Abstract
Here, we report the discovery and characterization of biominerals in the acorn worms Saccoglossus bromophenolosus and Ptychodera flava galapagos (Phylum: Hemichordata). Using electron microscopy, X-ray microprobe analyses and confocal Raman spectroscopy, we show that hemichordate biominerals are small CaCO3 aragonitic elements restricted to specialized epidermal structures, and in S. bromophenolosus, are apparently secreted by sclerocytes. Investigation of urchin biomineralizing proteins in the translated genome and expressed sequence tag (EST) libraries of Saccoglossus kowalevskii indicates that three members of the urchin MSP-130 family, a carbonic anhydrase and a matrix metaloprotease are present and transcribed during the development of S. kowalevskii. The SM family of proteins is absent from the hemichordate genome. These results increase the number of phyla known to biomineralize and suggest that some of the gene-regulatory ‘toolkit’, if not mineralized tissue themselves, may have been present in the common ancestor to hemichordates and echinoderms.
Keywords: hemichordate, biomineralization, aragonite, skeletal evolution
1. Introduction
Shells, carapaces, spines, ossicles, spicules and bone have been widely investigated by palaeontologists, physical chemists, material scientists and medical researchers under the common rubric of biomineralization since the late nineteenth century. There are so many minerals formed and so many morphologies produced that common threads connecting basic structures in different taxa are of considerable interest. Biomineralized tissues are the principal source of direct evidence for the ancestry of life on the Earth [1,2], are a rich source of morphological data that inform evolutionary hypotheses [3,4] and represent a remarkable interface between inorganic and organic worlds [5–9]. The generation of biominerals has been the subject of considerable theoretical and mechanistic exploration among the vertebrates, arthropods, molluscs and corals.
Among the invertebrate deuterostomes, biominerals have been reported to exist widely in tunicates [10] and echinoderms [8,9,11]. The oldest deuterostome fossils are the skeletal plates, or ossicles, of stylophoran echinoderms that appeared about 510 Ma [11]. Echinoderm ossicles are composed of numerous, single-calcite crystals that lie on the same crystal axis [6]. This skeletal tissue called stereom [3,12] forms intracellularly or extracellularly and is usually perforated by a network of mesh-like cavities. Morphologically, echinoderm ossicles form an endoskeleton, produced by mesenchymal cells and are usually covered by epidermis [4]. The majority of ossicles function in protection, positioned just under or within the ectoderm.
In sea urchins, ossicle minerals are occluded by secreted proteins that control the growth and physical properties of the material [4,5,13]. Some of these proteins are members of small gene families, which, at least in the purple sea urchin Strongylocentrotus purpuratus, are tightly clustered on the genome, suggesting that they expanded relatively recently by gene duplication [14]. Many of these proteins probably arose from ancestral C-lectin domain-containing proteins [14] (including the SM30 and SM50 families of proteins). Other, distantly related proteins, including the MSP-130 family of proteins, matrix metalloproteases and carbonic anhydrases are also implicated in urchin biomineralization. To what extent these molecules may be shared in the other echinoderm classes is poorly known [15] and in hemichordates is unknown.
Here, we provide the first description of biomineralized tissues from hemichordates, including their ultrastructure, and elemental constitution. Second, we searched for sea urchin biomineralization-related proteins in the hemichordate Saccoglossus kowalevskii genome. Third, we sought to shed some light on the possible evolutionary relationships between biominerals in echinoderms and hemichordates.
2. Material and methods
(a). Specimen collection
Saccoglossus bromophenolosus were collected in the mud adjacent to the Darling Marine Laboratory (University of Maine) in September 2008 and Ptychodera flava galapagos were collected in sand by S. A. Earl on Florena Island, Galapagos in December 2003.
(b). X-ray microprobe analyses and scanning electron microscopy
Ossicles were cleaned in 5 per cent NaOCl for 1 h, washed in several changes of distilled water and transferred to an aluminium SEM stub, carbon-coated (Devon D205A sputter coater), analysed and imaged using a JEOL 8200 Superprobe electron microprobe, with an accelerating voltage of 15 kV, beam current of 20 nA and a spot size of 1 μm. Semi-quantitative X-ray microprobe analyses were done in electron-dispersive spectrometer (EDS) mode. Quantitative analyses were done in wavelength-dispersive spectrometer (WDS) mode, with standards and background X-ray counts analysed every 40 samples. Count times were 20 s and 10 s for peaks and backgrounds, respectively. Matrix corrections were done using the ZAF method and analytical results reported as equivalent weight per cent oxides. The following elements were analysed and calibrated against their respective primary standards (diffraction crystal used in brackets): P (PETJ)-Apatite; Ba (PETJ)-Barite; Na (TAPH)-Jadeite; Si (TAP)-Sanidine; Mn (divalent) (LIFH)-Pyrolusite; K (PETJ)-Sanidine; S (PETJ)-Pyrr_239; Mg (TAPH)-Dolomite; Sr (TAP)-Celestite; Fe (divalent)(LIFH)-Garnet12442; Ca (PETJ)-Dolomite. Primary standards were from the American Society for Testing and Materials (ASTM) or the National Institute of Standards and Technology (NIST). Oxygen was calculated for each analysis on the basis of stoichiometry. For SEM analyses, ossicles were viewed on a Hitachi FE-SEM, model S-4700 with 10 kV acceleration voltage.
