Abstract
During vertebrate retinal development, the seven retinal cell types differentiate sequentially from a single population of retinal progenitor cells (RPCs) and organize themselves into a distinct laminar structure. The purpose of this study was to determine whether β-catenin, which functions both as a nuclear effector for the canonical Wnt signaling pathway and as a regulator of cell adhesion, is required for retinal neurogenesis or lamination. We used the Cre-loxP system to either eliminate β-catenin or to express a constitutively active form during retinal neurogenesis. Eliminating β-catenin did not affect cell differentiation, but did result in the loss of the radial arrangement of RPCs and caused abnormal migration of differentiated neurons. As a result, the laminar structure was massively disrupted in β-catenin-null retinas, although all retinal cell types still formed. In contrast to other neural tissues, eliminating β-catenin did not significantly reduce the proliferation rate of RPCs; likewise, activating β-catenin ectopically in RPCs did not result in overproliferation, but loss of neural retinal identity. These results indicate that β-catenin is essential during retinal neurogenesis as a regulator of cell adhesion but not as a nuclear effector of the canonical Wnt signaling pathway. The results further imply that retinal lamination and retinal cell differentiation are genetically separable processes.
Keywords: retina, retinal development, β-catenin, retinal lamination, cell adhsion, cell differentiation
Introduction
Tissue formation requires not only the specification and differentiation of functional cell types but also their correct organization. Although much has been learned about the molecular events regulating the differentiation of various cell types, far less is known about the events required to organize cells into tissues and how cellular organization is coordinated with cell-type differentiation.
The vertebrate retina is a highly representative neural tissue consisting of well-defined cell types that are organized into a distinct multilayered laminar structure. The retina has six neuronal cell types (RGCs, amacrine cells, bipolar cells, horizontal cells, and rod and cone photoreceptors) and one glial cell type (Müller glial cells). The nuclei of the different cell types are located in three layers: an outer nuclear layer (ONL), containing rod and cone photoreceptors; an inner nuclear layer (INL), containing horizontal cells, bipolar cells, Müller glial cells, and amacrine cells; and a ganglion cell layer (GCL) containing RGCs and displaced amacrine cells. In the mouse, retinal neurogenesis starts at embryonic day 11.5 (E11.5), after the optic cup has formed, and continues until postnatal day 11 (P11) (Young, 1985). All seven cell types derive from a single population of naïve RPCs following a distinct temporal birth order (Livesey and Cepko, 2001). Specification and differentiation of the individual cell types requires specific regulatory factors that act singly or in combination to determine cell fate (Hatakeyama and Kageyama, 2004; Mu and Klein, 2004).
In addition to adopting a specific cell fate, a differentiating retinal cell must also establish its spatial identity by migrating to the correct position within the retina (Hinds and Hinds, 1974; Hinds and Hinds, 1979; Hinds and Hinds, 1983). RPCs are oriented radially along the apical/basal axis, and their processes span the full thickness of the retina, with their end feet anchored at both sides (Hinds and Hinds, 1974). Cell differentiation starts with the detachment of the end foot from the apical surface so that the differentiating cell can migrate to the appropriate layer. For example, newly formed RGCs, the earliest cell type to differentiate (Mu and Klein, 2004), begin to migrate toward the basal (inner) side of the neural retina to form the future GCL (Hinds and Hinds, 1974). Similar migration of other newly formed cell types are also required to produce a mature, functional retina (Hinds and Hinds, 1979; Hinds and Hinds, 1983). Fundamental questions concerning retinal cell migration remain unanswered. For example, the basis by which the different cell types assume their appropriate location once their cell fate has been determined is unknown. Furthermore, the relationship between retinal cell-type specification and cell migration is not understood.
It has long been suggested that differential cell adhesion plays a key role in sorting different cell types into a specific cellular organization during development (Steinberg, 1970; Steinberg, 1978). This view has been supported by more recent studies. For example, genetic screens in zebrafish have identified mutations that have severe retinal lamination defects (Pujic and Malicki, 2004). The genes associated with several of these mutations have been identified, and the proteins they encode are invariably involved in cell adhesion (Erdmann et al., 2003; Horne-Badovinac et al., 2001; Jensen and Westerfield, 2004; Malicki et al., 2003; Masai et al., 2003; Wei and Malicki, 2002). Two of them, glass onion and parachute, are mutations in the coding region of the type I cadherin gene, N-cadherin (Erdmann et al., 2003; Malicki et al., 2003; Masai et al., 2003). Type I cadherins form adherens junctions mostly through homotypic interactions (Wheelock and Johnson, 2003). Cadherin molecules in cadherens junctions are linked to the actin cytoskeletal network by β-catenin through α-catenin. Adherens junctions function in various biological processes, such as cell migration, cell sorting, and boundary formation. β-catenin therefore plays a pivotal role, physically bridging adherens junctions to the cytoskeletal network.
β-catenin is also an effector of the canonical Wnt signaling pathway that is activated by binding of the Wnt ligands to their seven-transmembrane receptors, the frizzled proteins (Logan and Nusse, 2004). In the absence of Wnts, cytoplasmic β-catenin is phosphorylated by glycogen synthase kinase-3β (GSK-3β) and casein kinase I (CKI) bound to the adenomatosis polyposis coli (APC) complex and is targeted for degradation through the ubiquitination pathway. Activation of the Wnt pathway leads to the inhibition of GSK-3β, resulting in the stabilization and cytoplasmic accumulation of β-catenin. The stabilized β-catenin then interacts with Tcf/Lef transcription factors in the nucleus and activates downstream genes. In the nervous system, the Wnt-β-catenin pathway is involved in progenitor cell proliferation, cell-fate determination, and axon pathfinding (Charron and Tessier-Lavigne, 2005; Logan and Nusse, 2004).
In the developing mouse retina, many components of the Wnt pathway are expressed, and analysis of transgenic mice harboring a reporter transgene that responds to Wnt-β-catenin signaling, TCF/Lef-LacZ, suggests that the canonical pathway is activated during the early stages of retinogenesis (Liu et al., 2003). Other studies indicate that the Wnt pathway functions to promote RPC proliferation in Xenopus (Van Raay et al., 2005) and to prevent differentiation in the chick ciliary marginal zone (CMZ) (Kubo et al., 2003; Kubo et al., 2005). However, a definitive role of the Wnt-β-catenin pathway during retinal neurogenesis has not been established.
In this study, we directly determine the requirement for β-catenin in mouse retinal neurogenesis by specifically deleting the β-catenin gene in the developing retina. In our study, eliminating β-catenin led to a disorganized retina but did not affect the formation of any of the retinal cell types. In contrast, activating the nuclear function of β-catenin in the retina did not lead to overproliferation of RPCs, suggesting that the Wnt-β-catenin signaling pathway is not required for RPC proliferation during neurogenesis. Our results support the view that lamination and cell differentiation are genetically separable processes, and that during retinal neurogensis, β-catenin is required solely for cell adhesion.
Materials and methods
Mouse lines
Mice harboring a conditional β-catenin allele (here referred to as catnblox(e2-6)) (Brault et al., 2001) were obtained from The Jackson Laboratory (Bar Harbor, ME) and mice with a stabilized β-catenin allele (catnblox(e3)) were generously provided by Makoto M. Taketo (Harada et al., 1999). α-Cre mice, originally generated by Marquardt et al. (Marquardt et al., 2001), were obtained from Guillermo Oliver (St. Jude Children’s Research Hospital, Memphis, TN). Six3-Cre mice (Furuta et al., 2000; Mu et al., 2005) were kindly provided by Yas Furuta (M. D. Anderson Cancer Center, Houston, TX). These mice were bred into a 129/BL6 background. In all experiments using mice, the U. S. Public Health Service Policy on Humane Care and Use of Laboratory Animals was followed.
Genotyping
Genotyping for the β-catenin allele was performed by PCR following a previously described protocol (Brault et al., 2001). Genotyping for the α-Cre and six3-Cre mice was performed using PCR to detect the presence of the Cre sequence. The Cre primers used were 5’-CCT GAT CCT GGC AAT TTC GGC TA-3’ and 5’-TCC AAT TTA CTG ACC GTA CAC CAA-3’. The PCR conditions used were: pre-denaturing (94ºC, 5 min); 35 cycles of denaturing (94ºC, 30 sec), annealing (55ºC, 30 sec), and extension (72ºC, 40 sec); followed by 2 min of extra extension at 72ºC.
Histology and in situ hybridization
For morphological assessment of retinal phenotypes, embryos or eyes from different stages were fixed, paraffin-embedded, and sectioned at 10 μm. After de-waxing, the sections were stained with hematoxylin and eosin (H&E) as previously described (Mu et al., 2005).
In situ hybridization with 7-μm paraffin-embedded retinal sections was performed following a previously described protocol (Mu et al., 2004).
Fluorescence staining
Immunofluorescence staining on optimum cutting temperature medium (OCT)-embedded, 16 μm frozen retinal sections was carried out as previously described (Mu et al., 2005). The primary antibodies and the dilutions used in this study were as follows: anti-POU4f2 (previously called Brn3b) (Santa Cruz Biotechnology, Santa Cruz, CA, 1:50), anti-β-catenin (Cell Signaling, Beverly, MA, 1:500), anti-N-cadherin (BD Biosciences, San Jose, CA, 1:400), anti-Pax6 (Development Studies Hybridoma Bank, Iowa City, IA, 1:200), anti-Chx10 (Exalpha Biologicals, Maynard, MA, 1:400), anti-rhodopsin (Sigma-Aldrich, 1:400), anti-neurofilament 160 (NF160) (Sigma-Aldrich, 1:400), anti-PKCα (Sigma-Aldrich, 1:400), anti-Cralbp (Abcam, Cambridge, MA, 1:200), anti-cone arrestin (CAR) (Dr. Craft, UCLA, 1:300), anti-Par3 (Santa Cruz Biotechnology, 1:150), anti-Par6 (Santa Cruz Biotechnology, 1:150), anti-aPKCλ (Santa Cruz Biotechnology, 1:150). Fluorescent secondary antibodies were obtained from Molecular Probes (Eugene, OR) and were used at 1:800 dilution. Filamentous (F)-actin was stained with Alexa-488 conjugated phalloidin (Molecular Probes) at 1:1,000 dilution. Cell counting of individual cell types was performed as previously described (Mu et al., 2005). Briefly, total cells (based on propidium iodide staining) and positive cells for a specific marker were counted in the central retinal region within an arbitrary length unit and the percentage for each cell type was calculated. Effort was made to count cells from equivalent regions for different genotypes. Six sections from two different animals were counted for each marker and genotype. Statistical analysis was performed by two-sample t test with unequal variances.
