Abstract
Objectives:
The development of technologically advanced, expensive techniques has progressively reduced the value of chest X-ray in clinical practice for the assessment of left ventricular (LV) dilatation and dysfunction. Although controversial data are reported on the role of this widely available technique in cardiac assessment, it is known that the cardio-thoracic ratio is predictive of risk of progression in the NYHA Class, hospitalization, and outcome in patients with LV dysfunction. This study aimed to evaluate the reliability of the transverse diameter of heart shadow [TDH] by chest X-ray for detecting LV dilatation and dysfunction as compared to Magnetic Resonance Imaging (MRI) performed for different clinical reasons.
Materials and Methods:
In 101 patients, TDH was measured in digital chest X-ray and LV volumes and ejection fraction (EF) by MRI, both exams performed within 2 days.
Results:
A direct correlation between TDH and end-diastolic volumes (r = .75, P<0.0001) was reported. TDH cut-off values of 14.5 mm in females identified LV end-diastolic volumes >150 mL (sensitivity: 82%, specificity: 69%); in males a cut-off value of 15.5 mm identified LV end-diastolic volumes >210 mL (sensitivity: 84%; specificity: 72%). A negative relation was found between TDH and LVEF (r = -.54, P<0.0001). The above cut-off values of TDH discriminated patients with LV systolic dysfunction – LVEF <35% (sensitivity and specificity: 67% and 57% in females; 76% and 59% in males, respectively).
Conclusions:
Chest X-ray may still be considered a reliable technique in predicting LV dilatation by the accurate measurement of TDH as compared to cardiac MRI. Technologically advanced, expensive, and less available imaging techniques should be performed on the basis of sound clinical requests.
Keywords: Cardiac MRI, chest X-ray, left ventricular dilatation, left ventricular dysfunction
INTRODUCTION
Chest X-ray is one of the most widely used methods for population screening and patient evaluation in virtually all cardiac diseases.[1–4]
The American College of Cardiology/American Heart Association (ACC/AHA) and the European guidelines for the diagnosis and treatment of heart failure advocate looking for the presence of appropriate symptoms and signs and the objective evidence of cardiac dysfunction, as provided by electrocardiogram (ECG), chest X-ray, and cardiac imaging.[5] In this respect, chest X-ray represents a firm element of the diagnostic process in this group of patients.
However, the value of chest X-ray in detecting left ventricular (LV) size and function is still controversial, and it has been suggested that less than half of the patients with LV systolic dysfunction show a cardiothoracic ratio >55%.[6,7] Although comparison with other techniques has shown only a weak correlation between cardiothoracic ratio and radionuclide, echocardiographic, or magnetic resonance imaging (MRI)-derived volumes and ejection fraction (EF),[8,9] heart size on the chest X-ray is still of considerable prognostic value in the patients with low LV ejection fraction (LVEF).[10–12] Moreover, the measurement of the transverse chest dimensions could represent a source of error in the evaluation of the cardiothoracic ratio, which is the parameter considered for the assessment of heart size in most of the studies published so far. An index derived from cardiac silhouette, which does not take into account measures of the chest, could therefore provide more reliable parameters for definition of LV dimension and function.
Cardiac MRI – due to its good accuracy and superior reproducibility – is now considered as the Gold Standard for in vivo quantification of left and right ventricular volumes and EF.[13–18] MRI equipment, however, is not always available and its costs are considerable. On the other side, chest X-ray is fast, cheap, relatively safe in terms of radiation exposure, totally non-invasive, and is an easily available form of investigation. With these considerations in mind, the aim of this study was to evaluate in an unselected population undergoing cardiac MRI for clinical reasons the ability of a simple measurement derived from chest X-ray (transverse diameter of the heart shadow [TDH]) in detecting LV dilatation and systolic dysfunction, independently measured by cardiac MRI.
MATERIALS AND METHODS
Patients
A group of 101 consecutive patients admitted to our Institute and undergoing cardiac MRI and chest X-ray for clinical reasons were studied: 69 patients were males, mean age was 62±14 years. The indications for cardiac MRI were LV ischemic dysfunction, idiopathic cardiomyopathy, acute myo-pericarditis, valvular heart disease, hypertrophic cardiomyopathy, or arrhythmogenic right ventricular dysplasia in 50, 26, 6, 5, 2, and 12 patients, respectively. All patients were under stable clinical conditions at the time of the study.