(c). Confocal Raman spectroscopy
Confocal Raman spectra were acquired with a Renishaw InVia Raman microspectrometer equipped with a deep depletion CCD detector, 1800 l mm–1 grating and a holographic notch filter. Excitation was provided using a Spectra Physics argon ion 514.5 nm laser with 28 mW output and 5 mW at the sample. The spectrometer was attached to a Leica microscope with a 50X objective in a 180° backscatter collection configuration.
(d). Transmission electron microscopy
For transmission electron microscopy (TEM), S. kowelvskii specimens were fixed in 2.5 per cent glutaraldehyde, postfixed in 1 per cent osmium tetroxide, dehydrated and embedded in Epon 812. Ultrathin sections were stained with uranyl acetate and lead citrate and viewed on a Tecnai-12 TEM at 80 kV. We have no TEM data on Ptychodera because animals were damaged on collection, then fixed in 4 per cent formaldehyde and later transferred to alcohol.
(e). Informatics
See the electronic supplementary material.
3. Results
(a). Ossicle location, morphology and cellular environment
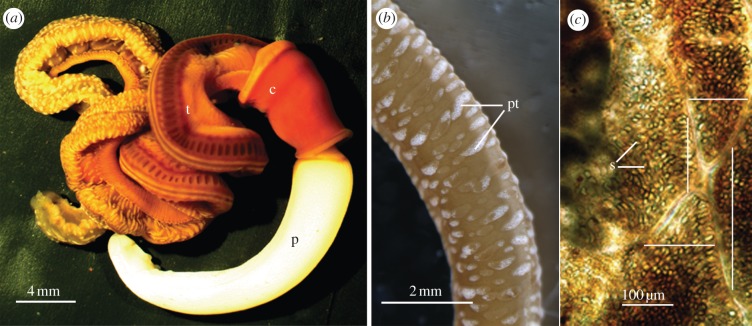
The enteropneust S. bromophenolosus (figure 1a) and the distantly related P. flava galapagos (not shown) contain in their epidermis numerous elevated protuberances that appear white (figure 1b). In S. kowalevskii anterior trunk, the protuberances are located in the grooves between the dorsal midline and the dorso-lateral gonads, and in the groove between the dorso-lateral gonads at the ventral midline (figure 1a). They are most abundant and well developed in the posterior intestinal trunk (figure 1b). Individual protuberances are composed of numerous fluid-filled vesicles (figure 1c) that are elliptical to ovoid and often pointed at the apical end. The vesicles measure 26 μm (mean of +8, n = 22) in depth and 15 μm (+5, n = 25) in width and the number of vesicles within a protuberance varies widely from 1 to 176 (75 + 65, n = 9). An individual vesicle may contain one or no ossicles. Trunk epidermal protuberances are found among the three major enteropneust families, including S. kowalevskii [16] (Family: Harrimanniidae), Spengelia alba [17] (Family: Spengeliidae), Ptychodera flava laysanica, P. flava caledoniensis [18] and Glossobalanus ruficollis [17] (Family: Ptychoderidae) suggesting that ossicles may be a general feature of the Enteropneusta.
Figure 1.
(a) Light micrographs of S. bromophenolosus. (p, anterior proboscis; c, central collar; t, posterior trunk). (b) The trunk is spotted with numerous elevated white protuberances (pt, protuberances). (c) Four abutting protuberances located in the groove between the dorsolateral gonads and the ventral midline. Each protuberance is composed of several superficial and transparent spaces, some of which contain a single ossicle (s, spaces). Each of the four lines crosses a single protuberance at one of its narrower termini.
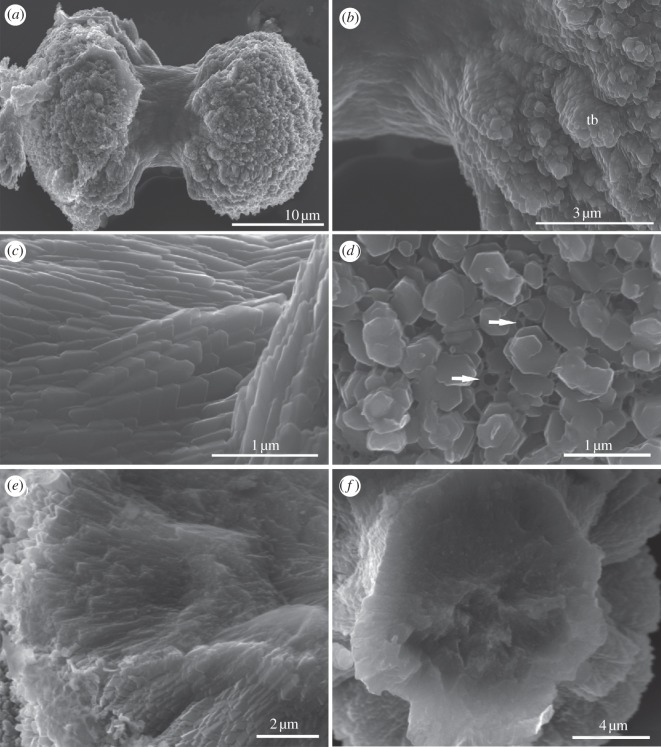
Although varied in structure, most S. bromophenolosus ossicles have a double-ended broccoli-like shape with a central shaft and flared tips (figure 2a). Larger ossicles are 30 μm in length, 10 μm across the narrow stalk and 20 μm across the widest point of the flared tips. The tips appear to be polycrystalline aggregates that form highly ordered, closely packed, expanding columns, or trabeculae, of plate-like crystals (figure 2b). The shaft region is also highly ordered and closely packed, but smooth and composed of overlapping laminar layers (figure 2c). Viewed terminally, the tips of the ossicles are perforated by a network of holes (figure 2d), interlaced with a net-like matrix (figure 2d, arrows). Internally, the ossicles are solid (figure 2e,f), similar to the narrow spicules of pluteus larvae [19] or small ossicles of holothuroid adults [20].
