Abstract
Objectives:
Our objective was to compare long-term outcomes of deep brain stimulation (DBS) of the globus pallidus interna (GPi) and subthalamic nucleus (STN) for patients with Parkinson disease (PD) in a multicenter randomized controlled trial.
Methods:
Patients randomly assigned to GPi (n = 89) or STN DBS (n = 70) were followed for 36 months. The primary outcome was motor function on stimulation/off medication using the Unified Parkinson's Disease Rating Scale motor subscale. Secondary outcomes included quality of life and neurocognitive function.
Results:
Motor function improved between baseline and 36 months for GPi (41.1 to 27.1; 95% confidence interval [CI] −16.4 to −10.8; p < 0.001) and STN (42.5 to 29.7; 95% CI −15.8 to −9.4; p < 0.001); improvements were similar between targets and stable over time (p = 0.59). Health-related quality of life improved at 6 months on all subscales (all p values significant), but improvement diminished over time. Mattis Dementia Rating Scale scores declined faster for STN than GPi patients (p = 0.01); other neurocognitive measures showed gradual decline overall.
Conclusions:
The beneficial effect of DBS on motor function was stable and comparable by target over 36 months. Slight declines in quality of life following initial gains and gradual decline in neurocognitive function likely reflect underlying disease progression and highlight the importance of nonmotor symptoms in determining quality of life.
Classification of Evidence:
This study provides Class III evidence that improvement of motor symptoms of PD by DBS remains stable over 3 years and does not differ by surgical target. Neurology® 2012;79:55–65
Deep brain stimulation (DBS) of the globus pallidus interna (GPi) or subthalamic nucleus (STN) is an accepted treatment for advanced Parkinson disease (PD) when symptoms are no longer managed adequately with medications. Recent publications have demonstrated that DBS is superior to best medical therapy to manage motor symptoms and improve quality of life,1–3 and that STN and GPi DBS are similarly effective in improving motor function and quality of life for patients with PD over 24 months.4
Reports of long-term (>24 months postsurgery) outcomes of DBS for PD are limited and none provide long-term comparisons of GPi and STN DBS. A few studies have examined long-term outcomes of STN DBS and describe generally stable motor responses.5–11 Long-term outcomes following GPi DBS are more uncertain because with one exception,4 the available studies were comprised of small numbers of patients in nonrandomized designs.12–16 Early reports of GPi DBS indicated loss of efficacy,12,13,15 raising questions about the durability of responses to GPi DBS.14 However, early reports of GPi DBS may not reflect contemporary practice. More recent nonrandomized studies find the effects of STN and GPi stimulation on motor function to be sustained up to 5–6 years following surgery.11,17
This article provides the first comparison of long-term (36-month) responses of motor and nonmotor symptoms to DBS in a carefully characterized population of patients with PD enrolled into a prospective, blinded, randomized controlled study comparing GPi and STN DBS.4
METHODS
Enrollment criteria, study design, and study procedures have been reported previously,1,4 and are only described briefly here. Patients were enrolled at 7 Veterans Affairs (VA) and 6 affiliated university medical centers. Eligibility requirements included patients with idiopathic PD who were Hoehn & Yahr stage ≥2 off medication18; levodopa-responsive; with persistent disabling symptoms despite optimal medication management (e.g., motor fluctuations, dyskinesia), ≥3 hours per 24-hour period with poor motor function/symptom control (“off” or “on with troubling dyskinesias”), stable medical therapy for ≥1 month; and age ≥21 years old. Patients were randomized to GPi or STN DBS and followed for 24 months. A subset of patients randomized before October 31, 2005, were eligible and consented for an additional 12 months follow-up and are reported here.
Baseline assessment included evaluation of motor symptoms off medications (in the “practically defined off state”)19 using the Unified Parkinson's Disease Rating Scale motor subscale (UPDRS III)20 and the stand-walk-sit test19 evaluated by study personnel and independently by movement disorders clinicians blinded to DBS target. In the on-medication state, patients were assessed again using the UPDRS III and stand-walk-sit tests; and on the Hoehn & Yahr, Schwab and England, UPDRS part I (mentation, behavior, and mood), part II (activities of daily living), and part IV (complications of therapy) subscales, the PD Questionnaire-39 (PDQ-39), and the Beck Depression Inventory II (BDI-II).19–22 The study nurse recorded patient medications, physical health status, and PD symptoms. A neurocognitive test battery was administered by a neuropsychologist (described previously).1,4
Motor diaries were used to assess self-reported function.23,24 Patients recorded which of 4 categories (on, on with troubling dyskinesia, off, or asleep) best reflected their predominant functioning for the prior 30 minutes in half-hour intervals for 2 days prior to each study visit.23 Time spent in each category was averaged over the 2 days.