BrdU labeling and TUNEL assay
For BrdU labeling, 100 μg BrdU per gram body weight was injected intraperitoneally into pregnant mice 1.5 hr before euthanization. Embryos were embedded in paraffin and then sectioned at 7 μm. The sections were de-waxed, treated as previously described (Mu et al., 2005), and stained with anti-BrdU primary antibody (Abcam, Cambridge, MA, 1:200) and Alexa 488-conjugated anti-rat IgG secondary antibody (Molecular Probes). The sections were then counter-stained with propidium iodide. The proportion of BrdU positive cells was calculated by counting the numbers of BrdU positive cells and total cells (based on propidium iodide staining) in an arbitrary length unit in the central region of the retinal sections and dividing by the total number of cells. For each genotype, six sections from two embryos were counted.
TUNEL assays were performed with the ApopTag plus fluorescein in situ apoptosis detection kit (Chemicon, Temecula, CA) following the manufacturer’s instructions.
Results
Dynamic expression patterns of β-catenin in retinal development
We and others previously showed that β-catenin is abundantly expressed in the developing retina (Liu et al., 2002; Mu et al., 2001). To better understand the function of β-catenin in the retina, we determined the spatiotemporal expression pattern of β-catenin protein throughout retinogenesis. Consistent with previous reports (Liu et al., 2002), we observed distinct spatial expression patterns for β-catenin at all developmental stages. At E12.5, when only a few differentiated cells are present, β-catenin was expressed in uniform levels across the retina (Fig. 1A). At E14.5 and E17.5, although all cells still expressed β-catenin, higher expression was observed in RGCs and their axons (Fig. 1B, C). In mature P16 retinas, expression of β-catenin was highest in the outer plexiform layer (OPL), inner plexiform layer (IPL), and optic fibers (Fig. 1D). It was also expressed in the INL and GCL, but not in the ONL (Fig. 1D). Moreover, at all the stages examined, β-catenin was localized mostly to the plasma membrane; nuclear staining was minimal. Neuronal cell processes had high levels of β-catenin, and from E12.5 to E17.5, β-catenin was strongly expressed at the apical and basal surfaces of the retina, where the end feet of RPCs are anchored (Fig. 1A-C).
Fig. 1.
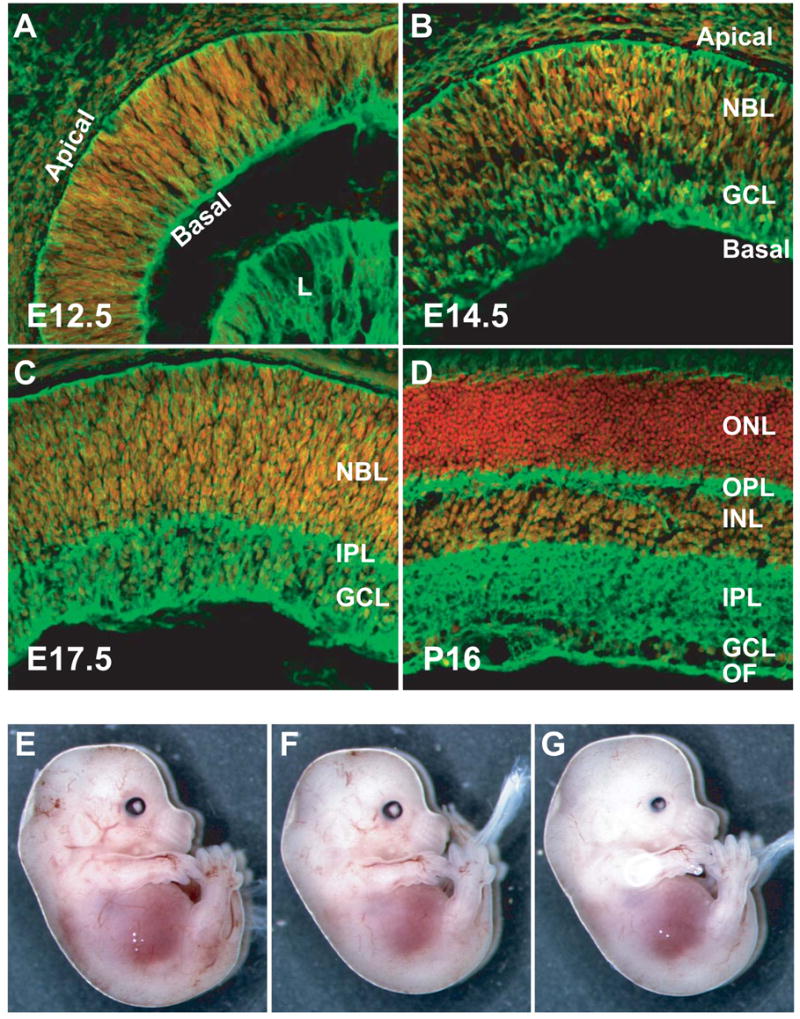
Expression patterns and conditional deletion of β-catenin in the developing retina. (A-D) Merged confocal images of β-catenin protein expression at E12.5 (A), E14.5 (B), E17.5 (C), and P16 (D) detected by an anti-β-catenin antibody (green). Nuclei were counter-stained red with propidium iodide. (E-G) Three E14.5 embryos of wild-type (E), catnblox(e2-6)/ lox(e2-6) (F), and catnblox(e2-6)/ lox(e2-6);six3-Cre (G). E and F have normal-sized eyes but G has smaller eyes. NBL, neural blast layer; GCL, ganglion cell layer; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; OF, optic fiber.
Loss of β-catenin disrupts retinal structure
We used mice homozygous for a floxed β-catenin allele (catnblox(e2-6)) (Brault et al., 2001) to specifically delete β-catenin in the developing retina. In this allele, loxP sites flank exons 2 to 6 of the β-catenin gene. The mice were bred to either six3-Cre or α-Cre transgenic mice. Six3-Cre is expressed throughout the developing retina, with highest expression in the central region (Furuta et al., 2000), whereas α-Cre is expressed only in the anterior-peripheral retina (Marquardt et al., 2001). The expression of Cre in both transgenic lines starts at around E9, when the optic vesicle has formed but neurogenesis has yet to begin. The two Cre transgenes allowed us to determine the effects of β-catenin’s absence at the neurogenesis stage, starting at around E11. Both catnblox(e2-6)/lox(e2-6);six3-Cre and catnblox(e2-6)/ lox(e2-6);α-Cre mice were viable and fertile, but their eyes were smaller compared to those of either catnb lox(e2-6)/ lox(e2-6) or wild-type mice, and the small-eye phenotype was apparent as early as E14.5 (Fig. 1E-G; for catnblox(e2-6)/ lox(e2-6); α-Cre mice, data not shown).
We first analyzed the cellular structure of β-catenin-deleted retinas at P16 when retinogenesis was completed and the multilayered mature retina had fully formed in the wild-type and catnblox(e2-6)/ lox(e2-6) retinas. In both catnblox(e2-6)/ lox(e2-6);six3-Cre and catnblox(e2-6)/ lox(e2-6);α-Cre retinas, the normal laminar structure was disrupted (Fig. 2). This disruption occurred in the central region in catnblox(e2-6)/ lox(e2-6);six3-Cre retinas (Fig. 2A-D) and in the peripheral region in catnblox(e2-6)/ lox(e2-6);α-Cre retinas (Fig. 2E-H). Cells in these regions formed either rosette structures or were randomly distributed (Fig. 2B, D, F, H). Despite the massive disruption, some localized patterning was observed within the rosette structures (Fig. 2D, H). The rosettes were typically comprised of an inner and outer layer separated by a thin non cellular layer. The inner layer resembled the ONL and outer layer was similar to the INL. Throughout the β-catenin-deleted region, patches of non cellular structures resembling the IPL of the mature wild-type retina could be observed (Fig. 2D, H).
Fig. 2.
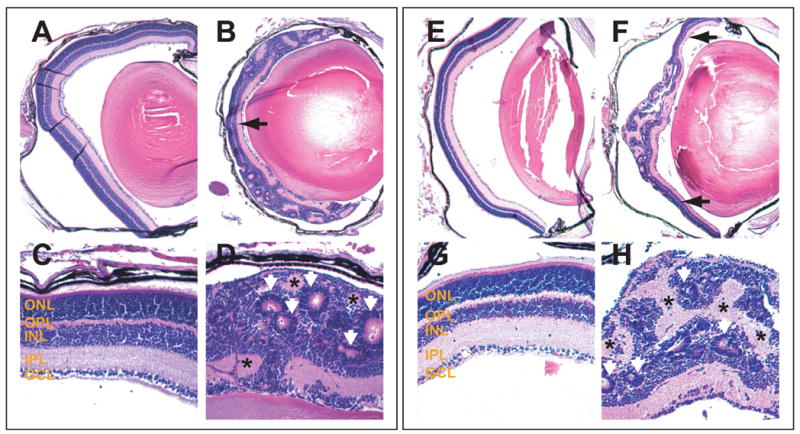
Deletion of β-catenin in P16 retinas. Retinal sections of different genotypes were analyzed by H&E staining. (A) Control catnblox(e2-6)/ lox(e2-6) retina. (B) catnblox(e2-6)/ lox(e2-6);α-Cre retina. (C and D) Higher magnification of A and B, respectively. (E) Control catnblox(e2-6)/ lox(e2-6) retina. (F) catnblox(e2-6)/ lox(e2-6);six3-Cre retina. (G and H) Higher magnification of E and F respectively. Black arrows point to the normal laminated structures in the central region of the catnblox(e2-6)/ lox(e2-6);α-Cre retina (B) and the peripheral region of the catnblox(e2-6)/ lox(e2-6);six3-Cre retina (F). White arrowheads in D and H point to rosette structures with inner and outer cell layers. Asterisks indicate the non cellular structures in the β-catenin-deleted retinas.
Identical defects were observed in the central region in catnblox(e2-6)/ lox(e2-6);six3-Cre retinas and the peripheral region in catnblox(e2-6)/ lox(e2-6);α-Cre retinas. We therefore performed further analyses solely with the catnblox(e2-6)/ lox(e2-6);six3-Cre and catnblox(e2-6)/lox(e2-6) mice.