Figure 2.
Scanning electron micrographs of ossicles isolated from S. bromophenolosus trunk epithelium. (a) Lateral view of a typical ossicle. The arrangement and shape of crystals differ between the central shaft and the termini. (b) Higher magnification of a terminus showing polycrystalline aggregates that form highly ordered, closely packed, expanding columns or trabeculae, of plate-like crystals (tb, trabeculae). (c) Higher magnification view showing the overlapping, smooth and laminar layers that constitute the central shaft portion of the ossicle in (a). (d) Terminal view of an ossicle, showing the arrangement of trabeculae, the perforated network of holes and a net-like matrix (arrows). (e) View of the inside of a fractured terminus. (f) View of the inside of a broken shaft.
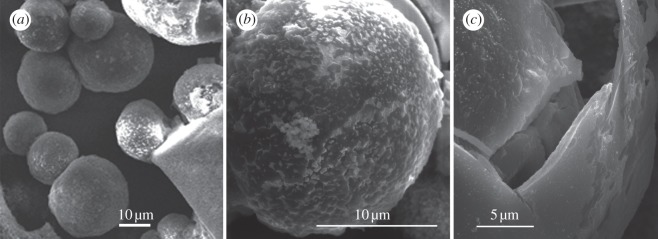
Ptychodera flava galapagos ossicles are smaller and much less abundant in the tissue than those of S. bromophenolosus. Ossicles are spherical with a mean diameter of 23.46 μm (±8.38, n = 20) and approximately 20 ossicles were isolated from a patch of epidermis measuring 4 mm by 4 mm. On the surface, they are smooth to rugose (figure 3). Ossicles that were fractured revealed an inner core that is porous, though not reminiscent of stereom (figure 3c). There are no crystalline plates or layers besides the outer spherical layer.
Figure 3.
Scanning electron micrographs of ossicles from the trunk epidermis of Ptychodera flava galapagos. (a) Ossicles are spherical with a mean diameter of 23.46 μm (±8.38, n = 20). (b) Ossicles are round and rugous. (c) They are composed of an outer layer that when fractured reveals a porous interior.
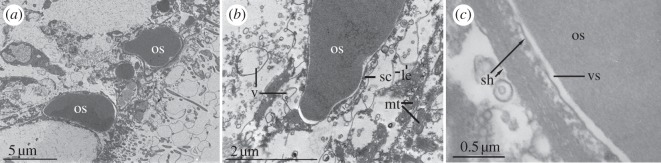
Hemichordate ossicles are positioned under or within a ciliated epidermis and are enveloped by sclerocyte cells in Saccoglossus (figure 4a). The cell cytoplasm is characterized by abundant vacuoles and mitochondria. The sclerocyte cell bodies are surrounded by an electron-dense boundary layer (lamina externa; figure 4b), which is not present in non-sclerocytes [3,21]. There is an electron-transparent space between the enveloping sheath of sclerocytes and the growing mineral surface (figure 4c). This cavity may be derived from cell vacuoles where the mineral is deposited extracellularly. In sea urchins, this space may be sealed off from the surrounding area to maintain a fluid-filled cavity of the necessary ionic composition and pH around the growing surface [3,22].
Figure 4.
Transmission electron micrographs of sections through the epithelium of S. bromophenolosus showing the ossicles and sclerocyte cells. (a) The ossicles are positioned in a heavily ciliated epidermis (os, ossicles). (b) The border between an ossicle and its surrounding sheath of sclerocytes indicates an extracellular position. The cytoplasm is filled with transparent vesicles and abundant mitochondria (v, vesicles, mt, mitochondria). An electron-dense boundary layer (le, lamina externe) surrounds the cell body. (c) Sclerocyte cells form a sheath around the ossicle (sh, sheath). The inner sheath is lined with matrix material that presumably crosses the vacuolar space to be incorporated in the ossicle (vs, vacuolar space).
