Abstract
Parasitic infections consist of a succession of steps during which hosts and parasites interact in specific manners. At each step, hosts can use diverse defence mechanisms to counteract the parasite's attempts to invade and exploit them. Of these steps, the penetration of parasites into the host is a key step for a successful infection and the epithelium is the first line of host defence. The shedding of this protective layer (moulting) is a crucial feature in the life cycle of several invertebrate and vertebrate taxa, and is generally considered to make hosts vulnerable to parasites and predators. Here, we used the crustacean Daphnia magna to test whether moulting influences the likelihood of infection by the castrating bacterium Pasteuria ramosa. This parasite is known to attach to the host cuticula before penetrating into its body. We found that the likelihood of successful parasite infection is greatly reduced if the host moults within 12 h after parasite exposure. Thus, moulting is beneficial for the host being exposed to this parasite. We further show that exposure to the parasite does not induce hosts to moult earlier. We discuss the implications of our findings for host and parasite evolution and epidemiology.
Keywords: moult, host, resistance, crustacean, ecdysozoan, Daphnia
1. Introduction
All multicellular organisms have an outer layer called cuticula or skin. This layer serves a protective role by forming a physical barrier against external biotic and abiotic attacks, as well as an immune shield (e.g. mammals [1]). This barrier has been shown to be effective against parasites, and mechanical damage correlates with an increase in the probability of infection [2]. Parasites evolve elaborate mechanisms to cross this barrier, such as the specialized ovipositors of parasitoids that lay eggs inside insect hosts, the unique adaptations of fungal pathogens to cross the cell wall of their plant hosts [3], the modified rostrum of blood-sucking arthropods that exploit vertebrate hosts and the harpoon-like invasion apparatus of microsporidia allowing the penetration into the host cell without ever attaching to the host [4]. These examples illustrate that parasites adapt to efficiently cross the host skin/cuticula barrier, while hosts evolve ways of reducing the likelihood of parasite invasion through the barrier.
The ecdysozoans (e.g. arthropods, nematodes) and the squamata (i.e. lizards and snakes) need to shed their skin/cuticula for growing, a process called moulting or ecdysis. There are costs and benefits to this process. Shortly after having shed the barrier, the new barrier is temporarily soft and thin, with individuals sometimes being unable to move. The individuals are, therefore, more vulnerable to predators, competitors and parasite penetrations until the barrier is fully re-established [2,5]. On the other hand, moulting at regular intervals benefits the host by removing the accumulation of epibionts [6,7] and helps in wound healing [8]. Given such costs and benefits, the timing of moulting is crucial and may be finely tuned to minimize the overall costs. For example, the crustacean Gammarus pulex adjusts the time of its moult cycle in response to parasitic infection risk, elongating it by several days when the individuals are exposed to ‘micro-organism-enriched’ water [9]. This example highlights the role of parasites on ecdysozoan development and at the same time supports the notion of the cuticular function to protect against infection.
Many ecto- and endoparasites need to attach to their hosts before penetrating the cuticula. If the moulting occurs when the parasite is already attached to the host epithelium but before it penetrates the barrier, this might interfere with its penetration and thus prevent infection. Contrary to the idea that moulting exposes hosts to parasitism, this idea states the opposite: moulting might protect the host from endoparasitic infections. Here, we test this hypothesis. Using the Gram-positive bacterium Pasteuria ramosa and its host Daphnia magna, we investigate the possibility that moulting interferes with the success of infection. Pasteuria parasites attach and penetrate the host cuticula before proliferating within the body in nematodes and crustaceans [10,11]. Infections proceed by the specific attachment of the parasite to the cuticula in the host oesophagus and the subsequent penetration into the host body cavity [11]. In arthropods, the oesophagus is part of the ectoderm and is shed during moulting [12]. Thus, we predicted that if moulting occurred shortly after the attachment of the parasite to the host, the parasite might be shed with the moult before penetrating into the host. If this is the case, moulting could be an effective mechanism for freeing hosts of attached parasites and would select for rapid endoparasite penetration speed. Moreover, if moulting interferes with parasite penetration, it is conceivable that hosts respond to parasite attachment by shortening the time to the next moulting. Here, we test these two hypotheses.
