Abstract
Adolescents have been hypothesized to exhibit an age-related partial anhedonia that may lead them to seek out natural and drug rewards to compensate for this attenuated hedonic sensitivity. In the present series of experiments, taste reactivity (TR) and 2 bottle choice tests were used to assess hedonic reactions to sucrose. In Exp 1, total positive taste responses to 10% sucrose solution were significantly higher in adolescent than adult rats during the infusion period. In Exp 2, adolescent animals exhibited a concentration-effect shift that was consistent with a greater hedonic sensitivity compared to adults. Conversely, adolescents exhibited fewer negative responses to quinine. Using a shortened infusion period, adolescents in Exp 3 exhibited a trend for greater positive TR than adults in response to 10 and 34% sucrose. Consistent with the TR results of Exp 1–3, adolescents consumed significantly more sucrose solution (ml/kg) than adults, although no significant age difference in sucrose preference rates emerged.
The results of the current series of experiments do not support the hypothesis that adolescents exhibit an age-related, partial anhedonia, with adolescent animals, under a number of test circumstances showing greater positive taste reactivity and reduced negative responding.
Keywords: Adolescence, Taste reactivity, Sucrose, Quinine, Reward, Rat, Hedonic responses
1. Introduction
Adolescence is a developmental period marked by both neural and behavioral changes. Adolescent-associated behavioral changes are surprisingly well conserved across species and include elevations in social interactions with peers, novelty seeking, and risk taking (Csikszentmihalyi et al., 1977; Primus and Kellogg, 1989; Pellis and Pellis, 1990; Pellis and Pellis, 1997; Trimpop et al., 1998). Adolescents exhibit both hyperdipsia and hyperphagia, with humans and rats exhibiting higher caloric intake relative to their body weight than any other time in the life span (Post and Kemper, 1993; Nance, 1983). In humans this developmental period is also the time during which drug use is typically initiated. The physiological mechanisms responsible for increases in consumption of natural reinforcers during adolescence might contribute to the propensity of adolescents to consume drug reinforcers as well, given that natural and drug reinforcer are thought to share common reward pathways (Di Chiara, 1999; Berridge and Robinson, 1998; Wise, 1989).
It is currently unclear if this adolescent-typical increase in the seeking and consumption of appetitive stimuli is related to increases or decreases in the incentive value attributed to rewarding stimuli. On one hand, adolescents might avidly seek out natural and drug rewards in an attempt to compensate for an attenuated sensitivity to hedonic stimuli (Spear, 2000). Some of the limited human data available supporting this hypothesis include the observation that human adolescents demonstrate less ventral striatal activation in response to reward cues and report a greater incidence of depressed mood compared to adults (Bjork et al., 2004; Petersen et al., 1993). Conversely, adolescent-typical increases in consummatory behaviors may be driven by increases in the hedonic value assigned to reinforcers (Ernst et al., 2006; Chambers et al., 2003). Supporting this notion, other functional magnetic resonance imaging (fMRI) work has shown adolescents to exhibit an exaggerated magnitude of activation in the nucleus accumbens (NAc) in response to visual stimuli associated with a monetary reward when compared with younger and older subjects (Galvan et al., 2006).
Traditionally, the consumption of appetitive stimuli has been used to index the hedonic value attributed to those stimuli, effects thought to be mediated through changes in mesolimbic dopamine function/sensitivity (Wise et al., 1978; Wise and Bozarth, 1982). The preference for mildly sweet sucrose solution (~1.0%) is one of the most extensively used behavioral measures of anhedonia, with anhedonia interpreted as a reduction in choice of the sucrose solution over water in two-bottle choice test (Willner et al., 1987).
More recently, Robinson and Berridge have proposed an incentive salience theory of reward that separates reward conceptually and functionally into two component psychological processes mediated by different neural mechanisms: “wanting” and “liking” (Berridge, 1996; Berridge and Robinson, 1998). Wanting refers to the motivational or craving element of reward, and is a reward component found to sensitize following repeated exposure to drugs of abuse. Liking, on the other hand refers to the hedonic or pleasure component of reward and is not thought to sensitize. Within this conceptual framework, dopamine is thought to be involved in assigning incentive motivational value to rewarding stimuli (i.e. wanting) (Berridge. 1996; Berridge and Robinson, 1998). Hedonic “liking” properties, on the other hand, are thought to be mediated by a hierarchical system involving opioid, cannabinoid, and to some extent GABA systems (Peciña et al., 2006).
One model that has been extensively employed to examine hedonic sensitivity to appetitive tastants is the taste reactivity (TR) test, which is thought to be a direct measure of the hedonic value attributed to stimuli. In the TR test, a solution is presented to the subject and the oral facial reactions to that tastant are proposed to reflect the palatability of the solutions. Palatable solutions, such as sucrose, elicit appetitive TR behaviors such as rhythmic and lateral tongue protrusions, whereas aversive solutions, such as quinine, elicit aversive TR behaviors (including gapes) (see methods for more details). Both appetitive and aversive taste reactions are highly conserved, with similar oral facial responses to appetitive and aversive stimuli seen in human infants as in mature primates and rodents (Steiner et al., 2001). Across-species differences emerging primarily in the timing at which particular TR behaviors are emitted, with humans and gorillas expressing behaviors much slower than rodents (Steiner et al., 2001).
The current series of experiments used the TR test, as developed for rats by Grill and Norgren (1978), to assess developmental differences in hedonic sensitivity to appetitive and aversive stimuli in adolescent and adult rats, with TR assessed both during and immediately following the infusion period. Developmental differences in voluntary consumption of an appetitive solution were also assessed using a two-bottle choice test.