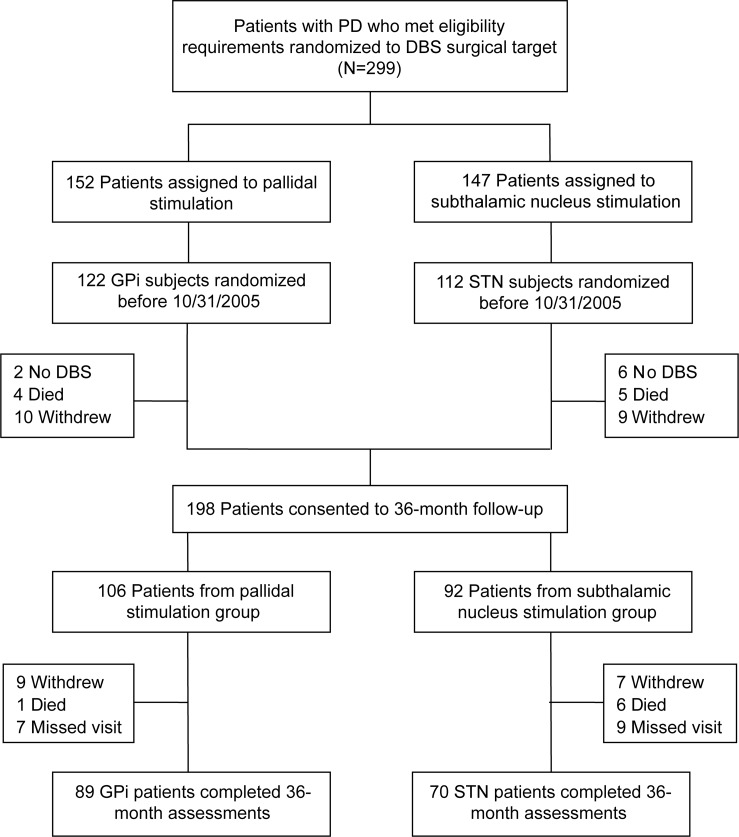
Randomization was stratified by study site and patient age (<70 vs ≥70 years old) using computer-generated assignment by the coordinating center. Patients underwent surgery within 1 month of randomization and remained blinded to DBS target for the study duration. Of the 299 patients randomized to surgical target, 198 were consented for 36-month follow-up (figure 1). Those who did not proceed to 36 months (n = 101) included 65 patients randomized after October 31, 2005, 9 deaths, 8 who did not receive DBS, and 19 withdrawals. A total of 159 patients completed the 36-month visit (80.3%); 39 patients died (7), withdrew (16), or missed the 36-month visit (16). For this article, analyses are based on these 159 patients.
Figure 1. Enrollment and outcome assessment for deep brain stimulation (DBS) in Parkinson disease (PD).
STN = subthalamic nucleus.
Standard protocol approvals, registrations, and patient consents.
The study was approved by all site institutional review boards and participants provided written informed consent. This study was registered at clinicaltrials.gov identifier: NCT00056563 and NCT01076452.
Follow-up.
Patients returned at 3, 6, 12, 18, 24, and 36 months for assessment. Baseline assessments including blinded motor evaluations were repeated at 6, 24, and 36 months with abbreviated assessments at 3, 12, and 18 months. Study personnel (unblinded) and movement disorders clinicians blinded to DBS targets independently assessed patients' motor scores on stimulation/off medication. Next, DBS was deactivated for 1 hour for a second assessment off stimulation/off medication. Then DBS was reactivated, patients took their medications, and returned approximately 1 hour later for a third set of assessments on stimulation/on medication, including the other UPDRS subscales, quality of life, and neurocognitive tests. Postoperative patient management was guided by study neurologists with the goal of achieving best symptom control regardless of DBS target, including medication adjustments and provision of nonpharmacologic treatments (e.g., physical therapy) as deemed appropriate.
Statistical analysis.
t Tests or Fisher exact tests were used for comparisons of baseline characteristics between treatment groups and subgroups. Medication was converted to levodopa equivalents for analysis.6 Mixed models analyses were conducted to account for the repeated measurements with random missing data. Time intervals treated as categorical variables were used to analyze changes over time or between 2 time points. Hypothesis tests with orthogonal polynomial contrasts were applied for the main time trend effect and its interaction with the treatment group. In addition, sensitivity analyses using mixed models treating time intervals as continuous variables were performed to verify the rate change (slope) over time treating groups as fixed effects, and the intercept and the linear time trend component as random effects. Analyses were performed using SAS software (version 9.2). All statistical tests were 2-sided and a p value ≤ 0.05 was considered statistically significant with no formal correction for multiple analyses.
This intervention study provides Class III evidence that GPi and STN DBS similarly improved and sustained motor function at 36 months postsurgery.
RESULTS
Eighty-nine GPi and 70 STN patients completed the 36-month assessment. Comparison of patients who completed the 36-month follow-up with patients from the full sample who did not complete this assessment (n = 140) showed the groups to be similar at baseline except completers were younger (60.6 vs 63.2 years; p = 0.01). Similarly, those who completed the 36-month follow-up were similar to those who consented but did not complete the 36-month assessment (n = 39) at baseline, except they were younger (60.6 vs 64.7 years; p = 0.01).
Baseline characteristics (n = 159) for GPi and STN groups, prior to surgery, were similar with the exception of quality of life and neurocognitive function. Differences in the PDQ-39 subscales for mobility (p = 0.01), emotional role function (p = 0.04), stigma (p = 0.03), social support (p = 0.01), and communication (p = 0.001) were worse (higher scores) for STN than GPi patients (table 1). GPi patients had better baseline verbal fluency–semantic (p = 0.02) and verbal learning total (p = 0.03) scores compared to STN patients (table e-1 on the Neurology® Web site at www.neurology.org).
Table 1.