(b). Elemental constitution of ossicles
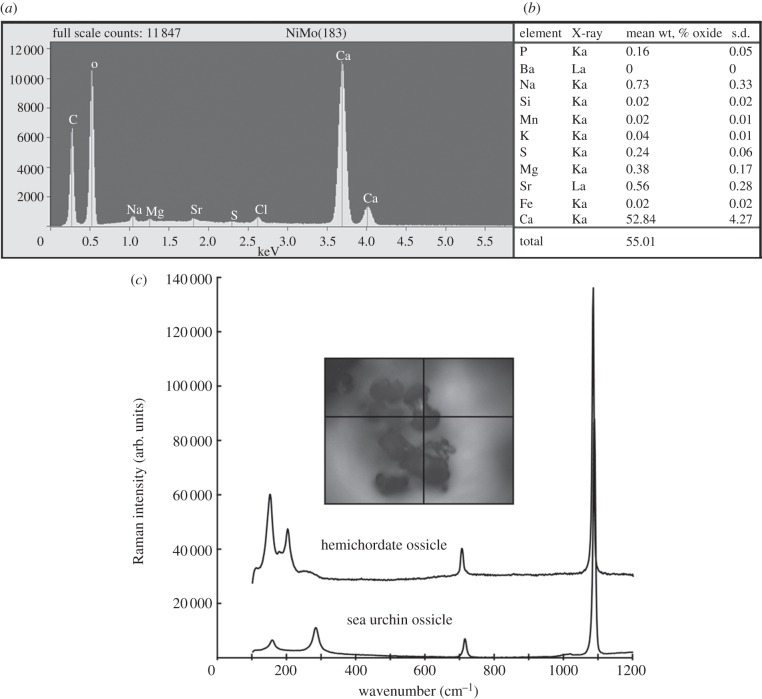
The elemental constitution of isolated ossicles was determined by X-ray microprobe analyses. Energy-dispersive analysis (using EDS) identified calcium, oxygen and carbon as principal elements (figure 5a). Wave-dispersive analysis quantified calcium and less-abundant elements identified by EDS (figure 5b), confirming that S. bromophenolosus and P. flava galapagos ossicles are composed of CaCO3 and minor (approx. 1%) quantities of strontium and magnesium.
Figure 5.
Determination of ossicle elemental constitution using X-ray microprobe analysis and crystal identity using confocal Raman spectroscopy. The ossicles of both species are composed of CaCO3, so only Saccoglossus data are shown. (a) Representative plot of EDS analysis of the central shaft of a single ossicle. (b) WDS analysis on 15 haphazardly chosen ossicles. Quantities are reported as mean weight % oxides. (c) Raman spectra for ossicles from S. bromophenolosus and a sea urchin test. The inset indicates the position on the hemichordate ossicle from which the spectral data were collected.
Because CaCO3 crystals can take the form of vaterite, aragonite or calcite, S. kowalevskii ossicles were subjected to confocal Raman spectroscopy to distinguish between these crystal polymorphs. Spectra collected from six haphazardly chosen ossicles exhibited peaks at 156 and 206 cm–1, a small peak at 708 cm–1 and a strong peak at 1086 cm–1 (figure 5c), which is characteristic of aragonite [23]. Exhaustive line map and depth scans at 1 μm resolution generated no evidence of a calcite peak at 285 cm–1. In contrast, Raman spectra collected from a sea urchin ossicle that clearly correspond to calcite (figure 5c). Although nitrogen was not detected by EDS analysis, protease treatment disintegrated the ossicles suggesting that they possess a protein matrix.
(c). Informatics
The genome of the hemichordate S. kowalevskii, sister taxon to S. bromophenolosus, contains homologues of five of 10 of the signalling molecules and extracellular matrix proteins involved in echinoderm biomineralization (table 1). See the electronic supplementary material for methods. A matrix metalloprotease (electronic supplementary material, figure S1) a carbonic anhydrase (electronic supplementary material, figure S2) and three MSP-130-like proteins (electronic supplementary material, figure S3) were recovered from hemichordate-translated gene sequences. Of the seven known MSP-130 urchin genes, five are expressed in primary mesenchyme cells (PMCs) [14] and are present in urchin test, spine, tooth and spicule [14,24,25]: Sp-Msp130L, Sp-Msp130, Sp-Msp130r1, Sp-Msp130r2, Sp-Msp130r3 (Glean3_entries 06387; 02088; 13822; 16506; 13823, respectively), but for completeness, we have included all known non-redundant urchin Msp130-like sequences [24] in our phylogenetic tree (electronic supplementary material, figure S3). Three homologues of this gene family, which we call S. kowalevskii MSP-1, 2 & 3 were recovered from both translated genome sequences as well as gastrula and early juvenile EST libraries of S. kowalevskii, indicating that the sequences recovered from the translated genomic sequences are transcribed. Significantly, the sister clade to the S. kowalevskii MSP130 sequences is composed exclusively of S. purpuratus sequences known to be expressed in PMCs. The monophyletic grouping of the three S. kowalevskii-translated proteins suggests that a single copy existed in the hemichordate ancestor, and has undergone two duplication events.
Table 1.
A list of 10 urchin spicule matrix proteins that were found in test, spine, tooth and spicule proteomes [24] and which (at least one member of a family) have been experimentally verified to be involved in urchin biomineralization. Amino acid sequences corresponding to proteins in bold have putative homologues in the S. kowalevskii-translated genome and mRNA encoding these sequences are present in S. kowalevskii EST libraries. E-values for the three S. kowalevskii MSP130s are based on comparison with urchin MSP130rel1.