2. Material and methods
(a). Biological material
We used different genotypes (clones) of the transparent crustacean D. magna (Kela 39-09, Kela 18-10 and Xinb3 from Finland, HO2 from Hungary and M10 from Belgium). Host clones were kept in standardized medium (ADaM [13], modified by using only 5% of the recommended Selenium) at 20°C, and fed daily with chemostat-cultured unicellular algae, Scenedesmus obliquus. The parasites used were P. ramosa clones C1 and C19, originally sampled from infected D. magna in natural populations in Moscow (Russia) and Gaarzerfeld (Germany), respectively [14]. Parasite suspensions for experimental exposure were produced from homogenized infected Daphnia.
(b). Parasite removal with host moulting
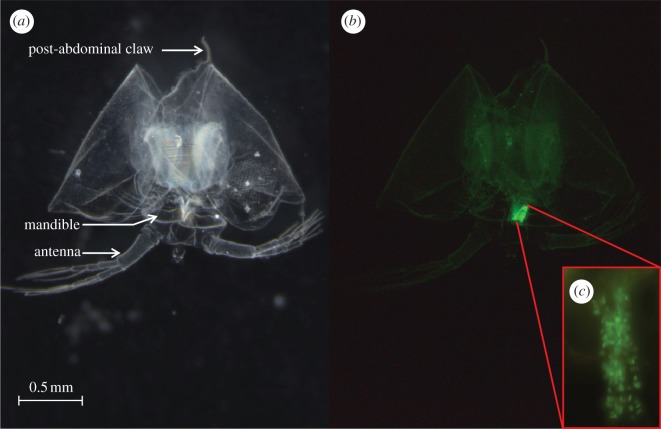
To test whether parasite spores attached to the host cuticula can be found in the oesophagus of the shed carapace after moulting, we exposed 23 D. magna adult females from the laboratory stock of clone Kela 39-09 and Kela 18-10 to 20 000 fluorescently labelled spores (cf. [11]) of each of the P. ramosa clones C1 and C19. The two Daphnia clones were chosen because they have opposite infection patterns for the two parasite clones. The clone Kela 39-09 is susceptible to P. ramosa C1 but not to C19, and the clone Kela 18-10 is susceptible to C19 but not to C1 [11]. Daphnia were raised in mass culture and then placed individually in 24-well plates, where exposure to the parasite took place in 1 ml of ADaM. Thirty-six hours after exposure to parasites, we checked all host individuals for moulting by visual inspection. For the 30 per cent of individuals that had moulted within the 36 h (susceptible combinations: Kela 39-09/C1 n = 5, Kela 18-10/C19 n = 13; resistant combinations: Kela 39-09/C19 n = 6, Kela 18-10/C1 n = 6), we checked for the presence or absence of parasite spores attached on the oesophagus of the shed cuticula (figure 1) under a fluorescence microscope (Leica DM 2500) with RGB filter cubes (Leica, bandpass filter excitation 420/30 nm, 495/15 nm, 570/20 nm; band pass filter suppression 465/20 nm, 530/30 nm, 640/40 nm).
Figure 1.
Shed cuticula of D. magna exposed to P. ramosa. Pictures represent the same cuticula under light (a) and fluorescent (b) stereomicroscopy. Inset (c) represents the magnification (200×) of the oesophageal region and the green spots are the spores attached to the cuticula. Spores were found attached to the oesophageal part of the shed cuticula in all susceptible hosts (Kela 39-09/C1 n = 5, Kela 18-10/C19 n = 13), but never in resistant hosts (resistant combinations: Kela 39-09/C19 n = 6, Kela 18-10/C1 n = 6).