Baseline characteristics of patients who received DBS and had 3-year assessments
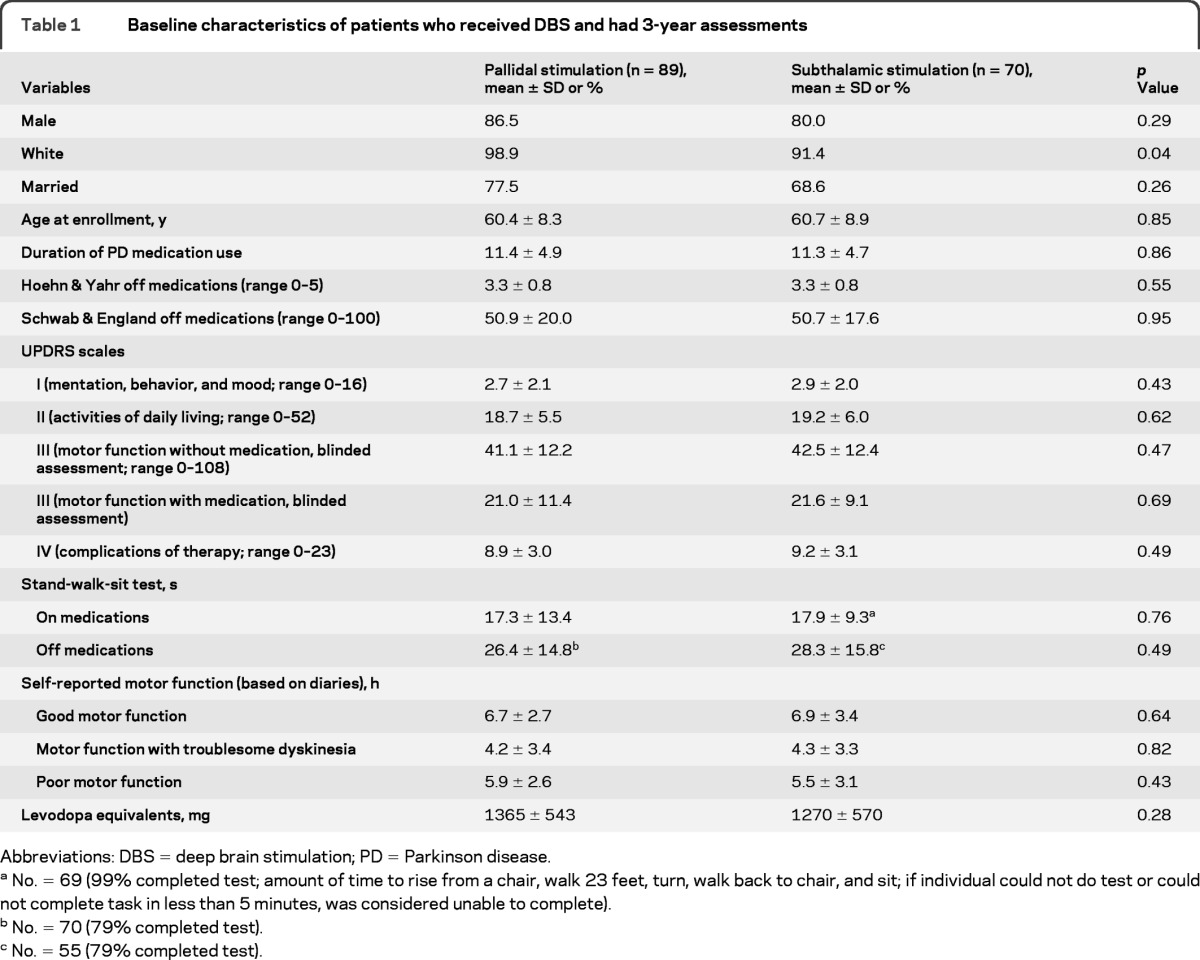
Abbreviations: DBS = deep brain stimulation; PD = Parkinson disease.
No. = 69 (99% completed test; amount of time to rise from a chair, walk 23 feet, turn, walk back to chair, and sit; if individual could not do test or could not complete task in less than 5 minutes, was considered unable to complete).
No. = 70 (79% completed test).
No. = 55 (79% completed test).
Motor function.
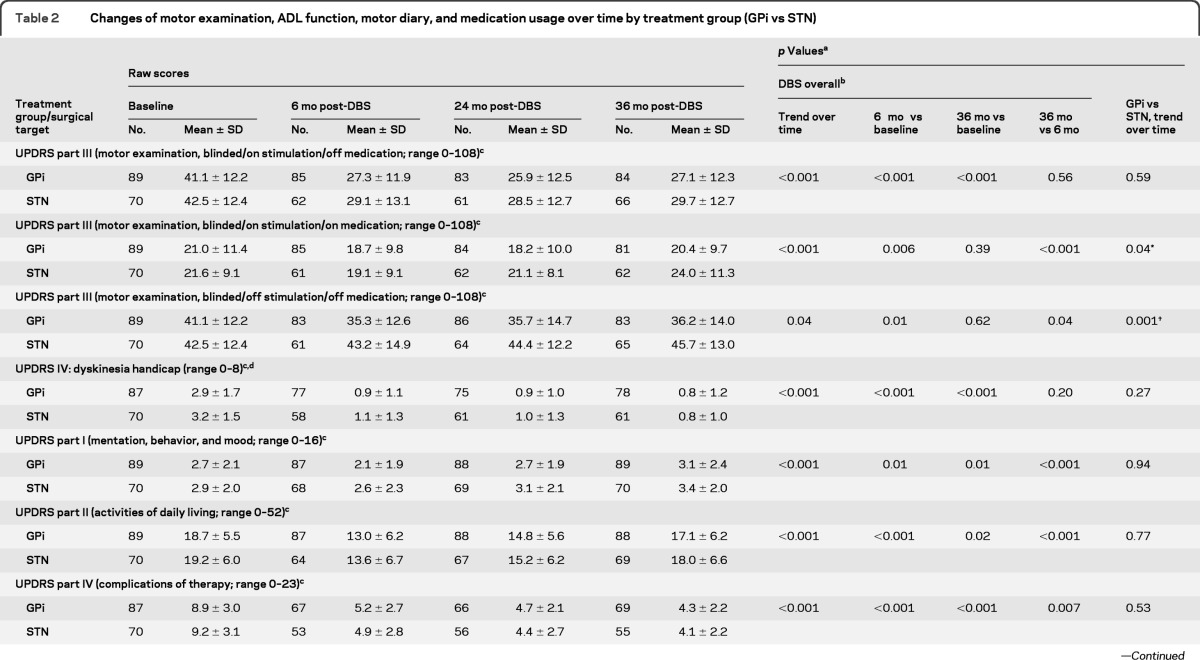
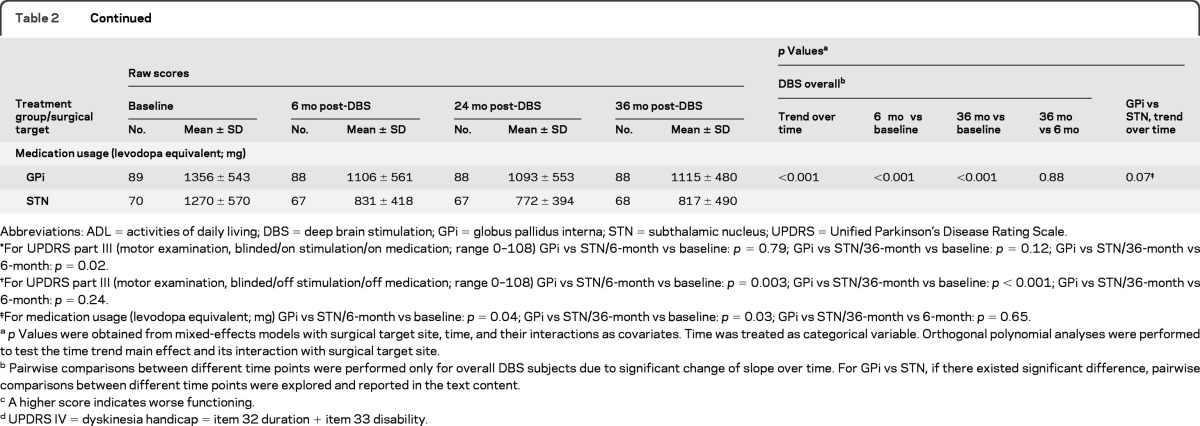
Change in UPDRS motor scores over time in the on stimulation/off medication state was the primary outcome. Motor function improved between baseline and 36 months for GPi (41.1 to 27.1; 95% confidence interval [CI] −16.4 to −10.8; p < 0.001) and STN (42.5 to 29.7; 95% CI −15.8 to −9.4; p < 0.001); improvements were similar between targets and stable over 36 months (table 2 and figure e-1). The pairwise comparisons of 6-month vs baseline (p < 0.001), 36-month vs baseline (p < 0.001), and 36-month vs 6-month (p = 0.56) scores supported the findings of improvement at 6 months and the stability of this improvement through 36 months. The interaction of main time trend effect with treatment group (p = 0.59) suggested a parallel trend (no change) over time for the 2 targets. Sensitivity analyses with time treated as a continuous variable with or without transformation were performed and the same conclusions were obtained. Differences in motor scores between 6, 24, and 36 months were less than 2 points in either target group in the on stimulation/off medication state. Motor subfeatures also improved to a similar extent between targets. Subscales for tremor, rigidity, akinesia, postural stability, and gait25 assessed on stimulation/off medication showed improvement at 6 months in both target groups which was maintained over time (table e-2).
Table 2.
Changes of motor examination, ADL function, motor diary, and medication usage over time by treatment group (GPi vs STN)
Abbreviations: ADL = activities of daily living; DBS = deep brain stimulation; GPi = globus pallidus interna; STN = subthalamic nucleus; UPDRS = Unified Parkinson's Disease Rating Scale.
For UPDRS part III (motor examination, blinded/on stimulation/on medication; range 0–108) GPi vs STN/6-month vs baseline: p = 0.79; GPi vs STN/36-month vs baseline: p = 0.12; GPi vs STN/36-month vs 6-month: p = 0.02.
For UPDRS part III (motor examination, blinded/off stimulation/off medication; range 0–108) GPi vs STN/6-month vs baseline: p = 0.003; GPi vs STN/36-month vs baseline: p < 0.001; GPi vs STN/36-month vs 6-month: p = 0.24.