| SpBase | urchin protein ID | Saccoglossus subject ID | e-value |
|---|---|---|---|
| SM proteins and other C-type lectin-like domain containing proteins | |||
| SM50 | Glean:18811 | not present | |
| PM27 | Glean:30147 | not present | |
| SM29 | Glean:05990 | not present | |
| SM32 | Glean:18810 | not present | |
| SM37 | Glean:18813 | not present | |
| SP-Clect_14 | Glean:05991 | not present | |
| matrix metalloproteases | |||
| Sp-Mt1-4 | Glean:13669/13670 | XP_002738586 | 3e−93 |
| Sp-Mt1-4/5 | Glean:28748/28749 | ||
| MSP130 proteins | |||
| Sp-Msp130′s | Glean:13821 | XP_002739468.1 | 3e−54 |
| XP_002733636.1 | 6e−37 | ||
| XP_002739469.1 | 3e−47 | ||
| carbonic anhydrases | |||
| Sp-Cara7LA | Glean:12518 | XP_002735813 | 4e−39 |
Matrix metalloproteinase inhibitors disrupt spicule formation by primary mesenchyme cells in the sea urchin embryo [26]. Four members of this gene family are expressed in all four urchin skeletal matrices and have a homologue in the S. kowalevskii genome and EST libraries. This translated protein is most similar to urchin Sp-Mt1-4/MmpL7, Glean3:28748 (tree not shown; electronic supplementary material, figure S1). Carbonic anhydrases are upregulated in the PMCs postgastrulation and localized to the leading edge of the growing larval arms of the urchin Heliocidaris tuberculata [27]. One, Sp-Clara7LA (Glean3:12518), is the most abundant in all four proteomically analysed skeletal matrices indicating that it may be required for mineralization in urchins [24]. This same protein has a gene homologue in the S. kowalevskii genome and gastrula and early juvenile EST libraries (electronic supplementary material, figure S2). We identified sequences containing C-type lectin domains in the genome of S. kowalevskii but bona fide SM proteins were not recovered and thus appear to be unique to urchins.
4. Discussion
The discovery of a new type of biomineral, heretofore unknown in Hemichordata, raises a number of new and interesting research avenues. First, these biominerals are structurally interesting, and present new opportunities for comparisons with known biominerals from other taxa. Second, hypotheses of function will need to be advanced and tested to explain the presence of these complex structures; presently, it is unknown how common these biominerals are within the hemichordate phylum, and whether their presence varies seasonally or with life cycle stage. Third, an investigation into the embryonic origin of these biominerals, with particular respect to the germ layers and cellular structures that produce them, is warranted. Fourth, the presence of a biomineral produced in the epidermis of animals that are the sister taxon to echinoderms formally raises the possibility that biomineralization in the Ambulacraria is a homologous feature. Fifth, fossil evidence, in the form of microscopic ossicles, for hemichordate ancestors may be present and worth searching for. We develop these latter two points in some detail.
One clear similarity between echinoderms and hemichordates is the CaCO3 composition of their skeletal elements. In contrast, hemichordate biominerals differ from those of echinoderms in that they are composed of aragonite, rather than the echinoderm polymorph calcite, and they have a lower quantity of magnesium. Notably, vaterite and aragonite can transform into calcite [28], both crystal polymorphs appear in closely related species of coral [5], tunicate [10] and bivalve [7], and are even present together in the bimineralic skeletons of bryozoans [29]. These observations suggest that the developmental pathways that produce CaCO3 polymorphs are similar, if not identical.
Saccoglossus kowalevskii ossicles develop in an extracellular space, and form a polycrystalline aggregate of laminar layers, with perforations that, on the surface, are reminiscent of a poorly developed stereom. This latter structural comparison is less concrete than the elemental one, because the central shaft of hemichordate ossicles is solid and because of the possibility that isolation and preparation of hemichordate ossicles introduced artefacts. The interior core of P. flava galapagos ossicles, though porous, does not resemble a stereom. Further work will be required to clarify these potentially informative structural details but, in the main, their homology with ossicles of echinoderms is equivocal.
In sea urchins, biomineralization is regulated by secreted proteins that are closely associated with the mineral element component of the biomineral. The proteins in the sea urchin S. purpuratus make only a minor contribution to its biomineral mass [15], but probably play an important role in regulating the growth and physical characteristics of the biomineral. The urchin larval skeleton is produced by the PMCs—a specialized population of embryonic mesodermal cells derived from the large micromere lineage. PMCs express a family of SM proteins, and a family of cell surface proteins consisting of at least five MSP130-related proteins and a matrix metalloproteinase [26] that may function in biomineralization [25,30,31]. Our analysis of the S. kowalevskii-translated genome, using 10 sea urchin proteins as queries, revealed five biomineralization genes: three MSP130 genes, a matrix metalloprotease and a carbonic anhydrase. The one urchin carbonic anhydrase probably required for biomineralization [24] is shown with its corresponding S. kowalevskii protein sequence (table 1; electronic supplementary material, figure S2). The one matrix metalloprotease is closest to urchin glean3_28748 (Sp-Mt1-4/MmpL7; table 1; electronic supplementary material, figure S1). These genes were also recovered from EST libraries developed from S. kowalevskii gastrulae and early juveniles. Illies et al. [25] conducted a sequence alignment of five MSP130 genes (three from S. purpuratus and one each from Heliocidaris tuberculata and H. erythrogramma) and found significant variation among those sequences in the presence and length of glycine-rich motifs. Despite the absence in S. purpuratus MSP130-related 1 and 2 of significant glycine-rich motifs, those genes are nevertheless annotated as spicule matrix genes because they are expressed at high levels in primary mesenchyme cells. Moreover, despite sharing relatively low overall sequence identity (in the 30% range), S. purpuratus MSP130-related 1 and 2 do unequivocally share short stretches of high sequence identity with MSP130, supporting the homology assignment. None of the three S. kowalevskii sequences that we found contains glycine-rich domains and, therefore, this appears to be an innovation of the urchins. By definition then, in S. kowalevskii, hits were based on sequence similarity outside this domain, obviating the possibility that, at least in the case of MSP130-like genes, hits were due to shared low-complexity regions. In contrast, SM-like sequences were not recovered and appear to be unique to echinoderms.
