., Gerlofsma J., Niblock H. 2007. Assessment of lower food web in Hamilton Harbour, Lake Ontario, 2002–2004. Canadian Technical Report of Fisheries and Aquatic Sciences 2729, pp. 182 See http://www.dfo-mpo.gc.ca/Library/332726.pdf
- 40.Eloire D., Somerfield P. J., Conway D. V. P., Halsband-Lenk C., Harris R., Bonnet D. 2010. Temporal variability and community composition of zooplankton at station L4 in the Western Channel: 20 years of sampling. J. Plankton Res. 32, 656–679 10.1093/plankt/fbq009 (doi:10.1093/plankt/fbq009) [DOI] [Google Scholar]
- 41.Longhi M. L., Beisner B. E. 2010. Patterns in taxonomic and functional diversity of lake phytoplankton. Freshwater Biol. 55, 1349–1366 10.1111/j.1365-2427.2009.02359.x (doi:10.1111/j.1365-2427.2009.02359.x) [DOI] [Google Scholar]
- 42.Aktan Y. 2011. Large-scale patterns in summer surface water phytoplankton (except picophytoplankton) in the Eastern Mediterranean. Estuar. Coast. Shelf Sci. 91, 551–558 10.1016/j.ecss.2010.12.010 (doi:10.1016/j.ecss.2010.12.010) [DOI] [Google Scholar]
- 43.Ghinaglia L. T., Herrera-Silveira J. A., Comín F. A. 2004. Structural variations of phytoplankton in the coastal seas of Yucatan, Mexico. Hydrobiologia 519, 85–102 10.1023/B:HYDR.0000026487.78497.b6 (doi:10.1023/B:HYDR.0000026487.78497.b6) [DOI] [Google Scholar]
- 44.Reid D. F., et al. 2007. A final report for the project ‘identifying, verifying, and establishing options for best management practices for NOBOB vessels’. Ann Arbor, MI: National Oceanic and Atmospheric Administration, Great Lakes Environmental Research Laboratory, and University of Michigan Cooperative Institute for Limnology and Ecosystems Research. See http://www.glerl.noaa.gov/res/Task_rpts/2004/aisreid04-1.html .
- 45.Lang I., Kaczmarska I. 2012. Morphological and molecular identity of diatoms retrieved from ship ballast tanks during trans-Pacific voyage 2. Nova Hedwigia, Beihf. 141, 515–534 [Google Scholar]
- 46.Hansen P. J. 1991. Quantitative importance and trophic role of heterotrophic dinoflagellates in a coastal pelagial food web. Mar. Ecol. Prog. Ser. 73, 253–261 10.3354/meps073253 (doi:10.3354/meps073253) [DOI] [Google Scholar]
- 47.Elton C. S. 1958. The ecology of invasions by animals and plants, 4th edn Chicago, IL: The University of Chicago Press [Google Scholar]
- 48.Mather L., MacIntosch K., Kaczmarska I., Klein G., Martin J. L. 2010. A checklist of diatom species reported (and presumed native) from Canadian coast waters. Canadian Technical Reports of Fisheries and Aquatic Science 2881, pp. 78 See http://www.dfo-mpo.gc.ca/Library/340286.pdf
- 49.Carlton J. T. 2009. Deep invasion ecology and the assembly of communities in historical time. In Biological invasions in marine ecosystems (eds Rilov G., Crooks J. A.), pp. 13–56 Berlin, Germany: Springer [Google Scholar]
- 50.Geller J. B., Darling J. A., Carlton J. T. 2010. Genetic perspectives on marine biological invasions. Annu. Rev. Mar. Sci. 2, 367–393 10.1146/annurev.marine.010908.163745 (doi:10.1146/annurev.marine.010908.163745) [DOI] [PubMed] [Google Scholar]