Chest radiography
Chest X-rays (postero-anterior and lateral projections), of patients in the erect position during an inspiratory breath-hold, with rib position on the 6th rib anteriorly and the 10th rib posteriorly, with focus-to-detector distance of approximately 2 m and very short exposure times, were analyzed. A digital system with Charged Coupled Device technology was used. The digital system IMIX-Thorax (Oy IMIX AB, Tampere, Finland), comprising a detector, scintillation camera, optical system, a system for converting X-ray photons, reconstructions computer, and post-processing computer, is capable of displaying the image in about 10 s. In the IMIX-Thorax, the detector system is strictly related to the ARCO COMB column (Arcoma AB, Vaxjo, Sweden). It includes an automatic exposure control system connected to the X-ray generator Medira 2064 (MEDIRA AB, Tampere, Finland), which ensures the constant and correct emission of radiation as a result of the automatic setting of the anode current at the X-ray tube.
The chest X-rays were obtained within 2 days of the cardiac MRI study. The films were independently read by two expert radiologists who were blinded to the patient's clinical and functional status and level of severity of cardiac disease. For the analysis, a standardized reading grid was used. TDH was measured by drawing a line near the middle of the heart shadow and the spine with a line from the right heart border to that line. Another line from the left heart border farthest from the line in the middle of the heart shadow was added. The two lengths were added together to derive the transverse diameter of the heart [Figure 1]. The cardiothoracic ratio (CTR) was also calculated as the ratio between the widest portion of the heart and the diameter of the chest, determined by drawing a line at the level of right leaf of the diaphragm and extending to the inner border of the rib cage on the right and on the left [Figure 1].
Figure 1.
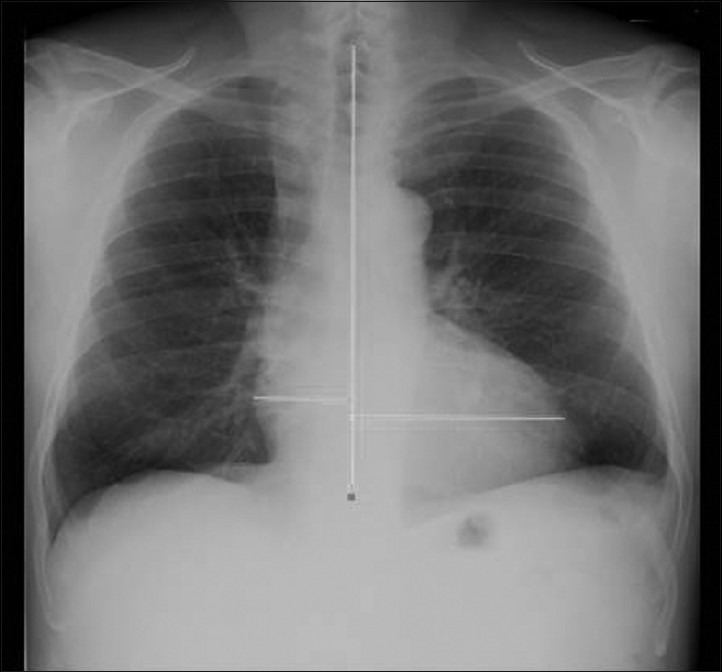
Postero-anterior chest X-ray projection where the measure of the transverse diameter of heart shadow (TDH) is reported. The measure was taken by drawing a line near the middle of the heart shadow and the spine and a line from the right border to that line. Another line from the left heart border, drawn to the middle, was added. The two lengths were added together to derive the TDH.