Together, these data permit an interpretation of an evolutionary relationship between the genetic programmes of biomineralization in echinoderms and hemichordates. Certainly, some of the genes are present and transcribed, but further study is needed to determine if they are involved in biomineral formation and occluded in acorn worm biomineralized elements.
The oldest echinoderm fossils, the stylophorans, appeared about 510 Ma [11]. Molecular clock data suggest that they are not the oldest echinoderms. Their well-developed stereom, ossicle disparity and body plan asymmetry suggest that they did not appear de novo, but represent a stem-group descendent of an ancestor that more plausibly possessed simpler, smaller and less well-organized ossicles. There is a rich and diverse fossil record of echinoderm skeletal elements [6] including very small Cambrian ossicles [1]. The enteropneust fossil record, by contrast, is poor and comparatively young [32] but further characterization of enteropneust ossicles may shed new light on interpretations of Early Cambrian and Proterozoic microfossils and may support molecular phylogenetic [33,34] and evolutionary developmental assertions [35] that the ancestral deuterostome resembled an enteropneust [33] and, considering the present results, that it possessed biominerals.
Based on our evidence, we cannot reject the hypothesis that biomineralization in the Ambulacraria is homologous at the mineral element, cellular or molecular levels. Model developmental systems such as S. purpuratus continue to provide significant new information about biomineralization in echinoderms. Insight into biomineralization has arisen from embryology [25,36], gene sequences and genomic arrangements [14,35,37], the localization and function of protein matrix molecules [24,37,38] and mineral elements [21,37–39], as well as the role of the cellular environment [11,21]. Comparative studies are necessary to rigorously reject the hypothesis that hemichordate and echinoderm ossicles are homologous, and that the regulatory developmental toolkit (if not biominerals per se) were present in the ancestral deuterostome.
An understanding of how, when and where minerals are deposited during hemichordate development and what proteins those minerals contain is central to understanding deuterostome skeletal evolution and the problem seems accessible.
Acknowledgements
Supported by NSERC Discovery Grants to C.B.C. and C.D.B. C.D.B. was supported by a NSERC Discovery Grant to Brian Hall (Dalhousie University) during the early stages of this work. We thank the director of the Darling Marine laboratory for providing facilities (C.B.C.). Patricia Scallion and Dan MacDonald (Dalhousie University) and Truis Smith-Palmer (Chemistry, Saint Francis Xavier University) provided expert assistance with SEM, X-ray microprobe and confocal Raman spectroscopy, respectively. The authors declare no conflict of interest.
References
- 1.Culver S. J., Pojeta J., Jr, Repetski J. E. 1988. First record of Early Cambrian shelly microfossils from west Africa. Geology 16, 596–599 10.1130/0091-7613(1988)016%3C0596:FROECS%3E2.3.CO;2 (doi:10.1130/0091-7613(1988)016<0596:FROECS>2.3.CO;2) [DOI] [Google Scholar]
- 2.Cohen P. A., Schopf J. W., Butterfield N. J., Kudryavtsev A. B., Macdonald F. A. 2011. Phosphate biomineralization in mid-Neoproterozoic protists. Geology. 39, 539–542 10.1130/G131833.1 (doi:10.1130/G131833.1) [DOI] [Google Scholar]
- 3.Smith A. B. 1990. Biomineralization in echinoderms. In Skeletal biomineralization: patterns, processes, and evolutionary trends (ed. Carter J. G.), pp. 413–443 New York, NY: Van Nostrand Reinhold [Google Scholar]
- 4.Wilt F. H. 2005. Developmental biology meets materials science: morphogenesis of biomineralized structures. Dev. Biol. 280, 15–25 10.1016/j.ydbio.2005.01.019 (doi:10.1016/j.ydbio.2005.01.019) [DOI] [PubMed] [Google Scholar]
- 5.Cohen A., McConnaughey T. A. 2003. Geochemical perspectives on coral mineralization. Rev. Mineral. Geochem. 54, 151–187 10.2113/0540151 (doi:10.2113/0540151) [DOI] [Google Scholar]
- 6.Berman A., Addadi L., Kvick Å., Leiserowitz L., Nelson M., Weiner S. 1990. Intercalation of sea urchin proteins in calcite: study of a crystalline composite material. Science 250, 664–667 10.1126/science.250.4981.664 (doi:10.1126/science.250.4981.664) [DOI] [PubMed] [Google Scholar]