For medication usage (levodopa equivalent; mg) GPi vs STN/6-month vs baseline: p = 0.04; GPi vs STN/36-month vs baseline: p = 0.03; GPi vs STN/36-month vs 6-month: p = 0.65.
p Values were obtained from mixed-effects models with surgical target site, time, and their interactions as covariates. Time was treated as categorical variable. Orthogonal polynomial analyses were performed to test the time trend main effect and its interaction with surgical target site.
Pairwise comparisons between different time points were performed only for overall DBS subjects due to significant change of slope over time. For GPi vs STN, if there existed significant difference, pairwise comparisons between different time points were explored and reported in the text content.
A higher score indicates worse functioning.
UPDRS IV = dyskinesia handicap = item 32 duration + item 33 disability.
Differences initially observed at 6 months postsurgery for motor function between GPi and STN groups off stimulation/off medication (p = 0.003) increased over time with GPi remaining stable and STN worsening over 36 months (p = 0.001; figure e-1). In the on stimulation/on medication condition, motor function improved at 6 months for DBS overall (p = 0.006), but this initial improvement diminished, and by 36 months scores diverged by target. Motor scores were worse than baseline for STN, but still better than baseline for GPi patients (GPi vs STN trend over time p = 0.04). The mentation/behavior/mood and activities of daily living UPDRS scores improved initially over baseline (ps < 0.001), however, by 36 months, scores increased (worsened) for both subscales and were worse than baseline for the UPDRS I (p < 0.01). Complications of therapy (UPDRS IV) subscale scores improved at 6 months and were maintained over 36 months (table 2). The initial decreases in postoperative medication dosages, greater for the STN than the GPi group, were sustained over 36 months (p = 0.07).
Self-reported motor function based on diaries showed that good function (on time without troublesome dyskinesia) improved following DBS and remained stable over 36 months (p = 0.48; table e-2). At 6 months, both groups experienced more than 5 hours gain in good functioning time; by 36 months, the gain dropped slightly to 4.6 hours/day for GPi and 4.1 hours/day for STN patients over baseline. Poor motor function (off time) and motor function with troubling dyskinesia decreased in both groups following DBS and remained stable through 36 months, while hours asleep increased for both groups and was sustained over time (all ps < 0.001).