Cardiac MRI
Cardiac MRI was performed using a 1.5 Tesla whole body scanner (GE Medical Systems, Milwaukee, Wisconsin, USA). A 4-element cardiac phased-array receiver surface coil was utilized for signal reception. A breath-hold segmented-gradient fast-imaging echo, employing steady-state acquisition, triggered with the ECG, was used to evaluate global LV function according to standard parameters. In each patient, a total of 9-12 short-axis views (depending on the LV volumes) and 2 long-axis views (one vertical and one horizontal) were acquired, with a minimum of 30 cine frames for each slice. To measure LV volumes, the endocardial borders were manually drawn in all the short axis end-diastolic and end-systolic images, excluding papillary muscles. End-diastolic and end-systolic LV volumes were calculated and LVEF was derived.[19]
Cut-off values of LV end-diastolic volume (LVEDV) were calculated on the basis of normal values reported in our MRI lab.[20] Therefore, an absolute LVEDV >210 mL was considered as abnormal in males and >150 mL as abnormal in females.
The procedures followed were in accordance with the ethical standards of the Institutional Committee on Human Experimentation and with the Declaration of Helsinki of 1974, as revised in 2000. Approval of the Institutional Committee was obtained and each patient gave informed consent for undergoing clinical examination and chest X-ray after cardiac MRI.
Statistical analysis
Quantitative data were expressed as mean ± standard deviation, qualitative data as percentage. The relationship between TDH and LV volume or LVEF at MRI was evaluated by least square linear regression analysis and the Pearson linear correlation (r).
The cut-off value of the TDH utilized to identify volumes >150 mL in females and >210 mL in males was computed using the receiver operating characteristics (ROC) curve and Jouden's J. The performance indices of the cut-off value were used to determine sensitivity, specificity, positive and negative likelihood ratio (LR+, LR-), and the area under the ROC curve (AUC). The AUC was estimated by a non-parametric approach. The comparison between females and males for the AUC was made using the method for independent sample. Inter-observer reliability in chest X-ray measurements of two observers was evaluated using the correlation coefficient of Lin,[21,22] the intra-class correlation coefficient (ICC), and the Bland and Altman method.[23] The ICC was calculated using a two-way random effects model where people and measure effects were random. A P value <0.05 was considered to be statistically significant. All tests were two-tailed. The statistical analysis was made by JMP statistical software, SAS Institute Inc, version 4.0.0, and by Stata SE statistical software, StataCorp, release 10.[24]
RESULTS
Inter-observer variability
The variability between the values of TDH measured by the two readers was very low. The correlation coefficient of Lin (r = 0,92, 95% C.I. = 0,87–0,97) and the intra-class correlation coefficient (ICC = 0,93, 95% C.I. = 0,86–0,96) were very high. The mean difference between readers was = 0.153 (s.d. = 0.572) and the Bland and Altman 95% Limits of Agreement were 1.275–0.969.
As far as the inter-observer variability of thoracic dimensions, the correlation coefficient of Lin was 0,86 (95% C.I. = 0,77–0,95) and the intra-class correlation coefficient was 0,86 (95% C.I. = 0,65–0,94). The mean difference between readers was 0.900 (s.d. = 1.479) while the Bland and Altman 95% Limits of Agreement were -1.999–3.799.
As far as CT ratio is concerned, the correlation coefficient of Lin was 0,84 (95% C.I. = 0,74–0,94) and the intra-class correlation coefficient was 0,84 (95% C.I. = 0,58–0,93). A mean difference between readers of -0.019 (s.d. = 0.029) and Bland and Altman 95% Limits of Agreement equal to -0.076–0.038 were observed.
Prediction of LV dilatation
As shown in Figure 2, a good correlation was found between TDH and absolute values of LVEDV by MRI (r = 0.75, P<0.0001).
Figure 2.
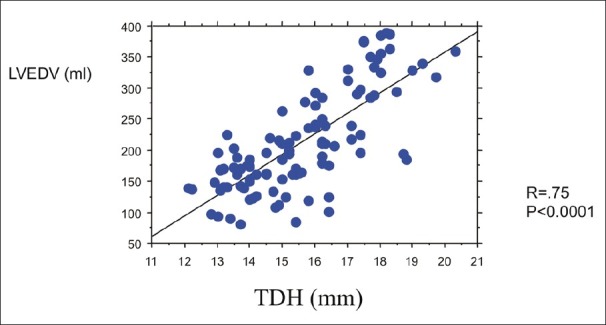
Relationship between the transverse diameter of heart shadow (TDH) and absolute values of left ventricular end-diastolic volume (LVEDV) by magnetic resonance imaging. A good positive correlation was found between the two indices (r = 0.75, P<0.0001).