To determine when defects in retinal lamination first occurred, catnblox(e2-6)/ lox(e2-6);six3-Cre retinas were examined at E12.5, 14.5, and 17.5 (Fig. 3). At E12.5, cellular defects were already obvious. These defects were restricted to the central retinal region, consistent with six3-Cre activity. In control retinas and in the peripheral region of catnblox(e2-6)/ lox(e2-6);six3-Cre retinas, RPCs had oval-shaped nuclei arranged radially along the apical-basal axis (Fig. 3A). In contrast, the nuclei in the central region of catnblox(e2-6)/ lox(e2-6);six3-Cre retinas lost their normal orientation and were round in shape (Fig. 3B). The apical surface of catnblox(e2-6)/ lox(e2-6);six3-Cre retinas was rough and folded rather than smooth and curved as seen in control retinas. These defects were more pronounced at E14.5 and E17.5 (Fig. 3C-F). Additionally, at E14.5, the GCL of catnblox(e2-6)/ lox(e2-6);six3-Cre retinas was greatly reduced in thickness or entirely absent in the central retinal region (Fig. 3C, D). At E17.5, the rosette structures were obvious (Fig. 3 E, F). Because the first sign of anomaly was the defective orientation and arrangement of RPCs, this could be the initial cause of the disorganized structure in the β-catenin-deleted retinas.
Fig. 3.
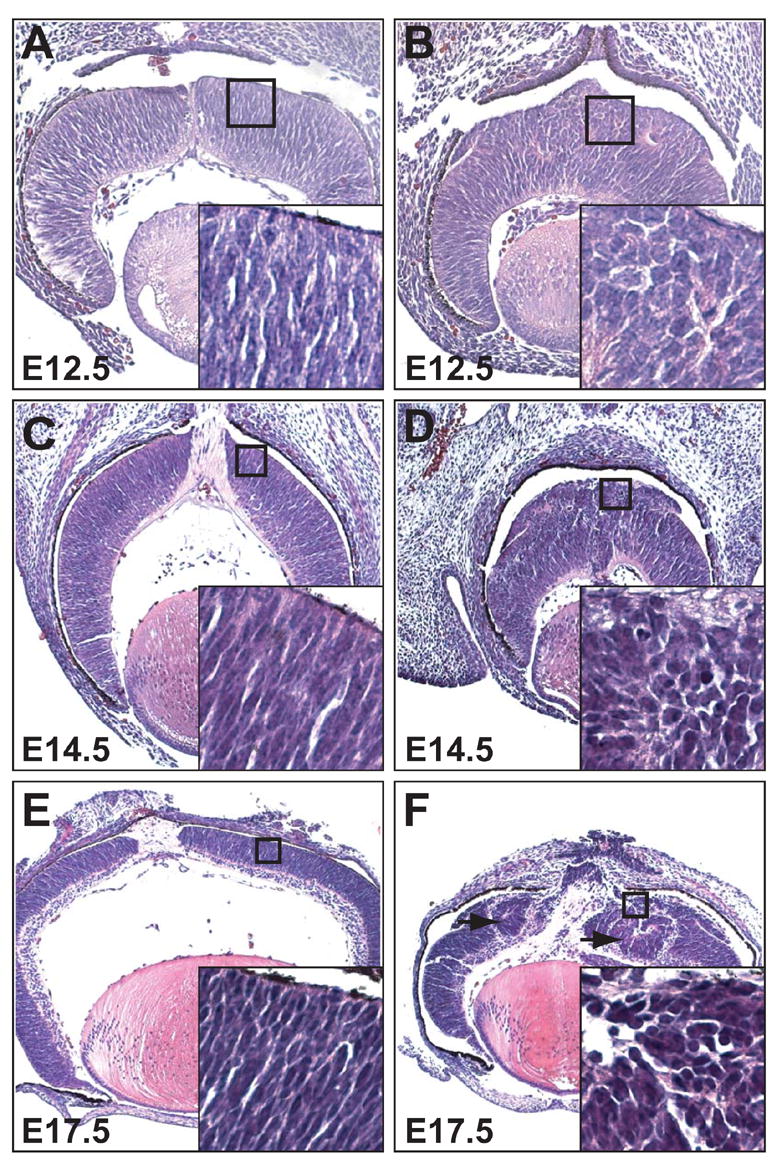
Progressive loss of radial orientation and cellular organization of RPCs during development. Shown are H&E-stained sections of E12.5 (A, B), E 14.5 (C, D), and E17.5 (E, F) retinas. (A, C, E) Control catnblox(e2-6)/ lox(e2-6) retinas showing RPCs with elongated nuclei and aligned radially along the apical-basal axis. (B, D, F) catnblox(e2-6)/ lox(e2-6);six3-Cre retinas. Insets at the lower right of each image show a higher magnification of the boxed region. Note that at as early as E12.5 (B), RPCs from β-catenin-deleted retinas lose their normal organization, and their nuclei are round. These defects become more severe at E14.5 (D) and E17.5 (F), and rosette structures begin to appear (arrowheads).
Loss of β-catenin leads to abnormal localization of N-cadherin, F-actin and the aPKCλ/Par3/Par6 complex during retinal development
Because β-catenin-mediated cell adhesion plays critical roles in cellular organization, we sought to determine whether cell adhesion was affected in β-catenin-deleted retinas. β-catenin physically interacts with N-cadherin and links it to F-actin. We therefore examined the expression of N-cadherin and F-actin during retinal development. In control retinas at E12.5, N-cadherin and F-actin were expressed in identical patterns as that of β-catenin (Fig. 4A, C, E). β-catenin, N-cadherin, and F-actin were expressed in all retinal cells, but expression was significantly higher at the apical and basal surfaces, and in the GCL (Fig. 4A, C, E). Expression of all three proteins was minimal in RPCs. The elevated expression of these proteins at both the apical and basal surfaces supports the notion that they have essential roles in maintaining the radial orientation and elongated morphology of RPCs. Moreover, their enhanced expression in the GCL suggests that differential cell adhesion is essential for proper migration of RGCs.
Fig. 4.
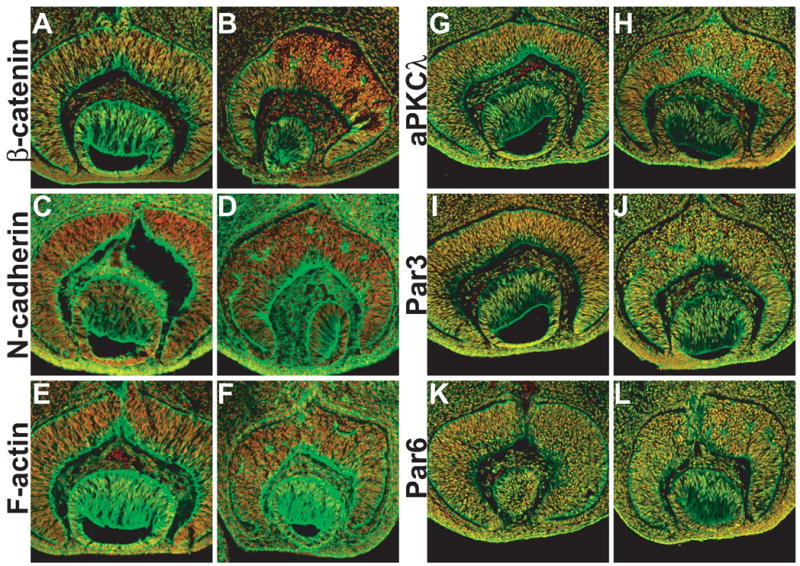
Expression of β-catenin, N-cadherin, F-actin, aPKCλ, Par3, and Par6 proteins in E12.5 retinas. Antibody staining is green, and nuclei are stained red with propidium iodide. (A, C, E, G, I, K) β-catenin (A), N-cadherin (C), F-actin (E), aPKCλ (G), Par3 (I), and Par6 (K) expression in catnblox(e2-6)/ lox(e2-6) control retinas. (B, D, F, H, J, L) Corresponding expression in catnblox(e2-6)/ lox(e2-6);six3-Cre retinas. In A, C, E, G, I, and K, high expression levels are observed at the apical and basal surfaces and the GCL. In the central region of catnblox(e2-6)/ lox(e2-6);six3-Cre retinas, β-catenin was not detected, and the normal N-cadherin, F-actin, aPKCλ, Par3, and Par6 expression patterns are disrupted. Note that the staining for N-cadherin, F-actin, aPKCλ, Par3, and Par6 on the apical surface in the central region of catnblox(e2-6)/ lox(e2-6);six3-Cre retinas disappears.
In catnblox(e2-6)/ lox(e2-6);six3-Cre retinas, β-catenin was efficiently deleted in the central region by E12.5, as shown by the virtual absence of β-catenin expression (Fig. 4B). Consequently, we observed abnormal expression patterns for N-cadherin and F-actin in this region (Fig. 4D, F). High-level expression of both proteins at the apical surface was largely lost. Instead, we observed only patchy staining in these regions (Fig. 4D, F). The abnormal expression patterns of N-cadherin and F-actin continued at later developmental stages (e.g. E14.5, data not shown). The disruption of normal expression patterns of N-cadherin and F-actin at as early as E12.5 was consistent with the observed cellular defects. These results suggest that β-catenin is essential for maintaining proper cell adhesion and linkage to the cytoskeletal network during retinal development. If so, abnormal cell adhesion might cause the aberrant cell shape and organization in β-catenin-deleted retinas.
Atypical protein kinase C (aPKC) forms a complex with Par3 and Par6 and regulates cell polarity in various biological processes (Henrique and Schweisguth, 2003; Ohno, 2001). Two components of the complex, aPKCλ and Par3 (Pard3 in zebrafish) have been implicated in the establishment and maintenance of cell polarity in the developing neural retina (Koike et al., 2005; Wei et al., 2004). There is also evidence suggesting that the aPKCλ/Par3/Par6 complex interacts with cell adherens junctions in regulating cell polarity (Koike et al., 2005). We therefore examined how the expression of this complex was affected by the deletion of β-catenin. At E12.5 in the control retinas, aPKCλ, Par3, Par6 are expressed in all retinal cells with the highest level at the apical and basal surface (Fig. 4G, I, K). In the β-catenin deleted retinas, although the overall level of these proteins did not change, their localization at the apical surface disappeared (Fig. 4H, J, L), suggesting that the function of the aPKCλ/Par3/Par6 complex is dependent on the cell adherens junctions in retinal development.