(c). Effect of moulting on parasite infection
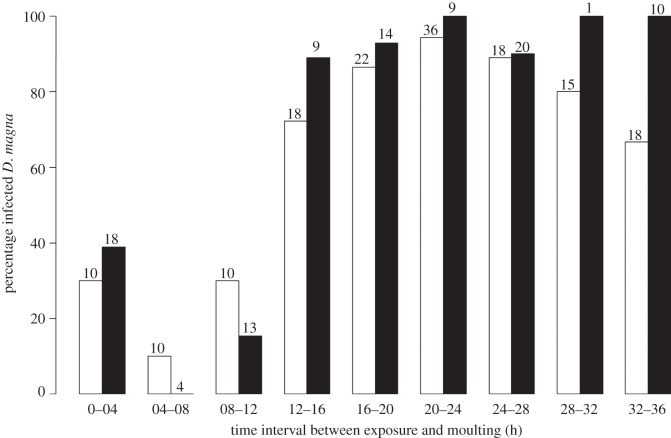
To test whether moulting interferes with the process of infection, we conducted two independent experiments in which we exposed 196 (experiment 1) and 160 (experiment 2) D. magna individuals from clone HO2 to P. ramosa clone C19. HO2 is known to be susceptible to C19 [11]. We used 28 additional Daphnia as control (non-exposed). Individual D. magna juveniles, not older than 3 days, were placed individually in 20 ml ADaM with 20 000 spores (juveniles moult approx. every 36 h at 20°C). As experiment 1 revealed a short time window for results to be observed, we conducted experiment 2 with a reduced duration for parasite exposure from 12 h (experiment 1) to 4 h (experiment 2). After the exposure, host individuals were transferred to 80 ml of parasite-free medium. In both experiments, each individual was checked for moulting every 4 h, between 0 and 36 h after exposure to P. ramosa spores. After 36 h, all individuals that had moulted were kept individually in 80 ml for 25 days during, which time the medium was renewed weekly. After this period, the individuals were checked for infection status by visual inspection. Infected animals are larger, lose their transparency and their haemolymph is packed full with parasite spores. The design of experiment 2 was modified based on the experience with experiment 1. First, in experiment 1, juveniles originated from a mass culture, thus, their mothers were unknown. In experiment 2, we took four juveniles per individually kept mother. Second, individuals that moulted during the exposure phase were excluded. And finally, to reduce the possibility that spores passing the gut are present in the medium, we transferred all host animals a second time, after 1 h, into parasite-free medium. We ended up with total sample sizes of 157 (experiment 1) and 98 (experiment 2) individuals. The number of moulting Daphnia for each 4 h interval varies between intervals, but was generally larger than 10 (figure 2).
Figure 2.
Percentage of infected D. magna according to the time period between exposure to P. ramosa and moulting event. Both of the independently replicated experiments show a low-infection success when the host moults within the first 12 h after exposure. The number on the top of each bar is the number of animals moulting in a given time interval. Unfilled bars, experiment 1; filled bars, experiment 2.
To study the influence of the time between exposure and moulting on the probability that the host became infected, we used a generalized linear model (GLM; [15]) with a binomial error distribution, and logit link constructed as: infection status ∼ experiment * time between exposure and moulting. ‘Infection status’ is either 0 (uninfected) or 1 (infected), and ‘*’ indicates that the effects were tested of both main factors as well as their interaction. The assumption on the error distribution was checked by estimating dispersion parameters in a GLM. No significant overdispersion was detected. For experiment 2, we tested separately the effect of the mother by taking ‘mother’ as a random factor in a general mixed model. As the factor ‘mother’ did not affect the outcome concerning the time period between exposure and moulting and its relationship to the probability of infection, we present here the simplest GLM combining the two experiments and excluding ‘mother’ as a factor.