In females, a cut-off value of 14.5 mm of TDH was able to identify LV volumes >150 mL with a sensitivity of 82%, a specificity of 69%, a LR+ of 2.63, and a LR- of 0.26. The AUC was 0.77 (95% C.I.: 0.59–0.94).
In males, a cut-off value of 15.5 mm identified absolute LV volumes >210 mL with a sensitivity of 84%, a specificity of 74%, a LR+ of 2.97, and a LR- of 0.22. The AUC was 0.83 (95% C.I.: 0.73–0.93). No significant difference in the AUC between females and males was observed (Chi-square=0.37, P=0.545).
When CTR was considered, a less significant relation was reported with absolute values of LVEDV (r=.46, P<0.001) and with LVEDV indexed for body surface area BSA (r=.52, P<0.001). A cut-off value of 0.50 identified pathologic absolute LV volumes with a sensitivity of 64%, a specificity of 66%, a LR+ of 2.34, and a LR- of 0.49.
Prediction of LV dysfunction
As illustrated in Figure 3, a negative relation was found between TDH and LVEF measured at MRI (r= - 0.54 P<0.001).
Figure 3.
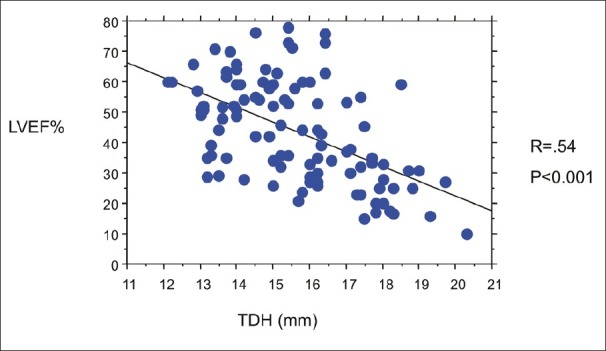
Relationship between the transverse diameter of heart shadow (TDH) and left ventricular ejection fraction (LVEF) measured at magnetic resonance imaging. A negative correlation was detected between the two indices (r= - 0.54 P<0.001).
The same cut-off values of the TDH were able to discriminate patients with a LVEF <35% with a sensitivity, a specificity, a LR+, and a LR- of 67%, 57%, 1.55, and 0.54 in females, and of 76%, 59%, 2.08, and 0.38 in males, respectively.
A mild relation was also seen between CTR and LVEF (r=.41, P<0.001).
DISCUSSION
In the present study, a good relationship was found between TDH measured on conventional chest X-ray and LVEDV measured by MRI in a consecutive unselected group of patients undergoing cardiac MRI.
The role of chest X-ray in the assessment of the heart is not univocal. Even American and European guidelines do not state the role in clinical practice of this easily available technique. Published data have shown that chest roentgenograms are not reliable in assessing and grading left ventricular systolic dysfunction in patients after acute MI[25,26] or in patients with chronic heart failure[27,28] when compared to radionuclide ventriculography or echocardiography.
Some authors have shown that the most informative radiological feature in detecting heart failure is actually cardiac enlargement[29] although other authors have shown that patients with a significant reduction in LV systolic function may have a normal heart size on the chest X-ray. At the same time, an enlarged heart may not reflect a reduced LVEF, mostly due to cardiac hypertrophy, pericardial effusion, or enlargement of atria. However, a higher CTR has been shown to be predictive of risk of progression in the NYHA Class and hospitalization.[10,30] At the same time, radiographic measures of cardiac size may represent predictors of outcome in patients with dilated cardiomyopathy.[12]
Most of the studies published so far on the assessment of cardiac dimensions by chest X-ray have used CTR as a marker of cardiac enlargement. In this study, we have shown that while a good relation was found between LVEDV and TDH, this relation is less significant for the CTR. As a matter of fact, it is understandable that the comparison of the CTR with LV volumes indexed for BSA may lead to poorer results. The normalization we apply to the cardiac silhouette when related to thoracic dimensions cannot be equalized to the normalization of LV volume we apply when indexing it to the BSA; a greater BSA can be reported in patients with increased abdominal circumferences and normal thoracic dimensions.