- 7.Falini G., Albeck S., Weiner S., Addadi L. 1996. Control of aragonite or calcite polymorphism by mollusk shell macromolecules. Science 271, 67–69 10.1126/science.271.5245.67 (doi:10.1126/science.271.5245.67) [DOI] [Google Scholar]
- 8.Aizenberg J., Tkachenko A., Weiner S., Addadi L., Hendler G. 2001. Calcitic microlenses as part of the photoreceptor system in brittlestars. Nature 412, 819–822 10.1038/35090573 (doi:10.1038/35090573) [DOI] [PubMed] [Google Scholar]
- 9.Raz S., Hamilton P. C., Wilt F. H., Weiner S., Addadi L. 2003. The transient phase of amorphous calcium carbonate in sea urchin larval spicules: the involvement of proteins and magnesium ion in its formation and stabilization. Adv. Funct. Mater. 13, 480–486 10.1002/adfm.200304285 (doi:10.1002/adfm.200304285) [DOI] [Google Scholar]
- 10.Lambert G., Lambert C. C., Lowenstam H. A. 1990. Protochordate biomineralization. In Skeletal biomineralization: patterns, processes, and evolutionary trends (ed. Carter J. G.), pp. 461–469 New York, NY: Van Nostrand Reinhold [Google Scholar]
- 11.Smith A. B. 2005. The pre-radial history of echinoderms. Geol. J. 40, 255–280 10.1002/gj.1018 (doi:10.1002/gj.1018) [DOI] [Google Scholar]
- 12.Bottjer D. J., Davidson E. H., Peterson K. J., Cameron R. A. 2006. Paleogenomics of echinoderms. Science 314, 956–960 10.1126/science.1132310 (doi:10.1126/science.1132310) [DOI] [PubMed] [Google Scholar]
- 13.Ettensohn C. A. 2009. Lessons from a gene regulatory network: echinoderm skeletogenesis provides insights into evolution, plasticity and morphogenesis. Development 136, 11–21 10.1242/dev.023564 (doi:10.1242/dev.023564) [DOI] [PubMed] [Google Scholar]
- 14.Livingston B. T., et al. 2006. A genome-wide analysis of biomineralization-related proteins in the sea urchin Strongylocentrotus purpuratus. Dev. Biol. 300, 335–348 10.1016/j.ydbio.2006.07.047 (doi:10.1016/j.ydbio.2006.07.047) [DOI] [PubMed] [Google Scholar]
- 15.Wilt F. H., Killian C. E., Livingston B. T. 2003. Development of calcareous skeletal elements in invertebrates. Differentiation 71, 237–250 10.1046/j.1432-0436.2003.7104501.x (doi:10.1046/j.1432-0436.2003.7104501.x) [DOI] [PubMed] [Google Scholar]
- 16.Agassiz A. 1873. The history of Balanoglossus and tornaria. Am. Acad. Arts Sci. Mem. 9, 421–436, 423 plates 10.2307/25058009 (doi:10.2307/25058009) [DOI] [Google Scholar]
- 17.van der Horst C. J. 1939. Hemichordata. In Klassen und Ordnungen des Tier-Reichs Vieter Band: Vermes. (ed. Bronn H. G.), Band 4, Abt. 4, Buch 2, Tiel 2 p. 737 Leipzig, Germany: Akademische Verlagsgesellschaft [Google Scholar]
- 18.Spengel J. W. 1903. Neue Beiträge zur Kenntniss der Enteropneusten. I. Ptychodera flava Eschsch. Von Laysan. Zoologischn Jahrbüchern, Abtheilung für Anatomie und Ontogenie der Thiere. Heft 2, 271–326 (29 plates) [Google Scholar]
- 19.Okazaki K., Inoué S. 1976. Crystal property of the larval sea urchin spicule. Dev. Growth Differ. 18, 413–434 10.1111/j.1440-169X.1976.00413.x (doi:10.1111/j.1440-169X.1976.00413.x) [DOI] [PubMed] [Google Scholar]
- 20.Massin C., Mercier A., Hamel J.-F. 2000. Ossicle change in Holothuria scabra with a discussion of ossicle evolution within the Holothuroidae (Echinodermata). Acta Zool. 81, 77–91 10.1046/j.1463-6395.2000.00039.x (doi:10.1046/j.1463-6395.2000.00039.x) [DOI] [Google Scholar]
- 21.Märkel K., Röser U. 1985. Comparative morphology of echinoderm calcified tissues: histology and ultraoid scales. Zoomorphology 105, 197–207 10.1007/BF00312157 (doi:10.1007/BF00312157) [DOI] [Google Scholar]
- 22.Guss K. A., Ettensohn C. A. 1997. Skeletal morphogenesis in the sea urchin embryo: Regulation of primary mesenchyme gene expression and skeletal rod growth by ectoderm-derived cues. Development 124, 1899–1908 [DOI] [PubMed] [Google Scholar]
- 23.Dandeu A., Humbert B., Carteret C., Muhr H., Plasari E., Bossoutrot J. M. 2006. Raman spectroscopy—a powerful tool for the quantitative determination of the composition of polymorph mixtures: application to CaCO3 polymorph mixtures. Chem. Eng. Technol. 29, 221–225 10.1002/ceat.200500354 (doi:10.1002/ceat.200500354) [DOI] [Google Scholar]