(d). Moulting as a passive defence against parasites
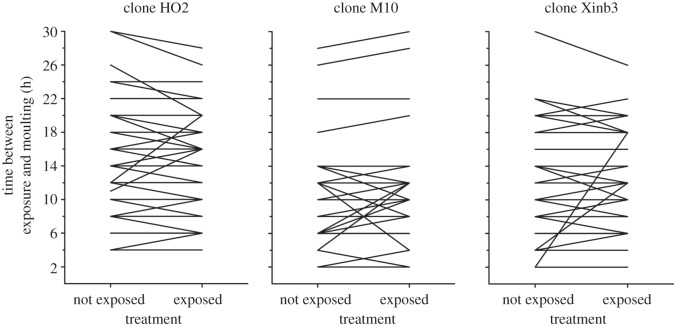
We investigated whether hosts exposed to P. ramosa shed their cuticula earlier than those not exposed. We used three Daphnia clones from very distinct geographical regions (HO2, M10 and Xinb3) and the P. ramosa clone C19. These combinations are known to be compatible [11]. For each host clone, we used 50 pairs of offspring, each taken from one clutch from a different mother, and exposed one offspring to the parasite and the other not (exposed to healthy Daphnia homogenized in ADaM to control for the exposure to Daphnia tissue; split-brood design). This paired design allows precise controlling for the stage and condition of the individuals at the time of parasite exposure because offspring of the same clutch hatch at the same time, moult at the same rate (at least for the first days when in the same conditions) and were exposed to the same maternal effect. Individuals were kept in 24-well plates and were checked for moulting every 2 h. The time before moulting seems to be the relevant biological metric as it is the time window in during which the host might remove the parasite before it penetrates into the host's body cavity. We followed the Daphnia individuals for moulting during 30 h after exposure to the parasite. The total number of replicates having moulted within the 30 h for the clones HO2, M10 and Xinb3 Daphnia were 43, 33 and 39 pairs, respectively.
3. Results
We used different Daphnia genotypes and protocols to test whether moulting can help reduce infection and whether moulting time can be shortened by hosts exposed for that purpose.
(a). Parasite removal with moulting
Because the cuticula of the Daphnia oesophagus is shed during moulting, we first tested whether spores attached to this part might be found in the moult. Microscopic examination of the moult of D. magna that had been exposed to parasites revealed that the parasite was still attached to the cuticula of the oesophagus in all susceptible host individuals (n = 18, figure 1) and in none of the resistant ones (n = 12).
(b). Effect of moulting on parasite infection
Our data showed that if the host moults within 12 h after exposure, the probability of infection is strongly reduced (figure 2). The time between exposure and moulting was a factor contributing significantly to the likelihood of infection (GLM; n = 255, d.f. = 1, deviance = 56.21, p < 0.0001), while its interaction with the factor Experiment (GLM; n = 255, d.f. = 1, deviance = 3.59, p = 0.06) was not significant. Both of the experiments showed the same results (GLM, n = 255, d.f. = 1, deviance = 0.2, p = 0.66; figure 2).
(c). Moulting as a passive defence against parasites
The time interval between parasite exposure and host moulting was not significantly different among the three host clones tested (ANOVA; n = 115, d.f. = 2, F = 2.15, p = 0.12). Thus, we tested whether the exposed group moulted before the non-exposed group without taking host clone into account. We found no significant difference in moult interval between Daphnia that were exposed versus those not exposed to the parasite (paired t-test; d.f. = 114, t = −0.41, p = 0.68; figure 3).
Figure 3.
Time between exposure and moulting for D. magna either exposed to P. ramosa or not exposed. The lines connect offspring from the same clutch. For clones HO2, M10 and Xinb3, the sample sizes were 43, 33 and 39 pairs, respectively. In cases of induction of moulting by parasite encounter, the offspring exposed should have shorter times to moulting than the offspring not exposed. The exposure to parasite did not affect the timing of moulting.
4. Discussion
We show that host moulting soon after parasite exposure does rid hosts from parasites attached to their cuticula (figure 1) and reduces the likelihood of successful infection (figure 2). To our knowledge, this is the first time that host moulting has been reported to interfere directly with the success of infection by a parasite. The attachment of the bacterial parasite P. ramosa to the oesophagus of its D. magna host was described before [11], but the mechanism the parasite uses to cross the cuticula after the attachment is still unknown. The strong increase in the likelihood of infection when hosts did not moult within the 12 h following the parasite exposure (figure 2) suggests that it takes about 12 h for this parasite to penetrate into the host's body cavity and penetration has to occur before host moulting. At 20°C, the interval between D. magna moults is about 36 h in juveniles and 3–4 days in adults [16]. Moulting-related disposal of parasites is therefore not trivial for parasites: considering a constant exposure to the parasite, about one-third of all spores would be ‘destined to fail’ before penetration in host juveniles, and 10–20% in adults. Thus, it is likely that moulting imposes selection on parasites to penetrate into the host body shortly after attaching to the body wall, especially because such ‘destined to fail’ spores are likely to be permanently lost, as they remain attached to the moult for several days (authors' unpublished results). Furthermore, these spores have lost their protective layer when they encounter the host [11] and are exposed to the environment, which might degrade them. In future work, it would be interesting to use different P. ramosa genotypes to search for polymorphism for the speed of penetration. Given the direct advantage of fast penetration, we speculate genetic variation when penetration speed is low. However, this simple prediction might change considering that (i) there are typically strong host genotype–parasite genotype interactions for the attachment of P. ramosa to its host's oesophagus [11] and (ii) there might be costs associated with attachment and penetration.