The X-ray reveals the cardiac silhouette, which encompasses all four cardiac chambers. A complete agreement between X-ray and LV volumes obtained by MRI cannot be expected since left atrial or right ventricular enlargement could be responsible for greater TDH measures.[31,32] Other variables, such as the presence of pericardial effusion, should be considered as the cause of an increased TDH unrelated to cardiac dimensions. In the present study, four patients with moderate LV dilatation showed a localized <3-mm pericardial effusion and none had important diffuse effusion.
A less significant relation was reported between TDH and MRI-assessed LVEF. Chest radiography is not a reliable indicator of the degree of left ventricular systolic dysfunction, as already reported by different authors, which compared CTR with echocardiography and radionuclide ventriculography.[4] In line with these results, the relation we found in the group of patients was only moderate. In this respect, chest X-ray could be of help when cardiac dimensions are integrated with other abnormalities, such as pulmonary congestion or edema. However, in the present study, 35/42 patients with EF <35% had a TDH >15 mm; this result may suggest that an increased TDH by itself may address the diagnosis of a reduced global LV function.
Limitations of the study
The study suffers from some limitations: first, a conventional digital X-ray was adopted. Probably an ECG-gated digital chest X-ray could have helped on timing the X-ray shot more precisely during the filling phase of the heart, so improving the relation observed between TDH and of LV volumes by MRI.[33]
Secondly, patients with significant pericardial effusion or severe pulmonary hypertension were not present in the studied population. Enlargement of the heart silhouette unrelated to LV dimensions like in the presence of significant diffuse pericardial effusion could have lead to poorer relations between TDH and LV volumes.
Finally, for the present study, TDH only was used to estimate LV dimensions and function. Other X-ray-derived parameters such as peribronchial cuffing or vascular pedicle have been reported in literature in heart failure patients, mostly for the assessment of intravascular volume status, although results are still not univocal.[34,35]
CONCLUSIONS
In conclusion, the results of this study show that chest X-ray, a widely available, low-cost, and low-risk technique – since radiological exposure is almost negligible (around 0.01 mSV, equivalent to the dose absorbed for 10 days due to the natural background radiation[36]) – should still be considered as the first approach for a rapid and reliable screening of cardiac dimensions. The use of highly technological, expensive, and much less available techniques should be devoted to specific clinical questions, which would be otherwise unanswered. This is particularly true when a properly executed and interpreted chest X-ray examination is integrated with clinical data collection and a careful physical examination.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2012/2/1/25/96540
REFERENCES
- 1.Ferguson EC, Krishnamurthy R, Oldham SA. Classic imaging signs of cardiovascular abnormalities. Radiographics. 2007;27:1323–4. doi: 10.1148/rg.275065148. [DOI] [PubMed] [Google Scholar]
- 2.Young JB. Imaging patients with heart failure: Expectations of the clinician. Heart Fail Clin. 2006;2:107–15. doi: 10.1016/j.hfc.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Maffessanti M, Berlot G, Bortolotto P. Chest roentgenology in the intensive care unit: An overview. Eur Radiol. 1998;8:69–78. doi: 10.1007/s003300050342. [DOI] [PubMed] [Google Scholar]