- 24.Mann K., Wilt F. H., Poustka A. J. 2010. Proteomic analysis of sea urchin (Strongylocentrotus purpuratus) spicule matrix. Proteome Sci. 8, 1–12 10.1186/1477-5956-8-1 (doi:10.1186/1477-5956-8-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Illies M. R., Peeler M. T., Dechtiaruk A. M., Ettensohn C. A. 2002. Identification and developmental expression of new biomineralization proteins in the sea urchin Strongylocentrotus purpuratus. Dev. Genes Evol. 212, 419–431 10.1007/s00427-002-0261-0 (doi:10.1007/s00427-002-0261-0) [DOI] [PubMed] [Google Scholar]
- 26.Ingersoll E. P., Wilt F. H. 1998. Matrix metalloproteinase inhibitors disrupt spicule formation by primary mesenchyme cells in the sea urchin embryo. Dev. Biol. 196, 95–106 10.1006/dbio.1998.8857 (doi:10.1006/dbio.1998.8857) [DOI] [PubMed] [Google Scholar]
- 27.Love A. C., Andrews M. E., Raff R. A. 2007. Gene expression patterns in a novel animal appendage: the sea urchin pluteus arm. Evol. Dev. 9, 51–68 10.1111/j.1525-142X.2006.00137.x (doi:10.1111/j.1525-142X.2006.00137.x) [DOI] [PubMed] [Google Scholar]
- 28.Tong H., Ma W., Wang L., Wan P., Hu J., Cao L. 2004. Control over the crystal phase, shape, size and aggregation of calcium carbonate via a l-aspartic acid inducing process. Biomaterials 25, 3923–3929 10.1016/j.biomaterials.2003.10.038 (doi:10.1016/j.biomaterials.2003.10.038) [DOI] [PubMed] [Google Scholar]
- 29.Taylor P. D., Kudryavstev A. B., Schoph J. W. 2008. Calcite and aragonite distributions in the skeletons of bimineralic bryozoans as revealed by Raman spectroscopy. Invert. Biol. 127, 87–97 10.1111/j.1744-7410.2007.00106.x (doi:10.1111/j.1744-7410.2007.00106.x) [DOI] [Google Scholar]
- 30.Leaf D. S., Anstrom J. A., Chin J. E., Harkey M. A., Showman R. M., Raff R. A. 1987. Antibodies to a fusion protein identify a cDNA clone encoding msp130, a primary mesenchyme-specific cell surface protein of the sea urchin embryo. Dev. Biol. 121, 29–40 10.1016/0012-1606(87)90135-7 (doi:10.1016/0012-1606(87)90135-7) [DOI] [PubMed] [Google Scholar]
- 31.Farach-Carson M. C., Carson D. D., Collier J. L., Lennarz W. J., Park H. R., Wright G. C. 1989. A calcium-binding, asparagine-linked oligosaccharide is involved in skeleton formation in the sea urchin embryo. J. Cell Biol. 109, 1289–1299 10.1083/jcb.109.3.1289 (doi:10.1083/jcb.109.3.1289) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kazmierczak J., Pszczolkowski A. 1969. Burrows of Enteropneusta in the Muschelkalk (Mid. Trias) of the Holy Cross Mountains, Poland. Acta Paleontol. Pol. 14, 299–324 [Google Scholar]
- 33.Cameron C. B., Swalla B. J., Garey J. R. 2000. Evolution of the chordate body plan: New insights from phylogenetic analysis of deuterostome phyla. Proc. Natl Acad. Sci. USA 97, 4469–4474 10.1073/pnas.97.9.4469 (doi:10.1073/pnas.97.9.4469) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cannon J. T., Rychel A. L., Eccleston H., Halanych K. M., Swalla B. J. 2009. Molecular phylogeny of Hemichordata, with updated status of deep-sea enteropneusts. Mol. Phylogenet. Evol. 52, 17–24 10.1016/j.ympev.2009.03.027 (doi:10.1016/j.ympev.2009.03.027) [DOI] [PubMed] [Google Scholar]
- 35.Lowe C., Wu M., Salic A., Evans L., Lander E., Stange-Thomann N., Gruber C. E., Gerhart J., Kirschner M. 2003. Anteroposterior patterning in hemichordates and the origins of the chordate nervous system. Cell 113, 853–865 10.1016/S0092-8674(03)00469-0 (doi:10.1016/S0092-8674(03)00469-0) [DOI] [PubMed] [Google Scholar]
- 36.Angerer L. M., Angerer R. C. 2003. Patterning the sea urchin embryo: Gene regulatory networks, signaling pathways and cellular interactions. Curr. Top. Dev. Biol. 53, 159–198 10.1016/S0070-2153(03)53005-8 (doi:10.1016/S0070-2153(03)53005-8) [DOI] [PubMed] [Google Scholar]
- 37.Melvin J. G. 2006. Bone: nature of the calcium phosphate crystals and cellular, structural, and physical chemical mechanisms in their formation. Rev. Mineral. Geochem. 64, 223–282 10.2138/rmg.2006.64.8 (doi:10.2138/rmg.2006.64.8) [DOI] [Google Scholar]
- 38.Killian C. E., Wilt F. H. 1996. Characterization of the proteins comprising the integral matrix of Strongylocentrotus purpuratus embryonic spicules. J. Biol. Chem. 271, 9150–9159 10.1074/jbc.271.15.9150 (doi:10.1074/jbc.271.15.9150) [DOI] [PubMed] [Google Scholar]
- 39.Beniash E., Aizenberg J., Addadi L., Weiner S. 1997. Amorphous calcium carbonate transforms into calcite during sea urchin larval spicule growth. Proc. R. Soc. Lond. B 264, 461–465 10.1098/rspb.1997.0066 (doi:10.1098/rspb.1997.0066) [DOI] [Google Scholar]