β-catenin is not required for the initiation of differentiation but is essential for the normal migration of differentiated cells
Retinal cell differentiation starts at E11.5 with the birth of the first RGCs (Young, 1985). RGC differentiation is preceded by expression of the proneural bHLH gene math5 in RPCs (Brown et al., 2001; Wang et al., 2001) and marked by expression of pou4f2 (brn3b), a gene encoding a class IV POU domain transcription factor (Xiang et al., 1995). RGC developmetn starts at the central retina and propagates toward the peripheral region as developments proceeds (Gan et al., 1999; Gan et al., 1996). The expression of both math5 and pou4f2 follows a central-to-peripheral pattern in control retinas as shown by in situ hybridization at E12.5 and E14.5 (Fig. 5A, C, E, G). In catnblox(e2-6)/ lox(e2-6);six3-Cre retinas, despite the abnormal cellular organization, expression of both math5 and pou4f2 initiated normally in the central retina (Fig. 5B, F) and propagated toward the periphery (Fig. 5D, H). In addition, the level of expression of the two genes in the central region of catnblox(e2-6)/ lox(e2-6);six3-Cre retinas was comparable to that in the peripheral region and in control retinas. These results suggest that the normal RPC orientation and organization was not required for the initiation of RGC differentiation, and hence neurogenesis.
Fig. 5.
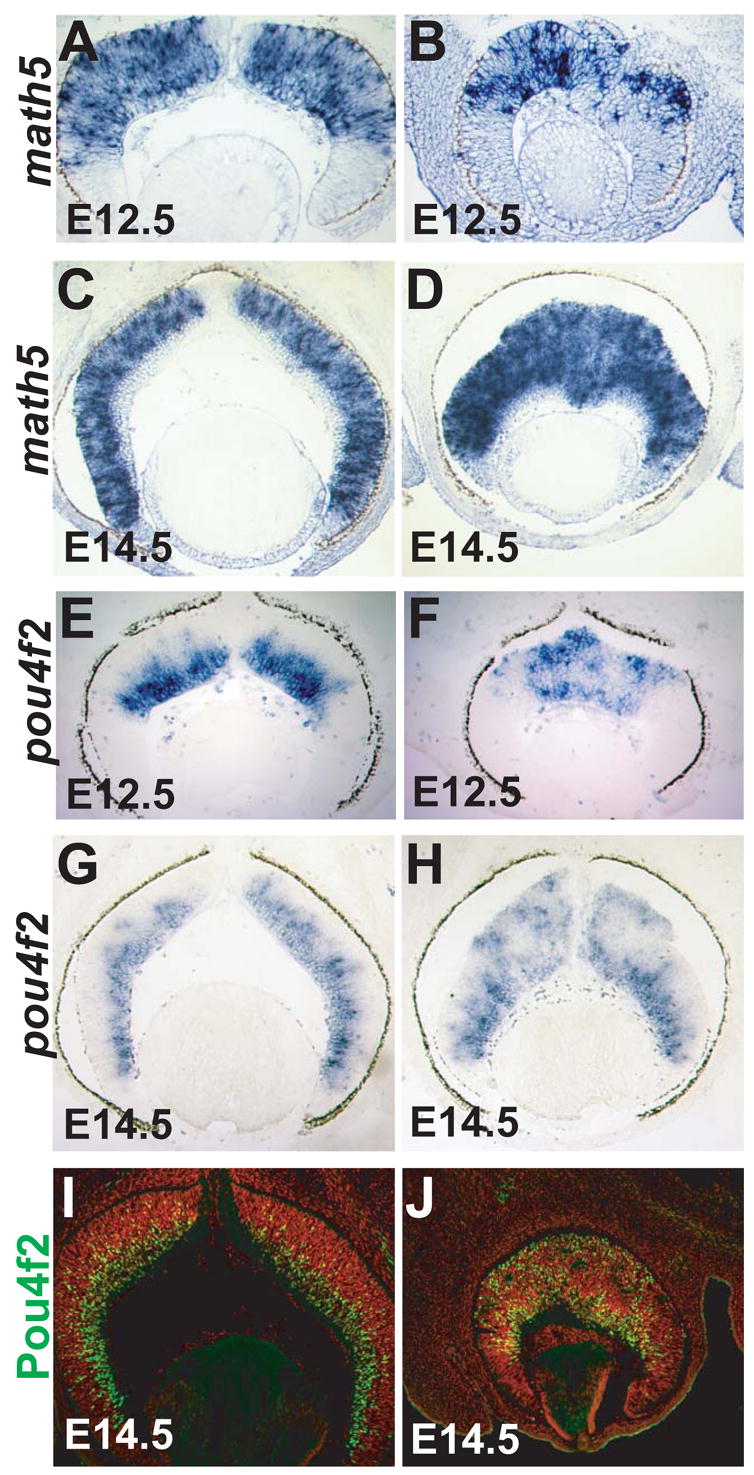
Math5 and pou4f2 expression in control and β-catenin-deleted retinas. (A, C, E, G, I) Control catnblox(e2-6)/ lox(e2-6) retinas. (B, D, F, H, J) catnblox(e2-6)/ lox(e2-6);six3-Cre retinas. Math5 and pou4f2 transcript expression are shown in A-H, and POU4f2 protein expression (green) is shown in I and J. Nuclei in I and J are stained red with propidium iodide. Similar to catnblox(e2-6)/ lox(e2-6) control retinas (A, C, E, G), expression of math5 and pou4f2 initiates normally at the center of catnblox(e2-6)/ lox(e2-6);six3-Cre retinas (B, F), and expands toward the periphery (D, H). Unlike the catnblox(e2-6)/ lox(e2-6) control retinas (E, G, I), newly formed RGCs, marked by pou4f2 expression, did not migrate normally to the GCL in β-catenin-deleted retinas (F, H, J).
However, the spatial expression pattern of pou4f2 was not normal in the β-catenin-deleted region. Unlike in control retinas, where pou4f2 expression was confined to the GCL on the basal side (Fig. 5E, G), pou4f2 mRNA was found in patches of cells that were located at different positions throughout the retina (Fig. 5F, H). This expression pattern was confirmed using an anti-POU4f2 antibody. At E14.5, in catnblox(e2-6)/ lox(e2-6) retinas, the majority of RGCs, as defined by POU4f2 protein expression, were in the GCL; only a few newly born RGCs were in the proliferation layer, and none of them were located on the apical side (Fig. 5I). In contrast, in catnblox(e2-6)/ lox(e2-6);six3-Cre retinas, RGCs were found throughout the retina, including the apical side, and the boundary between the proliferation zone and the GCL was lost (Fig. 5J). Because newly differentiated RGCs were not properly located to the GCL in the absence of β-catenin, it is possible that their cell adhesion properties were affected and, hence, that their directional migration was disrupted. Newly born RGCs either failed to migrate, or migrated randomly.
All retinal cell types form in the absence of β-catenin
The fact that the absence of β-catenin did not affect RGC differentiation but did affect their location in the retina prompted us to examine whether the other retinal cell types would also differentiate but be mislocalized. Antibodies corresponding to other retinal cell types were used to determine the extent of differentiation in β-catenin-deleted retinas. At P16, all cell types were observed in the central region of catnblox(e2-6)/ lox(e2-6);six3-Cre retinas, despite the fact that the retinal structure was severely disrupted in this region (Fig. 6A-P). Compared to catnblox(e2-6)/ lox(e2-6) retinas, the different cell types were mislocalized and observed across the thickness of the retina in the β-catenin-deleted region (Fig. 6A-P). However, mislocalization was not completely random. Consistent with our histological observations, cell types within the rosettes were partially laminated, with an organization similar to that of control retinas along the apical/basal axis. The rosette centers resembled the outer segment of photoreceptors and stained heavily for rhodopsin (Fig. 6H). Adjacent to these centers were the rod and cone photoreceptor nuclei (rhodopsin-positive cells) that resembled the ONL (Fig. 6G, H). At least two types of intermediate neurons, bipolar cells (Fig. 6O, P; Chx10 and PKCα) and amacrine cells (Fig. 6M, X; Pax6), were more distally located, forming INL-like structures. In areas where cells did not form rosettes, the different cell types were randomly distributed. The discontinuous non cellular structures stained positively for PKCα (Fig. 6P), Syntaxin (data not shown) and Cralbp (Fig. 6N), proteins normally expressed in the processes of rod bipolar cells, amacrine cells, and Müller glial cells, respectively. This suggests that these non cellular structures were indeed disorganized IPL. To determine whether the relative proportion of retinal cell types was altered in catnblox(e2-6)/ lox(e2-6);six3-Cre retinas, cell types were quantified using markers that allowed for distinction of individual cells because they were expressed predominantly in cell bodies. These included cones (CAR), bipolar cells (Chx10), amcrine cells (Pax6), and RGCs (Pou4f2). No significant changes were detected in any of these cell types in catnblox(e2-6)/ lox(e2-6);six3-Cre retinas (Fig. 6).
Fig. 6.
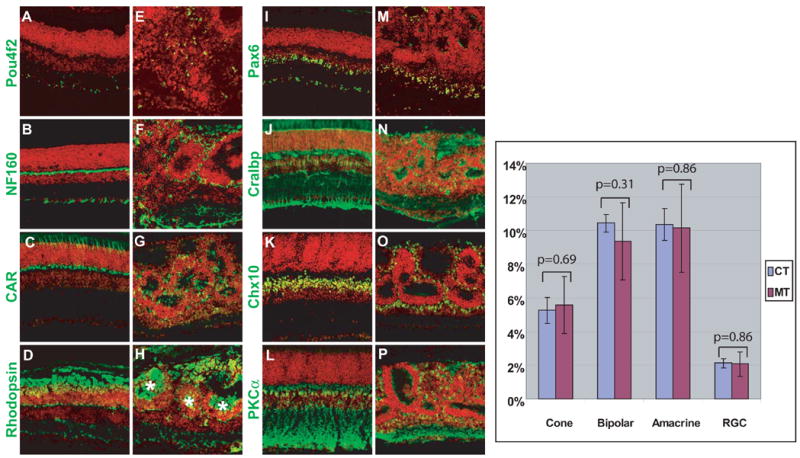
Differentiation of retinal cell types in control and β-catenin-deleted retinas at P16. Sections of P16 control catnblox(e2-6)/ lox(e2-6) (A-D, I-L) or catnblox(e2-6)/ lox(e2-6);six3-Cre (E-H, M-P) retinas were stained with individual antibodies against different retinal cell-type markers (green) and counter-stained red with propidium iodide. Pou4f2 (A, E): RGCs; NF160 (B, F): horizontal cells and RGCs; CAR (C, G): cones; rhodopsin (D, H): rods; Pax6 (I, M): amacrine cells and RGCs; Cralbp (J, N): Müller cells; Chx10 (K, O), bipolar cells; and PKCα (L, P), rod bipolar cells. Note that although all cell types are present, they lose their normal positions in catnblox(e2-6)/ lox(e2-6);six3-Cre retinas (E-H, M-P) compared to control retinas (A-D, I-L). Localized lamination of varying extents is observed around the rosettes with heavy staining of rhodopsin at the center (asterisks, H). Right panel: Quantification of four cell types (cone, bipolar, amacrine and RGC) in control (CT) and β-catenin-deleted (MT) retinas. Y axis is the percentage of individual cell types within an arbitrary length unit of retinal sections (see Material and methods). Standard deviations (error bars) are shown for each sample (n=6). There is no significant difference for any of the cell types between control and mutant retinas as indicated by the p values.