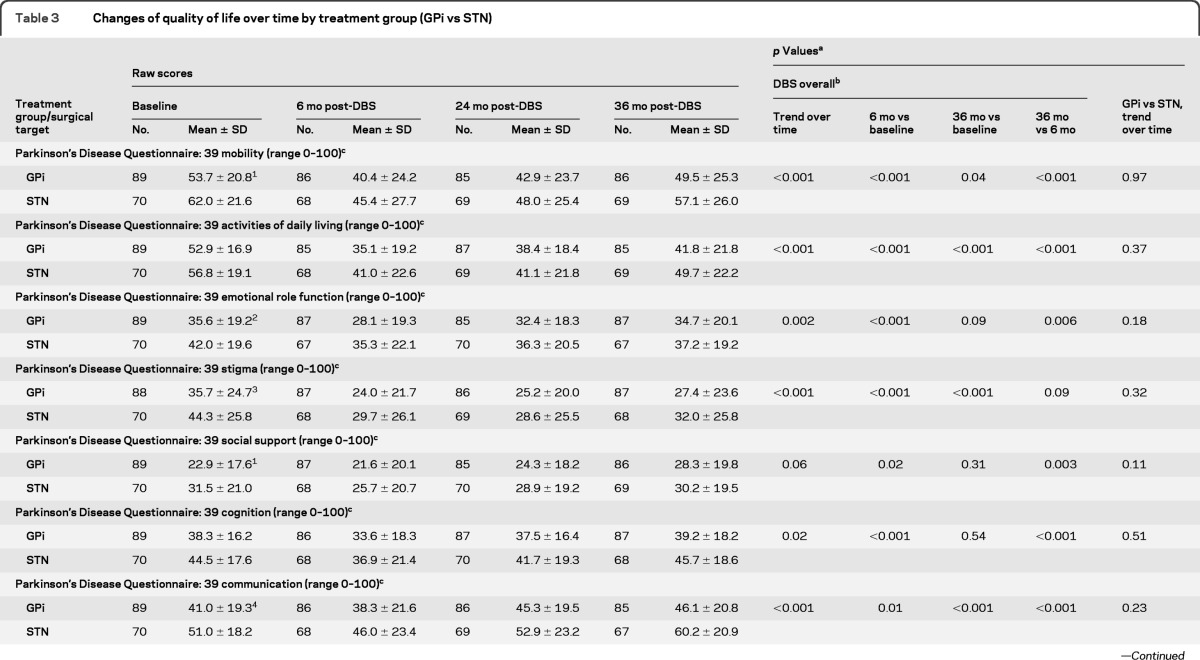
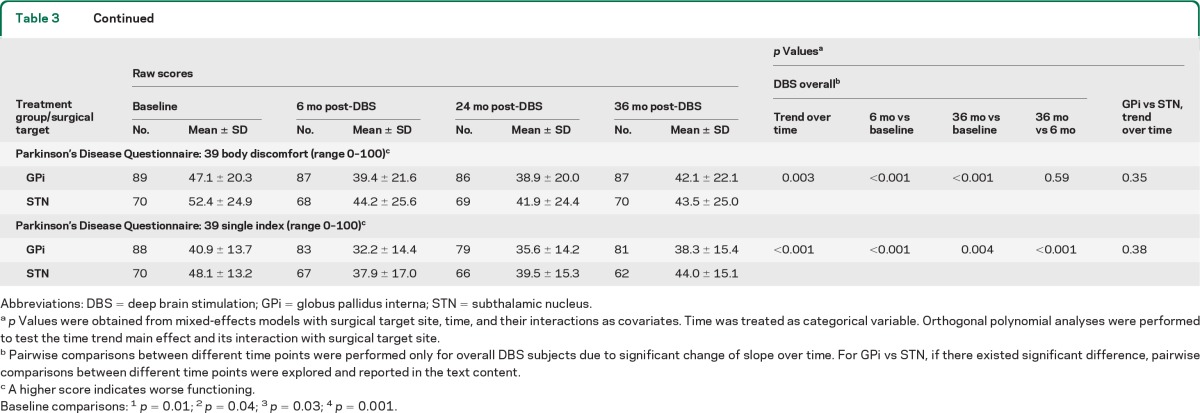
Changes in quality of life and neurocognitive measures (table 3 and figure e-2) were notable for a few items. All PDQ-39 subscales improved at 6 months following GPi and STN DBS, but there was some decrement in these gains over time, with 36-month scores worse than baseline for social support (p = 0.31) and cognition (p = 0.54). There were differences in the Mattis Dementia Scale. STN scores were slightly worse at 6 months vs no change in GPi (p = 0.03), followed by further gradual reductions in both groups over time, but STN more so than GPi (p = 0.01; table e-2). The Hopkins Verbal Learning Test Trials 1–3 Total scores (T score) differed by target (GPi group showed slight worsening at 6 months but then was stable, whereas STN was unchanged at 6 months but worsened by 36 months, p = 0.01). The Hopkins Verbal Learning Test Delayed Recall showed a similar pattern of results (trend over time p = 0.004). Other aspects of neurocognitive function showed either similar decline (trends over time for Wisconsin Card Sorting Test p = 0.03; Brief Visuospatial Memory Test Total Score, p = 0.001; Brief Visuospatial Memory Test Delayed Recall, p < 0.001), or no change in either target group. Depression scores did not change from baseline over 36 months, and no long-term target differences were noted.
Table 3.
Changes of quality of life over time by treatment group (GPi vs STN)
Abbreviations: DBS = deep brain stimulation; GPi = globus pallidus interna; STN = subthalamic nucleus.
p Values were obtained from mixed-effects models with surgical target site, time, and their interactions as covariates. Time was treated as categorical variable. Orthogonal polynomial analyses were performed to test the time trend main effect and its interaction with surgical target site.
Pairwise comparisons between different time points were performed only for overall DBS subjects due to significant change of slope over time. For GPi vs STN, if there existed significant difference, pairwise comparisons between different time points were explored and reported in the text content.
A higher score indicates worse functioning.
Baseline comparisons: 1p= 0.01; 2p= 0.04; 3p= 0.03; 4p= 0.001.
The same mixed model analyses were performed on the 198 patients who consented to the 36-month visit. Results were similar suggesting conclusions from the completer sample (n = 159) are representative of the larger sample.