The probability of the failure of parasite infection owing to host moulting has important implications for experiments with the Daphnia–Pasteuria system, which has advanced to a major system for studies of host–parasite evolutionary ecology [17–20]. Putative variation in host-moulting among experimental groups can lead to increased noise, or even spurious results, in infection rates. For example, poor resource intake lengthens the intermoult period in Daphnia [16] and would thus increase the likelihood of infection. Furthermore, moulting in cohorts of exposed animals may be synchronized (e.g. in groups of animals born in the same time) and thus can cause systematic biases, rather than just random noise. Our results suggest that, to minimize these effects, it seems appropriate to expose Daphnia at least twice to a smaller amount of parasite spores in 12–24 h intervals.
The protective role of moulting is likely to be relevant also in other host–parasite interactions. One of these interactions might involve vector-borne disease agents that take advantage of the adaptations of their bloodsucking vectors to cross the skin of their host. For example, in the case of the aetiological agents of Lyme disease, Borrelia burgdorferi s.s., the endoparasite requires its tick vector to be attached for around 78 h before being transmitted to its mammalian hosts [21]. Many vector-borne zoonoses (e.g. mites transmitting haemogregarian blood parasites, ticks transmitting Borrelia sp.) parasitize snakes and lizards [22]. The mechanisms discussed here suggest that regular moulting of these vertebrates might have consequences for their likelihood of becoming infected, especially when the time before transmission takes several days. If the host can shed its skin with the vector before the transfer of the endoparasite, it might explain, in part, the observation that lizards are less good hosts for certain parasites than other vertebrates [22].
Exposure to ‘micro-organism-enriched’ water has been shown to increase moulting intervals in another crustacean [9]. Therefore, we tested whether D. magna exposed to P. ramosa can accelerate their moulting cycles. The results represented in figure 3 suggest that this is not the case. The induction of moulting may be physiologically constrained either altogether or within the limit of 12 h, during which moulting could help reduce infection. However, somatic growth of crustaceans, and thus the moulting cycle, are known to be affected by environmental conditions (e.g. food [23] and temperature [24]) and the reaction norms are different between genotypes [25]. In parallel, in the Daphnia–Pasteuria system, as in many others, environmental factors are known to affect infection outcomes differently according to the host genotype, the parasite genotype or their combination [26]. Our results suggest that host moulting may contribute to this interaction between parasite success, host clone and environment.
In summary, we confirmed the hypothesis that when an Ecdysozoa host moults shortly after parasite exposure and attachment, the parasite infection process is compromised. Therefore, moulting can be advantageous to prevent parasite infections and might select for higher parasite penetration speed. It also shifts the cost–benefit calculation for moulting further in the direction of the benefits. We showed that, in our system, this process is not accelerated by the contact with the parasite, whereas in other host species this may be the case.
Acknowledgments
We thank Tim Janicke, Benjamin Lange, Flore Mas, Peter Sandner, Emilia Santos, Lukas Schärer, Lisa Schild, Dita Vizoso, Laura Walther and Thomas Zumbrunn for help in the laboratory; and Patrícia Beldade for comments on the manuscript. This study was supported by the Swiss National Science Foundation.