We also examined the possibility that hybrid cell types containing different cell markers might be formed in the absence of β-catenin, which would indicate abnormal differentiation. We used antibodies against five pairs of markers including CAR/Chx10 (Fig. 7A-C, G-I), Cralbp/Chx10 (Fig.7D-F, J-L), Cralbp/CAR (Fig. 7M-O, S-U), Pax6/Chx10 (Fig. 7P-R, V-X), and PKCa/Chx10 (data not shown). For each pair, the staining patterns of individual markers were different in both control and β-catenin –deleted retinas (Fig. 7). Although there was overlap of staining for some pairs (Fig. 7F, L, U), this was caused by overlapping neighboring cells or their processes, but not by staining of a single cell by both markers (inlets, Fig. 7 D-F, J-L, M-O, S-U). We observed no cells that were positive for two different cell type markers in either the control or β-catenin-deleted retinas.
Fig. 7.
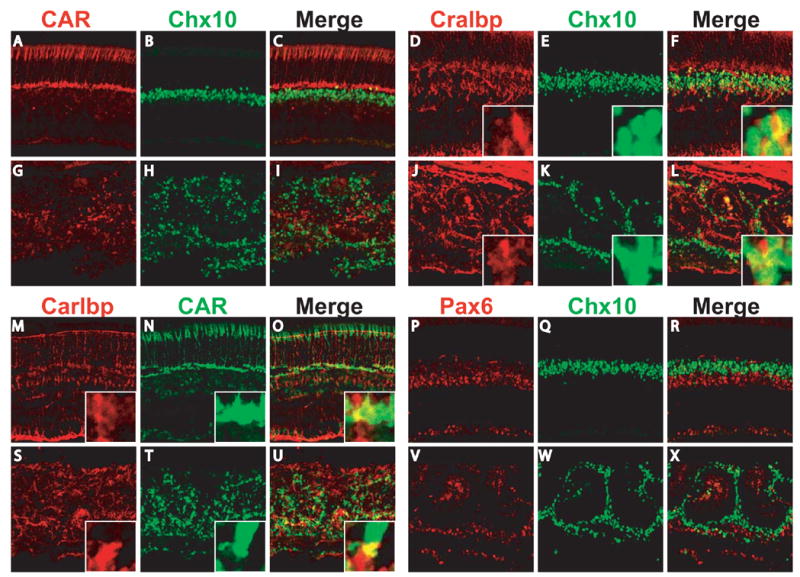
No hybrid retinal cell types were formed in the absence of β-catenin. P16 control catnblox(e2-6)/ lox(e2-6) (A-F, M-R) or catnblox(e2-6)/ lox(e2-6);six3-Cre (G-L, S-X) retinal sections were double stained with markers for two different cell types as indicated. For each pair of markers, images of individual and merged signals were shown. Inlets (D-F, J-L, M-O, and S-U) are high magnification of regions that appear yellow in the merged images. The yellow color was resulted from neighboring cells or their processes that overlap in the sections, but not from a single cell positive for both markers. No cell was observed double-positive for any pair of markers in either the control or β-catenin-deleted retinas.
These results indicate that β-catenin was not required for the development of any of the retinal cell types. Furthermore, the partially laminated rosettes observed in the absence of β-catenin indicate that localized areas of retinal lamination still form in retinas lacking β-catenin.
Cell proliferation and apoptosis in the absence of β-catenin
Both catnblox(e2-6)/ lox(e2-6);six3-Cre and catnblox(e2-6)/ lox(e2-6);α-Cre mice had smaller eyes than those of wild-type and catnblox(e2-6)/ lox(e2-6) controls (Fig. 1). The β-catenin-deleted regions in the retinas of the mutant mice were thicker than the corresponding regions of the control retinas (Fig. 2), but this alone could not account for the reduction in retinal size, because the total number of cells in the β-catenin-deleted retinas was also reduced. To determine the potential responsible mechanism, we examined the expression of cyclinD1, a gene expressed in RPCs and essential for their proliferation (Fantl et al., 1995; Sicinski et al., 1995). At E12.5 (data not shown) and E14.5 (Fig. 8A, B), no detectable changes in cyclinD1 expression were observed in catnblox(e2-6)/ lox(e2-6);six3-Cre retinas compared to control retinas. Additionally, no significant changes in cell proliferation were observed in the β-catenin deleted retinas when we used BrdU to chase-label S-phase RPCs at E14.5 (Fig. 8C, D) and compared the proportions of BrdU-positive cells in catnblox(e2-6)/ lox(e2-6);six3-Cre and control retinas (cell counting results not shown).
Fig. 8.
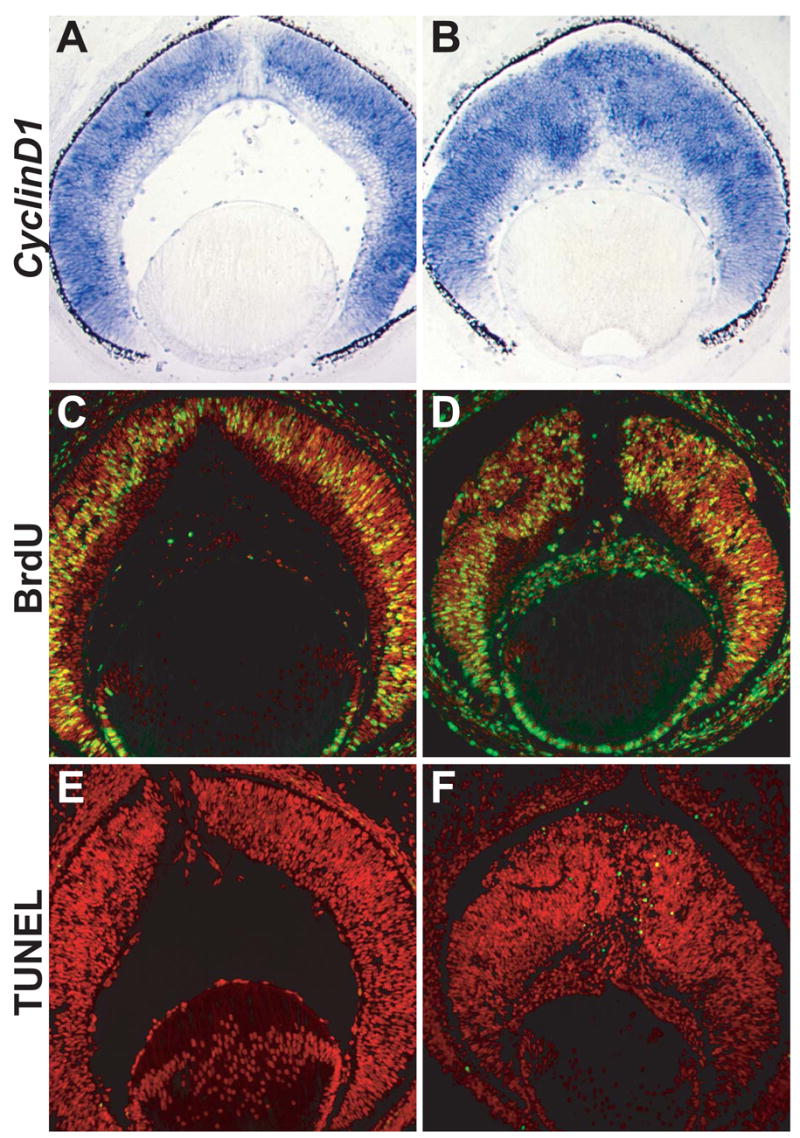
Cell proliferation and apoptosis in catnblox(e2-6)/ lox(e2-6) control (A, C, E) and catnblox(e2-6)/ lox(e2-6);six3-Cre (B, D, F) retinas at E14.5. (A, B) Expression of cyclinD1 transcripts. (C, D) BrdU chase-labeling (green) for S-phase cells. Nuclei are stained red with propidium iodide. (E, F) TUNEL assay for apoptotic cells (green).
We also examined whether retinal cells in β-catenin-deleted retinas underwent enhanced apoptosis at E12.5 and E14.5, because it is known that in wild-type retinas, little apoptosis occurs at these stages (Mu et al., 2005). TUNEL analysis showed that enhanced apoptosis was not observed in catnblox(e2-6)/ lox(e2-6);six3-Cre retinas at E12.5 (data not shown), but at E14.5, an increase in the number of apoptotic cells was clearly evident and these cells were restricted largely to the area where β-catenin was deleted (Fig. 8F). It was therefore likely that the reduced size of β-catenin-deleted retinas was caused in large part by enhanced apoptosis. Notably, enhanced apoptosis was also observed in retinas from zebrafish glass onion mutants (Pujic and Malicki, 2001), indicating that the loss of N-cadherin and the resulting defects in cell adhesion lead to a decrease in cell survival.
Ectopic activation of the canonical Wnt signaling pathway is detrimental to retinal development and leads to loss of neural retinal identity
The defects observed in β-catenin-deleted retinas during neurogenesis point to a role for β-catenin in cell adhesion. However, this fact does not rule out the possibility that the nuclear function of β-catenin is also required. To determine whether this was the case, we activated the nuclear function of β-catenin in the developing retina by breeding mice carrying the catnblox(e3) allele to six3-Cre or α-Cre transgenic mice. The catnblox(e3) allele contains floxed exon 3 sequences that encode the phosphorylation sites required for β-catenin degradation; deletion of exon 3 by Cre recombinase leads to the production of stabilized β-catenin and activation of the canonical Wnt pathway (Harada et al., 1999). This allele has been used widely to address the consequences of ectopic Wnt signaling in various tissues, and the ectopic stabilization of β-catenin often results in enhanced cell proliferation (Akiyama et al., 2004; Bierie et al., 2003; Harada et al., 1999; Zechner et al., 2003).