- 4.Sanchez O, Revel MP, Couchon S, Meyer GL. Imaging of pulmonary hypertension. Rev Mal Resp. 2007;24:155–69. [PubMed] [Google Scholar]
- 5.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic heart failure in the adult: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): Developed in Collaboration With the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: Endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 6.Petrie MC, Mc Murray JJ. It cannot be cardiac failure because the heart is not enlarged on the chest X ray. Editorial. Eur J Heart Fail. 2003;5:117–9. doi: 10.1016/s1388-9842(02)00239-8. [DOI] [PubMed] [Google Scholar]
- 7.The digitalis invest group: The effect of digoxin on mortality and morbidity in patients with heart failure. N EJM. 1997;336:525–33. doi: 10.1056/NEJM199702203360801. [DOI] [PubMed] [Google Scholar]
- 8.Clark AL, Coats AJ. Unreliability of cardiothoracic ratio as a marker of left ventricular impairment: Comparison with radionuclide ventriculography and echocardiography. Postgrad Med J. 2000;76:289–91. doi: 10.1136/pmj.76.895.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kono T, Suwa M, Hanada H, Hirota Y, Kawamura K. Clinical significance of normal cardiac silhouette in dilated cardiomyopathy--evaluation based upon echocardiography and magnetic resonance imaging. Jpn Circ J. 1992;56:359–65. doi: 10.1253/jcj.56.359. [DOI] [PubMed] [Google Scholar]
- 10.Kleber FX, Niemoller L, Fisher M, Doering W. Influence of severity of heart failure on the efficacy of angiotensin converting enzyme inhibition. Am J Cardiol. 1991;68:D121–6. doi: 10.1016/0002-9149(91)90269-q. [DOI] [PubMed] [Google Scholar]
- 11.Loeb HS, Johnson G, Henrick A, Smith R, Wilson J, Creno R, et al. Effect of enelapril, hydralazine plus isosorbide dinitrate, and prazosin on hospitalisation in patients with chronic congestive heart failure. Circulation. 1993;87:78–87. [PubMed] [Google Scholar]
- 12.Ernst E, Shub C, Bailey KR, Brown LR, Redfield MM. Radiographic measurements of cardiac size as predictors of outcome in patients with dilated cardiomyopathy. J Card Fail. 2001;7:13–20. doi: 10.1054/jcaf.2001.23244. [DOI] [PubMed] [Google Scholar]
- 13.Wang F, Zhang J, Fang W, Zhao SH, Lu MJ, He ZX. Evaluation of LV volumes and ejection fraction by gated SPECT and cardiac MRI in patients with dilated cardiomyopathy. Eur J Nucl Med Mol Imaging. 2009;36:1611–21. doi: 10.1007/s00259-009-1136-7. [DOI] [PubMed] [Google Scholar]
- 14.Selton-Suty C, Juilliere Y. Non invasive investigations of the right heart: How and why. Arch Cardiov Dis. 2009;102:219–32. doi: 10.1016/j.acvd.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 15.Marcu CB, Beek AM, van Rossum AC. Cardiovascular Magnetic Resonance Imaging for the assessment of right heart involvement in cardiac and pulmonary disease. Heart Lung Circ. 2006;15:362–70. doi: 10.1016/j.hlc.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Epstein FH. Magnetic resonance imaging of left ventricular function. J Nucl Cardiol. 2007;14:729–44. doi: 10.1016/j.nuclcard.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Kaji S, Yang PC, Kerr AB, Tang WM. Rapid evaluation of LV volume and mass without breath holding using real-time interactive cardiac MRI. J Am Coll Cardiol. 2001;38:527–33. doi: 10.1016/s0735-1097(01)01399-7. [DOI] [PubMed] [Google Scholar]
- 18.Alfakih K, Reid S, Jones T, Sivananthan M. Assessment of left ventricular function and mass by cardiac MRI. Eur Radiol. 2004;14:1813–22. doi: 10.1007/s00330-004-2387-0. [DOI] [PubMed] [Google Scholar]
- 19.Sironi AM, Lombardi M, Pepe A, De Marchi D. Study of heart function. In: Lombardi M, Bartolozzi C, editors. MRI of the Heart and Vessels. Milan: Springer; 2004. pp. 154–9. [Google Scholar]