References
- 1.Bernard J. J., Gallo R. L. 2011. Protecting the boundary: the sentinel role of host defense peptides in the skin. Cell. Mol. Life Sci. 68, 2189–2199 10.1007/s00018-011-0712-8 (doi:10.1007/s00018-011-0712-8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corteel M., Dantas-Lima J. J., Wille M., Alday-Sanz V., Pensaert M. B., Sorgeloos P., Nauwynck H. J. 2009. Molt stage and cuticle damage influence white spot syndrome virus immersion infection in penaeid shrimp. Vet. Microbiol. 137, 209–216 10.1016/j.vetmic.2009.01.018 (doi:10.1016/j.vetmic.2009.01.018). [DOI] [PubMed] [Google Scholar]
- 3.Mendgen K., Hahn M., Deising H. 1996. Morphogenesis and mechanisms of penetration by plant pathogenic fungi. Annu. Rev. Phytopathol. 34, 367–386 10.1146/annurev.phyto.34.1.367 (doi:10.1146/annurev.phyto.34.1.367). [DOI] [PubMed] [Google Scholar]
- 4.Xu Y., Weiss L. M. 2005. The microsporidian polar tube: a highly specialised invasion organelle. Int. J. Parasitol. 35, 941–953 10.1016/j.ijpara.2005.04.003 (doi:10.1016/j.ijpara.2005.04.003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Field R. H., Chapman C. J., Taylor A. C., Neil D. M., Vickerman K. 1992. Infection of the Norway lobster Nephrops norvegicus by Hematodinium-like species of Dinoflagellate on the west coast of Scotland. Dis. Aquat. Org. 13, 1–15 10.3354/dao013001 (doi:10.3354/dao013001). [DOI] [Google Scholar]
- 6.Gaiser E. E., Bachmann R. W. 1993. The ecology and taxonomy of epizoic diatoms on Cladocera. Limnol. Oceanogr. 38, 628–637 10.4319/lo.1993.38.3.0628 (doi:10.4319/lo.1993.38.3.0628). [DOI] [Google Scholar]
- 7.Threlkeld S. T., Chiavelli D. A., Willey R. L. 1993. The organization of zooplankton epibiont communities. Trends Ecol. Evol. 8, 317–321 10.1016/0169-5347(93)90238-K (doi:10.1016/0169-5347(93)90238-K). [DOI] [PubMed] [Google Scholar]
- 8.Plaistow S. J., Outreman Y., Moret Y., Rigaud T. 2003. Variation in the risk of being wounded: an overlooked factor in studies of invertebrate immune function? Ecol. Lett. 6, 489–494 10.1046/j.1461-0248.2003.00455.x (doi:10.1046/j.1461-0248.2003.00455.x). [DOI] [Google Scholar]
- 9.Moret Y., Rigaud T., Motreuil S., Troussard J. P., Moreau J. 2010. Condition-dependent ecdysis and immunocompetence in the amphipod crustacean, Gammarus pulex. Biol. Lett. 6, 788–791 10.1098/rsbl.2010.0234 (doi:10.1098/rsbl.2010.0234). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sayre R. M. 1993. Pasteuria, Metchnikoff, 1888. In Bacillus subtilis and other gram positive bacteria (eds Sonenshein A. L., Hoch J. A., Losick R.), pp. 101–112 Washington, DC: American Society for Microbiology [Google Scholar]
- 11.Duneau D., Luijckx P., Ben-Ami F., Laforsch C., Ebert D. 2011. Resolving the infection process reveals striking differences in the contribution of phylogeny, genetics and environment to host–parasite interactions. BMC Biol. 9, 11. 10.1186/1741-7007-9-11 (doi:10.1186/1741-7007-9-11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grassé P. P. 2006. The Crustacea, revised and updated from the Traité de zoologie. In Traité de zoologie (eds Forest J., Von Vaupel Klein J. C.). Leiden, The Netherlands: Brill Academic [Google Scholar]
- 13.Klüttgen B., Dulmer U., Engels M., Ratte H. T. 1994. ADaM, an artificial freshwater for the culture of zooplankton. Water Res. 28, 743–746 10.1016/0043-1354(94)90157-0 (doi:10.1016/0043-1354(94)90157-0). [DOI] [Google Scholar]