Ectopic activation of the Wnt-β-catenin pathway in the retina did not lead to an overproliferation of retinal cells; at E14.5, catnblox(e3)/+;six3-Cre and catnblox(e3)/+;α-Cre retinas were the same size as control retinas (Fig. 9A, E, data not shown). However, the choroid fissures in β-catenin-activated retinas failed to close (data not shown), and at later stages, the mutant eyes that were smaller than those of wild-type controls (Fig. 9F). β-catenin-activated retinas lost their normal structure as early as E14.5 in regions where Cre was expressed (Fig. 9E), and this defect became more severe at later stages (Fig. 9F). Furthermore, pigmented cells were abnormally intermingled with neural retinal cells in both catnblox(e3)/+;six3-Cre (inlet, Fig. 9F) and catnblox(e3)/+;α-Cre retinas (data not shown), suggesting that ectopic activation of the Wnt-β-catenin pathway leads to a transdifferentiation of neural retinal cells into pigmented cells. To further examine the possibility that transdifferentiation had occurred, we determined the expression patterns of Chx10 and Mitf, two transcription factors required for the development of the neural retina and pigmented epithelium, respectively (Fig. 9C, D). In the central region of catnblox(e3)/+;six3-Cre retinas, no Chx10 expression was detected indicating that neural retina development was compromised (Fig. 9G). Instead, the central region contained a sparse number of dispersed cells expressing Mitf (Fig. 8H). This result provided additional evidence that forced Wnt signaling altered the developmental pathway of retinal progenitor cells. These results suggest that activation of the canonical Wnt pathway does not facilitate neurogenesis, as might be expected, but rather compromises neural retinal integrity and identity.
Fig. 9.
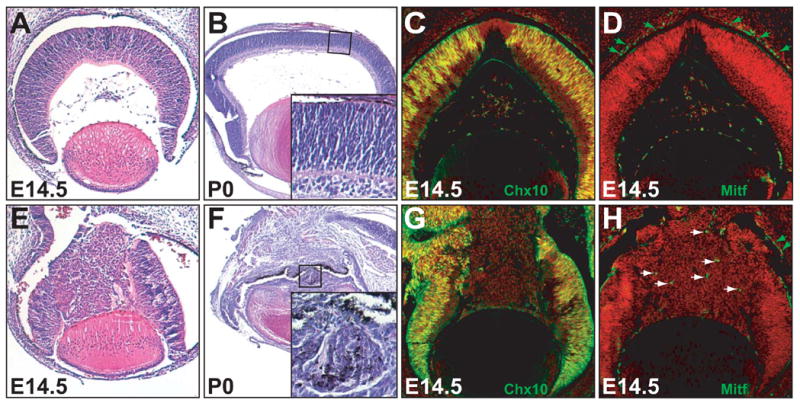
Effects of the ectopic expression of stabilized β-cateinin on retinal development. (A-D) control catnblox(e3)/ lox(e3) retinas. (E-H) catnblox(e3)/ lox(e3);six3-Cre retinas. (A, B, E, F) H&E staining of E14.5 and P0 retinas shows cellular disorganization in stabilized β-catenin-activated (catnblox(e3)/ lox(e3);six3-Cre) retinas. Note the presence of pigmented cells in the catnblox(e3)/ lox(e3);six3-Cre neural retina (inlet, F). At later stages (P0), catnblox(e3)/ lox(e3);six3-Cre eyes (F) are smaller than the control (B). (C, G, D, H) immunofluorescence staining (green) for Chx10 and Mitf of E14.5 control (C, D) and catnblox(e3)/ lox(e3);six3-Cre (H, G) retinas. In the central regions of catnblox(e3)/ lox(e3);six3-Cre retinas, Chx10 expression is lost, and some cells ( white arrowheads) express Mitf ectopically. Green arrowheads point to normal Mitf-expressing cells in the pigmented epithelia.
Discussion
Cell adhesion function of β-catenin in retinal development
In our study, we showed that retinogenesis is profoundly affected by the loss of β-catenin. These results provide direct in vivo evidence that β-catenin has essential functions in retinal development and that these functions are associated with retinal lamination rather than cell proliferation or cell differentiation. During retinal development, β-catenin, N-cadherin, and F-actin have virtually identical spatiotemporal expression patterns (Xu et al., 2002). The absence of β-catenin caused disruption in the normal expression pattern of N-cadherin, indicating that β-catenin is essential in maintaining normal cell adhesion during retinal development. Consistent with this, deletion of β-catenin led to retinal defects similar to those observed in N-cadherin mutations in zebrafish (Erdmann et al., 2003; Malicki et al., 2003; Masai et al., 2003) and N-cadherin loss-of-function perturbation in chick embryos (Matsunaga et al., 1988). In these cases as well as in the β-catenin-deleted retinas described here, cell shape and radial alignment of RPCs was disrupted, and the laminar structure of the mature retina failed to form. The disruption of RPC orientation and alignment occurs early, before neurogenesis begins, suggesting that N-cadherin-β-catenin-mediated cell adhesion is essential in establishing and maintaining the orientation, polarity, and alignment of RPCs in the vertebrate retina. As development proceeds, newly differentiated cells appear to rely on the pre-existing apical-basal orientation of RPCs for their migration. Although the precise mechanism that controls the directional migration of newly differentiated retinal cells is unknown, differences in cell adhesion between RPCs and differentiated retinal cells may be essential. This hypothesis is supported by the fact that there is higher expression of N-cadherin and β-cateinin in newly differentiated RGCs than there is in overlying RPCs. The defects in cellular organization seen in β-catenin-deleted retinas may be caused by changes in adhesion properties in both RPCs and newly differentiated retinal cells. Because localized cellular organization still occurred in β-catenin-deleted retinas, N-cadherin is probably not the only cell-adhesion molecule involved in retinal lamination. This is supported by the fact that β-catenin is not expressed in photoreceptors, which remain well-organized in β-catenin-deleted retinas. Indeed, many cell-adhesion molecules are expressed in the retina besides N-cadherin (Honjo et al., 2000), suggesting that non-β-catenin cell adhesion mechanisms (such as gap junction) are also important in the formation and maintenance of laminar retinal structures. Moreover, retinal lamination is an actively regulated process requiring both intra- and intercellular signaling. Mutations in genes for many molecules, including aPKCλ (Horne-Badovinac et al., 2001; Koike et al., 2005), Moe (a FERM domain protein) (Jensen and Westerfield, 2004), and Par3 (a PDZ domain protein) (Wei et al., 2004), cause defective lamination. Par3 and aPKC, together with Par6, function as a complex in the regulation of cell polarity in a variety of cell types in different species (Henrique and Schweisguth, 2003; Ohno, 2001).We found that the expression patterns of aPKCλ, Par3, and Par6 were disrupted in the absence of β-catenin, suggesting this complex may function through adherens junctions in the developing retina. At present, however, it is not understood how these molecules act together to coordinate cell orientation, cell polarity, cell migration, and lamination in the retina.
The function of β-catenin may not be limited to just lamination formation during retinal neurogenesis. There is much evidence suggesting that adherens junctions are also required for neurite extension (Elul et al., 2003; Riehl et al., 1996; Stone and Sakaguchi, 1996) and the canonical Wnt pathway inhibits this process (Ouchi et al., 2005).
Cell adhesion and cell differentiation are genetically separable processes in the developing retina
In the absence of β-catenin, RPCs lost their radial orientation and arrangement, but neurogenesis was not affected. This indicates that proper RPC arrangement is not a prerequisite for the differentiation of the retinal cell types. Similar observations have been made with zebrafish mutants with retinal lamination defects (Erdmann et al., 2003; Jensen and Westerfield, 2004; Koike et al., 2005; Malicki and Driever, 1999; Masai et al., 2003). Despite the fact that RGCs, the first retinal cells to differentiate, did not migrate to their proper position in the GCL, all later cell types still developed. These results imply that that the sequential development of the different retinal cell types is not dependent on the proper cellular organization. This is consistent with accumulating evidence suggesting that intrinsic mechanisms play a major role in retinal cell differentiation (Cayouette et al., 2003; Kay et al., 2005; Mu et al., 2005).
Although the loss of proper polarity and arrangement of RPCs was the earliest defect observed, it is possible that in the absence of β-catenin, the later aberrant migration of RGCs and other retinal cell types was not solely caused by polarity and arrangement defects in RPCs, but also by defective cell adhesion properties of the differentiated neurons. Migration may require proper adhesion between differentiated neurons and RPCs, as well as their interaction with the extracellular matrix or other substrates. In the developing cortex, differentiated neurons migrated along the processes of radial glial cells, which are the neural progenitor cells (Miyata et al., 2001; Noctor et al., 2001; Noctor et al., 2002). A similar scenario might exist in the developing retina in which the differentiated neuronal cell types rely on the oriented processes of RPCs for their proper unidirectional migration.
Nuclear function of β-catenin and the Wnt signaling pathway in retinal development
Several studies have suggested that the Wnt signaling pathway is important in retinal development in chickens (Kubo et al., 2003; Kubo et al., 2005), Xenopus (Van Raay et al., 2005), zebrafish (Cavodeassi et al., 2005; van de Water et al., 2001), and mice (Maretto et al., 2003). In the chick, Wnt2b (also know as Wnt13) is expressed at the tip of the cilliary marginal zone (CMV) of the retina. It promotes RPC proliferation and prevents precocious differentiation by suppressing the expression of proneural and neural genes (Kubo et al., 2003; Kubo et al., 2005). In Xenopus, Frizzled5 (Xfz5) has been shown to regulate the proliferation and neural potential of RPCs. In the mouse, knockout of the Wnt co-receptor Lrp6 gene leads to microophthalmia and retinal defects (Maretto et al., 2003). These findings combined with the fact that many components of the Wnt pathway are expressed in the developing mouse retina suggest that Wnt signaling is an important process in retinogenesis, although its specific role may vary among different vertebrate species. However, in all the above-mentioned studies, the role of the canonical Wnt pathway in retinal development was investigated by inactivating the pathway at stages prior to cell differentiation, thereby preventing an assessment of its role in cell type specification and differentiation. Transgenic analysis, using the TCF/Lef-LacZ transgene, which responds to canonical Wnt-β-catenin signaling, indicates that the Wnt-β-catenin pathway is largely inactive during neurogenesis (Liu et al., 2003). Although some residual LacZ-positive cells were still present at E14.5 in the retina of TCF/Lef-LacZ mice, this probably reflected the stability of LacZ rather than the activation of the canonic Wnt pathway. Therefore, Wnt-β-catenin signaling appears only to be required at early stages of retinal development to prevent precocious differentiation and is inactive during neurogenesis. Consistent with this idea, we found that β-catenin functions mainly in cell adhesion during retinal neurogenesis and not in the specification and differentiation of retinal cell types because these events were not affected in the absence of β-catenin. Moreover, we found that ectopic activation of the Wnt-β-catenin pathway did not lead to overproliferation, and was in fact detrimental to neurogenesis, consitent with previous reports both in zebrafish (van de Water et al., 2001) and mice (Ouchi et al., 2005). Retinal neurogenesis may require inactivation of this pathway, and this may be achieved by inhibitory molecules such as Axin (van de Water et al., 2001), Sfrp1, Sfrp2 (Liu et al., 2003; Mu et al., 2001) and WIF-1 (Hunter et al., 2004), which are all expressed in the developing retina. This differs from other neural tissues in which the canonical Wnt-β-catenin pathway is required for cell proliferation (Chenn and Walsh, 2002; Zechner et al., 2003) and cell-fate determination (Hari et al., 2002; Lee et al., 2004). Notably, in Xenopus, overexpression of Sfrp2 and Frzb, two inhibitory molecules of the Wnt pathway, also cause retinal lamination defects (Ladher et al., 2000). However, because these genes were overexpressed very early, the defects observed may be indirect. It is also possible that Wnt ligands act through non-canonical signaling pathways in the retina (Esteve et al., 2003; Yu et al., 2004) and that non-canonical Wnt signaling and cell adherens junctions are both required for retinal lamination.