DISCUSSION
We reported previously the results of a prospective, randomized trial comparing outcomes of GPi and STN DBS at 2 years.4 In that analysis, motor function improved following DBS, the degree of improvement was comparable by target, and we did not observe a change in motor responses over 24 months. In the current extended follow-up of a subset of patients, blinded assessment of motor responses to STN and GPi DBS remained stable through 36 months.
Our findings are consistent with other reports of extended follow-up of STN DBS, which generally describe stable responses over time,5–11 but differ from some early reports of long-term follow-up of GPi DBS. Several small uncontrolled case series indicated that efficacy of bilateral GPi DBS wanes over 1–3 years.12–15 Other studies show stable responses up to 6 years postsurgery,11,17,26–28 consistent with the motor outcomes we observed in our study population. Most of the case series describing GPi failures were from the 1990s. The failure rate of GPi DBS in those series, up to 80% over 3 years,15 is too great to attribute exclusively to study design (uncontrolled case series vs our randomized trial) and may reflect differences in patient selection, targeting, implantation technique, or postoperative management that have evolved since those studies were published.
We observed a significant divergence of GPi and STN motor outcomes at 36 months in the on stimulation/on medication condition despite stable doses of medications (albeit differentially reduced dosages from baseline levels for GPi and STN) in both groups over time. The synergistic effect of DBS plus medications seen in both groups at 6 months was largely maintained in the GPi group over time. In contrast, the combined medications and DBS effect became significantly less in the STN group by 36 months, at which time the average motor score on stimulation/on medication was worse than the on-medication score at baseline, before DBS. The reduced synergistic effect of medications plus DBS in the setting of preserved motor responses to STN DBS alone (i.e., on stimulation/off medication scores) indicates reduced medication effectiveness as the cause of deteriorating motor scores in the on stimulation/on medication state, perhaps because dopaminergic medication was reduced to a greater extent in the STN group initially and was not increased over time. The GPi group had consistently lower (better) motor scores compared to the STN group off medication/off stimulation, and the motor scores remained stable throughout the 36-month follow-up period. This could be due to prolonged residual benefits of higher daily doses of dopaminergic medications in the GPi group (e.g., longer washout required for larger doses of medications). Alternatively, this could be an artifact of the evaluation paradigm, in which DBS was deactivated for 1 hour prior to the off stimulation assessment. If GPi DBS has a longer “washout” period following deactivation compared to STN DBS (i.e., a washout period >1 hour), the off stimulation score 1 hour following DBS deactivation would be lower (better) than the off stimulation score for STN subjects. However, we observed that motor scores for the STN group in the off stimulation/off medication condition gradually worsened over time despite a stable test paradigm and stable medication doses in both groups, suggesting that difference between GPi and STN DBS is not related exclusively to a longer medication washout period or for DBS deactivation. The gradual worsening of off stimulation/off medication scores in the STN group cannot be attributed exclusively to disease progression, because a similar worsening should have affected the GPi group as well. Whether GPi DBS or the higher doses of medications used in the GPi population alter progression of PD symptoms cannot be determined from our data. We identified some neurocognitive differences between GPi and STN DBS groups over time, as well as overall longitudinal declines that occur in both groups; close to 0.5 standard deviations on several measures over 36 months. Most studies of DBS in PD report small declines in neurocognitive function over time, but these declines are generally considered acceptable given the gains in motor function,10 and some continued longitudinal decline in test performance is expected given the known neurodegenerative changes associated with PD.
While similar rates of decline were observed in GPi and STN groups on many measures of cognitive functioning, we observed statistically significant differences between GPi and STN groups on the Mattis Dementia Rating Scale and the Hopkins Verbal Learning Test, both of which showed no change in the GPi group but worsening by 36 months in the STN group. The importance of these target differences must be interpreted cautiously in light of a few differences at baseline (STN patients were slightly worse on average than GPi patients on some baseline neurocognitive tests), because we did not adjust for these and other covariates as confounders. The precise determinants, clinical significance, and functional impact of these small group differences are not known. In our original study cohort, we observed slight improvement in depression scores for the GPi group with slight worsening in the STN group at 24 months.4 The present study suggests that by 36 months depression scores for both targets are comparable to baseline, with no group differences present.
The quality of life (PDQ-39) subscales show improvement in both groups at 6 months followed by diminishing improvement in several subscales over the remainder of the 36-month period. Emotional well-being, social support, and cognition subscales showed no difference between 36-month scores and baseline. Other subscales showed significant improvement over baseline but generally the improvement was small and of questionable clinical benefit. Activities of daily living showed a similar trend, with improvement early after surgery followed by gradual loss of improvement over the subsequent assessments. Similar loss of improvement of activities of daily living despite stable motor responses to DBS have been reported previously.9,10
A potential limitation of these data is that our sample at 36 months is approximately half the size of the original study cohort; yet baseline comparisons with the full sample show these groups to be very similar, particularly with respect to our primary outcome (UPDRS motor score).
Our data indicate that GPi and STN DBS improve motor function in patients with PD, the extent of improvement in motor function does not differ by DBS target, and the motor benefit is maintained over 36 months. In contrast, some early gains in quality of life and activities of daily living are gradually lost, and decline in neurocognitive function is seen over serial evaluations, most likely reflecting disease progression and worsening or emergence of DBS- and medication-resistant symptoms. The greater worsening in cognition and on stimulation/on medication motor scores over time for STN needs further evaluation, which could include efforts to determine whether increasing medication doses over time could attenuate these differences between GPi and STN. Our findings indicate that GPi and STN are both viable DBS targets for treatment of motor symptoms, but highlight the importance of nonmotor symptoms as determinants of quality of life in people with PD.29,30
Supplementary Material
GLOSSARY
- BDI-II
Beck Depression Inventory II
- CI
confidence interval
- DBS
deep brain stimulation
- GPi
globus pallidus interna
- PD
Parkinson disease
- PDQ-39
PD Questionnaire-39
- RCT
randomized controlled trial
- STN
subthalamic nucleus
- UPDRS III
Unified Parkinson's Disease Rating Scale motor subscale
- VA
Veterans Affairs
Footnotes
Editorial, page 19
Supplemental data at www.neurology.org.