- 20.Lombardi M, Sironi AM, Monti L, Deiana M, Ghedin P. Heart: MRI quantification of left and right ventricular dimensions: Accuracy and reproducibility. In: Lombardi M, Bartolozzi C, editors. MRI of the heart and the vessels. Germany: Springer Publisher; 2004. pp. 162–4. [Google Scholar]
- 21.Lin, LI-K A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45:255–68. [PubMed] [Google Scholar]
- 22.Lin, LI-K A note on the concordance correlation coefficient. Biometrics. 2000;56:324–5. [Google Scholar]
- 23.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10. [PubMed] [Google Scholar]
- 24.College Station, TX: Stata Corp LP; 2007. Stata Corp. Stata Statistical Software: Release 10. [Google Scholar]
- 25.Madsen EB, Gilpin E, Slutsky RA, Ahnve S, Henning H, Ross Jl. Usefulness of the chest X-ray for predicting abnormal left ventricular function after acute myocardial infarction. Am Heart J. 1984;108:1431–6. doi: 10.1016/0002-8703(84)90688-4. [DOI] [PubMed] [Google Scholar]
- 26.Alam M, Rosenhamer G, Hoglund C. Comparability of echocardiography and chest X-ray following myocardial infarction. J Int Med. 1989;226:171–5. doi: 10.1111/j.1365-2796.1989.tb01375.x. [DOI] [PubMed] [Google Scholar]
- 27.Chakko S, Woska D, Martinez H, De Marchena E, Futterman L, Kessler KM, et al. Clinical, radiographic, and hemodynamic correlations in chronic congestive heart failure: Conflicting results may lead to inappropriate care. Am J Med. 1991;90:353–9. doi: 10.1016/0002-9343(91)80016-f. [DOI] [PubMed] [Google Scholar]
- 28.Thomas JT, Kelly RF, Thomas SJ. Utility of history, physical examination, electrocardiogram, and chest radiograph for differentiating normal from decreased systolic function in patients with heart failure. Am J Med. 2002;112:437–45. doi: 10.1016/s0002-9343(02)01048-3. [DOI] [PubMed] [Google Scholar]
- 29.Fonseca C. Diagnosis of heart failure in primary care. Heart Fail Rev. 2006;11:95–107. doi: 10.1007/s10741-006-9481-0. [DOI] [PubMed] [Google Scholar]
- 30.Cohn JN, Johnson G, Ziesche S, Francis G, Tristani F, Smith R, et al. A comparison of enalapril with hydralazine isosorbide dinitrate in the treatment of chronic congestive heart failure. N Engl J Med. 1991;325:303–10. doi: 10.1056/NEJM199108013250502. [DOI] [PubMed] [Google Scholar]
- 31.Garq S, Mittal SR. Status of chest X-ray in diagnosing right ventricular infarction. Int J Cardiol. 1996;57:283–5. doi: 10.1016/s0167-5273(96)02788-x. [DOI] [PubMed] [Google Scholar]
- 32.Mikami T, Kudo T, Hashimoto M, Sugawara T, Sakamoto S, Tanabe Y, Yasuda H. Re-evaluation of determinants of the cardiothoracic ratio using two-dimensional echocardiography. J Cardiol. 1988;18:395–402. [PubMed] [Google Scholar]
- 33.Tanaka R, Sanada S, Suzuki M, Kobayashi T, Matsui T, Inoue H, et al. Breathing chest radiography using a dynamic flat-panel detector (FPD) with computer analysis for a screening examination. Med Phys. 2004;31:2254–62. doi: 10.1118/1.1769351. [DOI] [PubMed] [Google Scholar]
- 34.Studler U, Kretzschmar M, Christ M, Breidthardt T, Noveanu M, Schoetzau A, et al. Accuracy of chest radiographs in the emergency diagnosis of heart failure. Eur Radiol. 2008;18:1644–52. doi: 10.1007/s00330-008-0930-0. [DOI] [PubMed] [Google Scholar]
- 35.Miller RR, Ely EW. Radiographic measures of intravascular volume status: The role of vascular pedicle width. Curr Opin Crit Care. 2006;12:255–62. doi: 10.1097/01.ccx.0000224871.31947.8d. [DOI] [PubMed] [Google Scholar]
- 36.American College of Radiology. ACR.SPR Practice Guideline for the Performance of Pediatric and Adult Portable (Mobile Unit) Chest Radiography. [Last accessed on 2010 Aug 17]. Available from: http://www.acr.org/SecondaryMainMenuCategories/quality_safety/guidelines/dx/Chest/portable_chest.aspx .