The canonical Wnt-β-catenin pathway may be required in the separation of the neural retinal and pigmented epithelial fates. Chx10 and Mitf function to establish and maintain these two fates respectively (Horsford et al., 2005; Rowan et al., 2004). Our observation that activated β-catenin leads to loss of Chx10 expression and gain of ectopic Mitf expression, and hence an apparent change in the pathway of neural retinal cells into pigmented cells, suggests that the Wnt-β-catenin pathway has a role in pigmented epithelial fate.
Acknowledgments
We thank Drs. Makoto M. Taketo, Peter Gruss, Guillermo Oliver, and Yas Furuta for the mouse lines used in this work. This work was supported by a National Eye Institute grant (EY011930) to W.H.K., Robert A. Welch Foundation Chair (G-0010) to W.H.K., and a grant from the E. Matilda Ziegler Foundation for the Blind to X.M. X.F. is a recipient of the Schissler Foundation Fellowship. The University of Texas M. D. Research Animal Support Facility is supported in part by National Cancer Institute grant CA16672.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akiyama H, Lyons JP, Mori-Akiyama Y, Yang X, Zhang R, Zhang Z, Deng JM, Taketo MM, Nakamura T, Behringer RR, McCrea PD, de Crombrugghe B. Interactions between Sox9 and beta-catenin control chondrocyte differentiation. Genes Dev. 2004;18:1072–1087. doi: 10.1101/gad.1171104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierie B, Nozawa M, Renou JP, Shillingford JM, Morgan F, Oka T, Taketo MM, Cardiff RD, Miyoshi K, Wagner KU, Robinson GW, Hennighausen L. Activation of beta-catenin in prostate epithelium induces hyperplasias and squamous transdifferentiation. Oncogene. 2003;22:3875–3887. doi: 10.1038/sj.onc.1206426. [DOI] [PubMed] [Google Scholar]
- Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- Brown NL, Patel S, Brzezinski J, Glaser T. Math5 is required for retinal ganglion cell and optic nerve formation. Development. 2001;128:2497–2508. doi: 10.1242/dev.128.13.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavodeassi F, Carreira-Barbosa F, Young RM, Concha ML, Allende ML, Houart C, Tada M, Wilson SW. Early stages of zebrafish eye formation require the coordinated activity of Wnt11, Fz5, and the Wnt/beta-catenin pathway. Neuron. 2005;47:43–56. doi: 10.1016/j.neuron.2005.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayouette M, Barres BA, Raff M. Importance of intrinsic mechanisms in cell fate decisions in the developing rat retina. Neuron. 2003;40:897–904. doi: 10.1016/s0896-6273(03)00756-6. [DOI] [PubMed] [Google Scholar]
- Charron F, Tessier-Lavigne M. Novel brain wiring functions for classical morphogens: a role as graded positional cues in axon guidance. Development. 2005;132:2251–2262. doi: 10.1242/dev.01830. [DOI] [PubMed] [Google Scholar]
- Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297:365–369. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- Elul TM, Kimes NE, Kohwi M, Reichardt LF. N- and C-terminal domains of beta-catenin, respectively, are required to initiate and shape axon arbors of retinal ganglion cells in vivo. J Neurosci. 2003;23:6567–6575. doi: 10.1523/JNEUROSCI.23-16-06567.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann B, Kirsch FP, Rathjen FG, More MI. N-cadherin is essential for retinal lamination in the zebrafish. Dev Dyn. 2003;226:570–577. doi: 10.1002/dvdy.10266. [DOI] [PubMed] [Google Scholar]
- Esteve P, Trousse F, Rodriguez J, Bovolenta P. SFRP1 modulates retina cell differentiation through a beta-catenin-independent mechanism. J Cell Sci. 2003;116:2471–2481. doi: 10.1242/jcs.00452. [DOI] [PubMed] [Google Scholar]
- Fantl V, Stamp G, Andrews A, Rosewell I, Dickson C. Mice lacking cyclin D1 are small and show defects in eye and mammary gland development. Genes Dev. 1995;9:2364–2372. doi: 10.1101/gad.9.19.2364. [DOI] [PubMed] [Google Scholar]
- Furuta Y, Lagutin O, Hogan BL, Oliver GC. Retina- and ventral forebrain-specific Cre recombinase activity in transgenic mice. Genesis. 2000;26:130–132. [PubMed] [Google Scholar]
- Gan L, Wang SW, Huang Z, Klein WH. POU domain factor Brn-3b is essential for retinal ganglion cell differentiation and survival but not for initial cell fate specification. Dev Biol. 1999;210:469–480. doi: 10.1006/dbio.1999.9280. [DOI] [PubMed] [Google Scholar]
- Gan L, Xiang M, Zhou L, Wagner DS, Klein WH, Nathans J. POU domain factor Brn-3b is required for the development of a large set of retinal ganglion cells. Proc Natl Acad Sci U S A. 1996;93:3920–3925. doi: 10.1073/pnas.93.9.3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, Taketo MM. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. Embo J. 1999;18:5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari L, Brault V, Kleber M, Lee HY, Ille F, Leimeroth R, Paratore C, Suter U, Kemler R, Sommer L. Lineage-specific requirements of beta-catenin in neural crest development. J Cell Biol. 2002;159:867–880. doi: 10.1083/jcb.200209039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama J, Kageyama R. Retinal cell fate determination and bHLH factors. Semin Cell Dev Biol. 2004;15:83–89. doi: 10.1016/j.semcdb.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Henrique D, Schweisguth F. Cell polarity: the ups and downs of the Par6/aPKC complex. Curr Opin Genet Dev. 2003;13:341–350. doi: 10.1016/s0959-437x(03)00077-7. [DOI] [PubMed] [Google Scholar]
- Hinds JW, Hinds PL. Early ganglion cell differentiation in the mouse retina: an electron microscopic analysis utilizing serial sections. Dev Biol. 1974;37:381–416. doi: 10.1016/0012-1606(74)90156-0. [DOI] [PubMed] [Google Scholar]
- Hinds JW, Hinds PL. Differentiation of photoreceptors and horizontal cells in the embryonic mouse retina: an electron microscopic, serial section analysis. J Comp Neurol. 1979;187:495–511. doi: 10.1002/cne.901870303. [DOI] [PubMed] [Google Scholar]
- Hinds JW, Hinds PL. Development of retinal amacrine cells in the mouse embryo: evidence for two modes of formation. J Comp Neurol. 1983;213:1–23. doi: 10.1002/cne.902130102. [DOI] [PubMed] [Google Scholar]
- Honjo M, Tanihara H, Suzuki S, Tanaka T, Honda Y, Takeichi M. Differential expression of cadherin adhesion receptors in neural retina of the postnatal mouse. Invest Ophthalmol Vis Sci. 2000;41:546–551. [PubMed] [Google Scholar]
- Horne-Badovinac S, Lin D, Waldron S, Schwarz M, Mbamalu G, Pawson T, Jan Y, Stainier DY, Abdelilah-Seyfried S. Positional cloning of heart and soul reveals multiple roles for PKC lambda in zebrafish organogenesis. Curr Biol. 2001;11:1492–1502. doi: 10.1016/s0960-9822(01)00458-4. [DOI] [PubMed] [Google Scholar]
- Horsford DJ, Nguyen MT, Sellar GC, Kothary R, Arnheiter H, McInnes RR. Chx10 repression of Mitf is required for the maintenance of mammalian neuroretinal identity. Development. 2005;132:177–187. doi: 10.1242/dev.01571. [DOI] [PubMed] [Google Scholar]
- Hunter DD, Zhang M, Ferguson JW, Koch M, Brunken WJ. The extracellular matrix component WIF-1 is expressed during, and can modulate, retinal development. Mol Cell Neurosci. 2004;27:477–488. doi: 10.1016/j.mcn.2004.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen AM, Westerfield M. Zebrafish mosaic eyes is a novel FERM protein required for retinal lamination and retinal pigmented epithelial tight junction formation. Curr Biol. 2004;14:711–717. doi: 10.1016/j.cub.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Kay JN, Link BA, Baier H. Staggered cell-intrinsic timing of ath5 expression underlies the wave of ganglion cell neurogenesis in the zebrafish retina. Development. 2005;132:2573–2585. doi: 10.1242/dev.01831. [DOI] [PubMed] [Google Scholar]
- Koike C, Nishida A, Akimoto K, Nakaya MA, Noda T, Ohno S, Furukawa T. Function of atypical protein kinase Clambda in differentiating photoreceptors is required for proper lamination of mouse retina. J Neurosci. 2005;25:10290–10298. doi: 10.1523/JNEUROSCI.3657-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo F, Takeichi M, Nakagawa S. Wnt2b controls retinal cell differentiation at the ciliary marginal zone. Development. 2003;130:587–598. doi: 10.1242/dev.00244. [DOI] [PubMed] [Google Scholar]
- Kubo F, Takeichi M, Nakagawa S. Wnt2b inhibits differentiation of retinal progenitor cells in the absence of Notch activity by downregulating the expression of proneural genes. Development. 2005;132:2759–2770. doi: 10.1242/dev.01856. [DOI] [PubMed] [Google Scholar]
- Ladher RK, Church VL, Allen S, Robson L, Abdelfattah A, Brown NA, Hattersley G, Rosen V, Luyten FP, Dale L, Francis-West PH. Cloning and expression of the Wnt antagonists Sfrp-2 and Frzb during chick development. Dev Biol. 2000;218:183–198. doi: 10.1006/dbio.1999.9586. [DOI] [PubMed] [Google Scholar]
- Lee HY, Kleber M, Hari L, Brault V, Suter U, Taketo MM, Kemler R, Sommer L. Instructive role of Wnt/beta-catenin in sensory fate specification in neural crest stem cells. Science. 2004;303:1020–1023. doi: 10.1126/science.1091611. [DOI] [PubMed] [Google Scholar]