Coinvestigators and Contributors are listed on the Neurology® Web site at www.neurology.org.
The full protocol is available from the Department of Veterans Affairs, Cooperative Studies Program Office within the Office of Research & Development, Washington, DC, CSP@va.gov or 202-461-1700.
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the National Institute of Neurological Disorders and Stroke.
AUTHOR CONTRIBUTIONS
F. Weaver: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, obtaining funding. K. Follett: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, study supervision, obtaining funding. M. Stern: study concept or design, analysis or interpretation of data, study supervision. P. Luo: drafting/revising the manuscript, analysis or interpretation of data, statistical analysis. C. Harris: analysis or interpretation of data, acquisition of data, study supervision. K. Hur: study concept or design, acquisition of data, study supervision. W. Marks: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, study supervision. J. Rothlind: drafting/revising the manuscript, study concept or design. O. Sagher: drafting/revising the manuscript, study concept or design, analysis or interpretation of data. C. Moy: drafting/revising the manuscript. R. Pahwa: drafting/revising the manuscript, study supervision, served on steering committee. K. Burchiel: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, study supervision. P. Hogarth: drafting/revising the manuscript, acquisition of data, study supervision. E. Lai: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, study supervision. J. Duda: drafting/revising the manuscript, acquisition of data, study supervision. K. Holloway: drafting/revising the manuscript, acquisition of data, study supervision. A. Samii: drafting/revising the manuscript, acquisition of data. S. Horn: drafting/revising the manuscript, acquisition of data, study supervision. J. Bronstein: drafting/revising the manuscript, study concept or design, acquisition of data, study supervision, obtaining funding. G. Stoner: study concept or design, study supervision. P. Starr: study concept or design, contribution of vital reagents/tools/patients, acquisition of data. R. Simpson: drafting/revising the manuscript, study concept or design, acquisition of data, study supervision. G. Baltuch: drafting/revising the manuscript, study concept or design, contribution of vital reagents/tools/patients, acquisition of data, study supervision, performed procedure, pre and postop follow-up. A. DeSalles: drafting/revising the manuscript, acquisition of data, obtaining funding, surgeon for my group and providing data. G. Huang: drafting/revising the manuscript, study supervision, obtaining funding. D. Reda: drafting/revising the manuscript, study concept or design, study supervision, obtaining funding. Ping Luo, PhD, had full access to all data in the study and takes responsibility for data integrity and accuracy of the data analysis.
DISCLOSURE
F. Weaver reports no disclosures relevant to the manuscript. K. Follett reports serving as a consultant for Medtronic Neurological (safety monitoring panel). M. Stern reports receiving consulting fees from Teva, Ipsen, Medtronic, Adamas Pharma, Schering-Plough, payment for speakers bureaus from Teva, Novartis, and the Movement Disorder Society, payment for development of educational presentations from Teva and Medtronic. P. Luo, C. Harris, and K. Hur report no disclosures relevant to the manuscript. W. Marks reports receiving consulting fees from Medtronic and lecture fees from Medtronic. J. Rothlind, O. Sagher, and C. Moy report no disclosures relevant to the manuscript. R. Pahwa reports receiving consulting fees from Teva, GSK, GE Healthcare, EMD Serono, Boehringer Ingelheim, Medtronic, St Jude Medical, Impax Pharma, Novartis, Adamas Pharma, and Biogen, providing expert testimony, grants/grants pending from Teva, Novartis, Xenoport, Impax Pharma, Boehringer Ingelheim, Solvay, EMD Serono, Eisai, NIH/NINDS, NPF, Allon, Acadia, GSK, Valeant, Kyowa, Johnson & Johnson, Santhera, and Schering, payment for lectures from Teva, GSK, and Medtronic, royalties from Oxford Press and Informa Healthcare, payment for development of educational presentations from Medscape, Co-Editor in Chief International Journal of Neuroscience, and DSMB member (Ceregene). K. Burchiel reports grants/grants pending from Medtronic, and owning stock/stock options in Medtronic. P. Hogarth, E. Lai, and J. Duda report no disclosures relevant to the manuscript. K. Holloway reports receiving consulting fees and grant/grants pending from Medtronic. A. Samii reports receiving payment for lectures from Teva, Ipsen, and Boehringer Ingelheim. S. Horn, J. Bronstein, and G. Stoner report no disclosures relevant to the manuscript. P. Starr reports receiving consulting fees from Medtronic. R. Simpson reports participating on advisory board and receiving consulting fees from Medtronic, and providing expert testimony. G. Baltuch, A. DeSalles, G. Huang, and D. Reda report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1. Weaver FM, Follett K, Stern M, et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA 2009; 301: 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Deuschl G, Schade-Brittinger C, Krack P, et al. A randomized trial of deep-brain stimulation for Parkinson's disease. N Engl J Med 2006; 355: 896–908. [DOI] [PubMed] [Google Scholar]
- 3. Williams A, Gill S, Varma T, et al. Deep brain stimulation plus best medical therapy versus best medical therapy alone for advanced Parkinson's disease (PD SURG trial): a randomised, open-label trial. Lancet Neurol 2010; 9: 581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Follett KA, Weaver FM, Stern M, et al. Pallidal versus subthalamic deep-brain stimulation for Parkinson's disease. N Engl J Med 2010; 362: 2077–2091. [DOI] [PubMed] [Google Scholar]