- 14.Luijckx P., Ben-Ami F., Mouton L., Du Pasquier L., Ebert D. 2011. Cloning of the unculturable parasite Pasteuria ramosa and its Daphnia host reveals extreme genotype–genotype interactions. Ecol. Lett. 14, 125–131 10.1111/j.1461-0248.2010.01561.x (doi:10.1111/j.1461-0248.2010.01561.x). [DOI] [PubMed] [Google Scholar]
- 15.R Development Core Team 2008. R: a language and environment for statistical computing, 2.8.1 edn. Vienna, Austria: R foundation for statistical computing [Google Scholar]
- 16.Ebert D. 1994. A maturation size threshold and phenotypic plasticity of age and size at maturity in Daphnia magna. Oikos 69, 309–317 10.2307/3546152 (doi:10.2307/3546152). [DOI] [Google Scholar]
- 17.Altermatt F., Ebert D. 2008. Genetic diversity of Daphnia magna populations enhances resistance to parasites. Ecol. Lett. 11, 918–928 10.1111/j.1461-0248.2008.01203.x (doi:10.1111/j.1461-0248.2008.01203.x). [DOI] [PubMed] [Google Scholar]
- 18.Decaestecker E., Gaba S., Raeymaekers J. A. M., Stoks R., Van Kerckhoven L., Ebert D., De Meester L. 2007. Host–parasite ‘Red Queen’ dynamics archived in pond sediment. Nature 450, 870–873 10.1038/nature06291 (doi:10.1038/nature06291). [DOI] [PubMed] [Google Scholar]
- 19.Ben-Ami F., Mouton L., Ebert D. 2008. The effects of multiple infections on the expression and evolution of virulence in a Daphnia–endoparasite system. Evolution 62, 1700–1711 10.1111/j.1558-5646.2008.00391.x (doi:10.1111/j.1558-5646.2008.00391.x). [DOI] [PubMed] [Google Scholar]
- 20.Jensen K. H., Little T., Skorping A., Ebert D. 2006. Empirical support for optimal virulence in a castrating parasite. PLoS Biol. 4, 1265–1269 10.1371/journal.pbio.0040197 (doi:10.1371/journal.pbio.0040197). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hojgaard A., Eisen R. J., Piesman J. 2008. Transmission dynamics of Borrelia burgdorferi s.s. during the key third day of feeding by nymphal Ixodes scapularis (Acari: Ixodidae). J. Med. Entomol. 45, 732–736 10.1603/0022-2585(2008)45[732:tdobbs]2.0.co;2 (doi:10.1603/0022-2585(2008)45[732:tdobbs]2.0.co;2) [DOI] [PubMed] [Google Scholar]
- 22.Giery S. T., Ostfeld R. S. 2007. The role of lizards in the ecology of Lyme disease in two endemic zones of the northeastern United States. J. Parasitol. 93, 511–517 10.1645/GE-1053R1.1 (doi:10.1645/GE-1053R1.1). [DOI] [PubMed] [Google Scholar]
- 23.Ebert D. 1992. A food-independent maturation threshold and size at maturity in Daphnia magna. Limnol. Oceanogr. 37, 878–881 10.4319/lo.1992.37.4.0878 (doi:10.4319/lo.1992.37.4.0878). [DOI] [Google Scholar]
- 24.Stoner A. W., Ottmar M. L., Copeman L. A. 2010. Temperature effects on the molting, growth, and lipid composition of newly-settled red king crab. J. Exp. Mar. Biol. Ecol. 393, 138–147 10.1016/j.jembe.2010.07.011 (doi:10.1016/j.jembe.2010.07.011). [DOI] [Google Scholar]
- 25.Mitchell S. E., Lampert W. 2000. Temperature adaptation in a geographically widespread zooplankter, Daphnia magna. J. Evol. Biol. 13, 371–382 10.1046/j.1420-9101.2000.00193.x (doi:10.1046/j.1420-9101.2000.00193.x). [DOI] [Google Scholar]
- 26.Lazzaro B. P., Little T. J. 2009. Immunity in a variable world. Phil. Trans. R. Soc. B 364, 15–26 10.1098/rstb.2008.0141 (doi:10.1098/rstb.2008.0141). [DOI] [PMC free article] [PubMed] [Google Scholar]