- Liu H, Mohamed O, Dufort D, Wallace VA. Characterization of Wnt signaling components and activation of the Wnt canonical pathway in the murine retina. Dev Dyn. 2003;227:323–334. doi: 10.1002/dvdy.10315. [DOI] [PubMed] [Google Scholar]
- Liu X, Sarthy VP, Craft CM, Honda Y, Ide C. Developmental expression of beta-catenin in mouse retina. Anat Sci Int. 2002;77:182–188. doi: 10.1046/j.0022-7722.2002.00026.x. [DOI] [PubMed] [Google Scholar]
- Livesey FJ, Cepko CL. Vertebrate neural cell-fate determination: lessons from the retina. Nat Rev Neurosci. 2001;2:109–118. doi: 10.1038/35053522. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Malicki J, Driever W. oko meduzy mutations affect neuronal patterning in the zebrafish retina and reveal cell-cell interactions of the retinal neuroepithelial sheet. Development. 1999;126:1235–1246. doi: 10.1242/dev.126.6.1235. [DOI] [PubMed] [Google Scholar]
- Malicki J, Jo H, Pujic Z. Zebrafish N-cadherin, encoded by the glass onion locus, plays an essential role in retinal patterning. Dev Biol. 2003;259:95–108. doi: 10.1016/s0012-1606(03)00181-7. [DOI] [PubMed] [Google Scholar]
- Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, Volpin D, Bressan GM, Piccolo S. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci U S A. 2003;100:3299–3304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt T, Ashery-Padan R, Andrejewski N, Scardigli R, Guillemot F, Gruss P. Pax6 is required for the multipotent state of retinal progenitor cells. Cell. 2001;105:43–55. doi: 10.1016/s0092-8674(01)00295-1. [DOI] [PubMed] [Google Scholar]
- Masai I, Lele Z, Yamaguchi M, Komori A, Nakata A, Nishiwaki Y, Wada H, Tanaka H, Nojima Y, Hammerschmidt M, Wilson SW, Okamoto H. N-cadherin mediates retinal lamination, maintenance of forebrain compartments and patterning of retinal neurites. Development. 2003;130:2479–2494. doi: 10.1242/dev.00465. [DOI] [PubMed] [Google Scholar]
- Matsunaga M, Hatta K, Takeichi M. Role of N-cadherin cell adhesion molecules in the histogenesis of neural retina. Neuron. 1988;1:289–295. doi: 10.1016/0896-6273(88)90077-3. [DOI] [PubMed] [Google Scholar]
- Miyata T, Kawaguchi A, Okano H, Ogawa M. Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron. 2001;31:727–741. doi: 10.1016/s0896-6273(01)00420-2. [DOI] [PubMed] [Google Scholar]
- Mu X, Beremand PD, Zhao S, Pershad R, Sun H, Scarpa A, Liang S, Thomas TL, Klein WH. Discrete gene sets depend on POU domain transcription factor Brn3b/Brn-3.2/POU4f2 for their expression in the mouse embryonic retina. Development. 2004;131:1197–1210. doi: 10.1242/dev.01010. [DOI] [PubMed] [Google Scholar]
- Mu X, Fu X, Sun H, Liang S, Maeda H, Frishman LJ, Klein WH. Ganglion cells are required for normal progenitor- cell proliferation but not cell-fate determination or patterning in the developing mouse retina. Curr Biol. 2005;15:525–530. doi: 10.1016/j.cub.2005.01.043. [DOI] [PubMed] [Google Scholar]
- Mu X, Klein WH. A gene regulatory hierarchy for retinal ganglion cell specification and differentiation. Semin Cell Dev Biol. 2004;15:115–123. doi: 10.1016/j.semcdb.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Mu X, Zhao S, Pershad R, Hsieh TF, Scarpa A, Wang SW, White RA, Beremand PD, Thomas TL, Gan L, Klein WH. Gene expression in the developing mouse retina by EST sequencing and microarray analysis. Nucleic Acids Res. 2001;29:4983–4993. doi: 10.1093/nar/29.24.4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Flint AC, Weissman TA, Wong WS, Clinton BK, Kriegstein AR. Dividing precursor cells of the embryonic cortical ventricular zone have morphological and molecular characteristics of radial glia. J Neurosci. 2002;22:3161–3173. doi: 10.1523/JNEUROSCI.22-08-03161.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno S. Intercellular junctions and cellular polarity: the PAR-aPKC complex, a conserved core cassette playing fundamental roles in cell polarity. Curr Opin Cell Biol. 2001;13:641–648. doi: 10.1016/s0955-0674(00)00264-7. [DOI] [PubMed] [Google Scholar]
- Ouchi Y, Tabata Y, Arai K, Watanabe S. Negative regulation of retinal-neurite extension by beta-catenin signaling pathway. J Cell Sci. 2005;118:4473–4483. doi: 10.1242/jcs.02575. [DOI] [PubMed] [Google Scholar]
- Pujic Z, Malicki J. Mutation of the zebrafish glass onion locus causes early cell-nonautonomous loss of neuroepithelial integrity followed by severe neuronal patterning defects in the retina. Dev Biol. 2001;234:454–469. doi: 10.1006/dbio.2001.0251. [DOI] [PubMed] [Google Scholar]
- Pujic Z, Malicki J. Retinal pattern and the genetic basis of its formation in zebrafish. Semin Cell Dev Biol. 2004;15:105–114. doi: 10.1016/j.semcdb.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Riehl R, Johnson K, Bradley R, Grunwald GB, Cornel E, Lilienbaum A, Holt CE. Cadherin function is required for axon outgrowth in retinal ganglion cells in vivo. Neuron. 1996;17:837–848. doi: 10.1016/s0896-6273(00)80216-0. [DOI] [PubMed] [Google Scholar]
- Rowan S, Chen CM, Young TL, Fisher DE, Cepko CL. Transdifferentiation of the retina into pigmented cells in ocular retardation mice defines a new function of the homeodomain gene Chx10. Development. 2004;131:5139–5152. doi: 10.1242/dev.01300. [DOI] [PubMed] [Google Scholar]
- Sicinski P, Donaher JL, Parker SB, Li T, Fazeli A, Gardner H, Haslam SZ, Bronson RT, Elledge SJ, Weinberg RA. Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell. 1995;82:621–630. doi: 10.1016/0092-8674(95)90034-9. [DOI] [PubMed] [Google Scholar]
- Steinberg MS. Does differential adhesion govern self-assembly processes in histogenesis? Equilibrium configurations and the emergence of a hierarchy among populations of embryonic cells. J Exp Zool. 1970;173:395–433. doi: 10.1002/jez.1401730406. [DOI] [PubMed] [Google Scholar]
- Steinberg MS. Cell-cell recognition in multicellular assembly: levels of specificity. Symp Soc Exp Biol. 1978;32:25–49. [PubMed] [Google Scholar]
- Stone KE, Sakaguchi DS. Perturbation of the developing Xenopus retinotectal projection following injections of antibodies against beta1 integrin receptors and N-cadherin. Dev Biol. 1996;180:297–310. doi: 10.1006/dbio.1996.0302. [DOI] [PubMed] [Google Scholar]
- van de Water S, van de Wetering M, Joore J, Esseling J, Bink R, Clevers H, Zivkovic D. Ectopic Wnt signal determines the eyeless phenotype of zebrafish masterblind mutant. Development. 2001;128:3877–3888. doi: 10.1242/dev.128.20.3877. [DOI] [PubMed] [Google Scholar]
- Van Raay TJ, Moore KB, Iordanova I, Steele M, Jamrich M, Harris WA, Vetter ML. Frizzled 5 signaling governs the neural potential of progenitors in the developing Xenopus retina. Neuron. 2005;46:23–36. doi: 10.1016/j.neuron.2005.02.023. [DOI] [PubMed] [Google Scholar]
- Wang SW, Kim BS, Ding K, Wang H, Sun D, Johnson RL, Klein WH, Gan L. Requirement for math5 in the development of retinal ganglion cells. Genes Dev. 2001;15:24–29. doi: 10.1101/gad.855301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Cheng Y, Luo Y, Shi X, Nelson S, Hyde DR. The zebrafish Pard3 ortholog is required for separation of the eye fields and retinal lamination. Dev Biol. 2004;269:286–301. doi: 10.1016/j.ydbio.2004.01.017. [DOI] [PubMed] [Google Scholar]
- Wei X, Malicki J. nagie oko, encoding a MAGUK-family protein, is essential for cellular patterning of the retina. Nat Genet. 2002;31:150–157. doi: 10.1038/ng883. [DOI] [PubMed] [Google Scholar]
- Wheelock MJ, Johnson KR. Cadherins as modulators of cellular phenotype. Annu Rev Cell Dev Biol. 2003;19:207–235. doi: 10.1146/annurev.cellbio.19.011102.111135. [DOI] [PubMed] [Google Scholar]
- Xiang M, Zhou L, Macke JP, Yoshioka T, Hendry SH, Eddy RL, Shows TB, Nathans J. The Brn-3 family of POU-domain factors: primary structure, binding specificity, and expression in subsets of retinal ganglion cells and somatosensory neurons. J Neurosci. 1995;15:4762–4785. doi: 10.1523/JNEUROSCI.15-07-04762.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Overbeek PA, Reneker LW. Systematic analysis of E-, N- and P-cadherin expression in mouse eye development. Exp Eye Res. 2002;74:753–760. doi: 10.1006/exer.2002.1175. [DOI] [PubMed] [Google Scholar]
- Young RW. Cell differentiation in the retina of the mouse. Anat Rec. 1985;212:199–205. doi: 10.1002/ar.1092120215. [DOI] [PubMed] [Google Scholar]
- Yu J, He S, Friedman JS, Akimoto M, Ghosh D, Mears AJ, Hicks D, Swaroop A. Altered expression of genes of the Bmp/Smad and Wnt/calcium signaling pathways in the cone-only Nrl-/- mouse retina, revealed by gene profiling using custom cDNA microarrays. J Biol Chem. 2004;279:42211–42220. doi: 10.1074/jbc.M408223200. [DOI] [PubMed] [Google Scholar]
- Zechner D, Fujita Y, Hulsken J, Muller T, Walther I, Taketo MM, Crenshaw EB, 3rd, Birchmeier W, Birchmeier C. beta-Catenin signals regulate cell growth and the balance between progenitor cell expansion and differentiation in the nervous system. Dev Biol. 2003;258:406–418. doi: 10.1016/s0012-1606(03)00123-4. [DOI] [PubMed] [Google Scholar]