- 5. Schüpbach WM, Chastan N, Welter ML, et al. Stimulation of the subthalamic nucleus in Parkinson's disease: a 5 year follow up. J Neurol Neurosurg Psychiatry 2005; 76: 1640–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Krack P, Batir A, Van Blercom N, et al. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Engl J Med 2003; 349: 1925–1934. [DOI] [PubMed] [Google Scholar]
- 7. Gervais-Bernard H, Xie-Brustolin J, Mertens P, et al. Bilateral subthalamic nucleus stimulation in advanced Parkinson's disease: five year follow-up. J Neurol 2009; 256: 225–233. [DOI] [PubMed] [Google Scholar]
- 8. Piboolnurak P, Lang AE, Lozano AM, et al. Levodopa response in long-term bilateral subthalamic stimulation for Parkinson's disease. Mov Disord 2007; 22: 990–997. [DOI] [PubMed] [Google Scholar]
- 9. Romito LM, Contarino MF, Vanacore N, Bentivoglio AR, Scerrati M, Albanese A. Replacement of dopaminergic medication with subthalamic nucleus stimulation in Parkinson's disease: long-term observation. Mov Disord 2009; 24: 557–563. [DOI] [PubMed] [Google Scholar]
- 10. Fasano A, Romito LM, Daniele A, et al. Motor and cognitive outcome in patients with Parkinson's disease 8 years after subthalamic implants. Brain 2010; 133: 2664–2676. [DOI] [PubMed] [Google Scholar]
- 11. Moro E, Lozano AM, Pollak P, et al. Long-term results of a multicenter study on subthalamic and pallidal stimulation in Parkinson's disease. Mov Disord 2010; 25: 578–586. [DOI] [PubMed] [Google Scholar]
- 12. Ghika J, Villemure J-G, Fankhauser H, et al. Efficiency and safety of bilateral contemporaneous pallidal stimulation (deep brain stimulation) in levodopa-responsive patients with Parkinson's disease with severe motor fluctuations: a 2-year review. J Neurosurg 1998; 89: 713–718. [DOI] [PubMed] [Google Scholar]
- 13. Bacia-Salorio JL, Roldan R, Talamantes F, Fascual-Leone A. Electrical inhibition of basal ganglia nuclei in Parkinson's disease: long-term results. Stereotact Funct Neurosurg 1999; 72: 202–207. [DOI] [PubMed] [Google Scholar]
- 14. Volkmann J, Allert N, Voges J, et al. Long-term results of bilateral pallidal stimulation in Parkinson's disease. Ann Neurol 2004; 55: 871–875. [DOI] [PubMed] [Google Scholar]
- 15. Houeto JL, Bejjani PB, Damier P, et al. Failure of long-term pallidal stimulation corrected by Subthalamic stimulation in PD. Neurol 2000; 55: 728–730. [DOI] [PubMed] [Google Scholar]
- 16. Volkmann J, Albanese A, Kulisevsky J, et al. Long-term effects of pallidal or subthalamic deep brain stimulation on quality of life in Parkinson's disease. Mov Disord 2009; 24: 1154–1161. [DOI] [PubMed] [Google Scholar]
- 17. Rodriguez-Oroz MC, Obeso JA, Houeto J-L, et al. Bilateral deep brain stimulation in Parkinson's disease: a multicentre study with 4 years follow-up. Brain 2005; 128: 2240–2249. [DOI] [PubMed] [Google Scholar]
- 18. Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology 1967; 17: 427–442. [DOI] [PubMed] [Google Scholar]
- 19. Langston JW, Widner H, Goetz CG, et al. Core assessment program for intracerebral transplantations (CAPIT). Mov Disord 1992; 7: 2–13. [DOI] [PubMed] [Google Scholar]
- 20. Fahn S, Elton RL. members of the UPDRS Development Committee. Unified Parkinson's Disease Rating Scale. In: Fahn S, Marsden CD, Goldstein M.eds. Recent Developments in Parkinson's Disease, vol 2 Florham Park, NJ: Macmillan Health Care Information; 1987: 153–164. [Google Scholar]
- 21. Schwab RS, England AC. Projection Technique for Evaluating Surgery in Parkinson's Disease: Third Symposium on Parkinson's Disease. Edinburgh: Gillingham and Donaldson; 1969. [Google Scholar]
- 22. Peto V, Jenkinson C, Fitzpatrick R. PDQ-39: a review of the development, validation and application of a Parkinson's disease quality of life questionnaire and its associated measures. J Neurol 1998; 245 (suppl 1): S10–S14. [DOI] [PubMed] [Google Scholar]
- 23. Hauser RA, Deckers F, Lehert P. Parkinson's disease home diary: further validation and implications for clinical trials. Mov Disord 2004; 19: 1409–1413. [DOI] [PubMed] [Google Scholar]
- 24. Goetz CG, Stebbins GT, Blasucci LM, et al. Efficacy of a patient-training videotape on motor fluctuations for on-off diaries in Parkinson's disease. Mov Disord 1997; 12: 1039–1041. [DOI] [PubMed] [Google Scholar]
- 25. Lozano AM, Lang AE, Galvez-Jimenez N, Miyasaki J, Duff J, Hutchinson D. Effect of GPi pallidotomy on motor function in Parkinson's disease. The Lancet 1995; 346: 1383–1387. [DOI] [PubMed] [Google Scholar]
- 26. Durif F, Lemaire J-J, Debilly B, Dordain G. Long-term follow-up of globus pallidus internalis in Parkinson's disease. Mov Disord 2002; 4: 803–807. [DOI] [PubMed] [Google Scholar]
- 27. Gross C, Rougier A, Guehl D, et al. High-frequency stimulation of the globus pallidus chronic stimulation in Parkinson's disease: a study of seven cases. J Neurosurg 1997; 87: 491–497. [DOI] [PubMed] [Google Scholar]
- 28. Lyons K, Wilkinson S, Tröster AI, Pahwa R. Long-term efficacy of globus pallidus stimulation in advanced Parkinson's disease. Stereotact Funct Neurosurg 2002; 79: 214–220. [DOI] [PubMed] [Google Scholar]
- 29. Schrag A, Jahanshahi M, Quinn N. What contributes to quality of life in patients with Parkinson's disease? J Neurol Neurosurg Psychiatry 2000; 69: 308–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zahodne LB, Okun MS, Foote KD, et al. Greater improvement in quality of life following unilateral deep brain stimulation surgery in the globus pallidus as compared to the subthalamic nucleus. J Neurol 2009; 256: 1321–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.