2. General methods
2.1. Subjects
Male Sprague–Dawley rats (Taconic Farms) bred in our colony were used. All animals were maintained in a temperature-controlled (22 °C) vivarium on a 14-h/10-h light cycle (lights on 0700) with ad libitum access to food (Purina Rat Chow, Lowell, MA) and water. On postnatal day 1 (P1), litters were culled to 7–10 pups. Animals were weaned on P21 and pair-housed with same-sex littermates until the time of the experimental procedures. No more than one animal from a given litter was used in any experimental group. At all times, rats used in these experiments were maintained and treated in accordance with guidelines for animal care established by the National Institutes of Health (1986).
2.2. Taste reactivity
2.2.1. Surgery
At the onset of each experiment, beginning on P 28–29 for adolescents and P 69–70 for adults, animals were anesthetized with isoflurane prior to the implantation of an oral cannula. Each cannula consisted of polyethylene tubing (PE-50) with a heat-flanged tip. A guide needle was used to insert the cannula through the cheek at the level of the 1st maxillary molar. Another guide needle was then used to tunnel the cannula subcutaneously to the dorsal skull surface where the cannula was secured with surgical glue. Following surgery, animals were singly housed to avoid across-animal damage to the cannulas.
2.2.2. Apparatus
Testing was conducted in a Plexiglas cylinder (adolescent – height = 16.0 in, diameter = 8.0 in; adult – height = 16.0 in, diameter = 10.0 in). A mirror was placed at a 45° angle below the Plexiglas floor, providing a ventral view of the rat for video recording. Solutions were infused into animals' cannulas via connection to a calibrated syringe pump controlling rate, duration and volume of the infusion.
2.2.3. Behavioral measures
All taste reactivity behaviors during the infusion period and 30 s post-infusion were scored frame-by-frame. Scoring criteria for each behavior, and rationale for the classification of components into hedonic or aversive categories were based on Berridge and Grill (1983). Positive hedonic reactions included: (a) rhythmic tongue protrusions — the anterior tip of the tongue visibly emerges directly on the midline, covering the upper incisors and is then retracted — movements repeated at an approximate rate of 8.8 cycles/s; (b) lateral tongue protrusions — the tongue protrudes (nonrhythmic) from the side of the mouth followed by a forward extension, generally occurring on alternating sides of the mouth or in one single protrusion; (c) paw licks — any instance of the rat licking its paws, with the exception of paw licking occurring within a grooming sequence; (d) lateral tongue movements — the tongue emerges on the side of the mouth, extending the upper lip/cheek laterally without protruding from the mouth. Aversive reaction patterns included; (a) gapes — the mandible lowers, while the corners of the mouth contract, forming a triangle shaped mouth opening that is held for approximately 83 ms; (b) chin rubbing — the animal rubs its chin on the floor while projecting the body forward; face washing — single or several wipes of the face with the forepaws; head shake — a bout (typically with a duration of <1 s) of rapid (>60 cycles/s) side to side movements of the head; (e) forelimb flails — a brief bout (<1 s) of shaking of the forelimbs at a rate greater than 60 cycles/s; (f) paw treads — planting of the forelimbs on the floor and alternating forceful strokes forward and back. Unless otherwise noted, scoring criterion used was based on those of Berridge (Berridge, 1996, 2000). Discrete actions such as lateral tongue protrusions, gapes, and chin rubbing as well as bouts of head shaking, forelimb flails and paw treads were scored as a single count for each occurrence. Behaviors that typically persist for >1 s were recorded as follows: paw licks, rhythmic mouth movements, grooming and face washing were recorded in 5-s bins (any occurrence of these behaviors within each 5-s time bin was scored as a single count). Rhythmic tongue protrusions were scored similarly, except 2-s bins were used. The final total positive hedonic score was composed of the sum of all rhythmic tongue protrusions, lateral tongue protrusions, paw licking, and lateral tongue movements, whereas total negative hedonic scores were composed of the sum of all gapes, chin rubs, face washing, head shakes, forelimb flails and paw treads.
The experimenter was present during the test sessions to videotape, using a digital camera (JVS, HDD), a close-up view of the oral region during the infusion period and for 30 sec thereafter. An investigator blind to both solution type and age later analyzed the sessions frame-by-frame.
2.2.4. Experiment 1
Following surgery (Experiment [Exp] Day 0), all animals were given 3 days to recover, after which the taste reactivity test was conducted (Exp Day 3—P 33–34 for adolescents (n = 5); P 72–73 for adults (n = 6)). On test day, following a 15 min habituation to the chamber, each animal received a 45 s continuous infusion of 10% sucrose solution at a rate of 1 ml/min. Total number of positive and negative responses during the 45 s infusion period and for 30 s thereafter were recorded. Number of negative responses to this appetitive tastant was negligible at both ages and assessment periods. Consequently, only positive response data were analyzed.
Total positive taste responses (i.e., the sum of paw licks, rhythmic tongue protrusions, lateral tongue protrusions and lateral tongue movements) to the 10% sucrose solution were significantly higher in adolescent than adult rats during the 45-s infusion period (t = 2.6; df = 9; p<0.028)(seeFig. l).
Fig. 1.
Total positive responses to 10% sucrose in adolescents and adults during the 45-s infusion period of Exp 1, Total positive responses = paw licks + lateral tongue protrusion + rhythmic tongue protrusions. Asterisk (*) indicates significant difference between adolescent and adult animals (p<0.05). Bars reflect standard error of the mean.
Total positive taste responses during the 30-s post-infusion period were relatively low and did not differ significantly with age, despite a seeming trend for adults to exhibit more responses compared to adolescents (adults: 1.3 ±0.4; adolescents: 0.6±0.3) (t = 0.9; df = 9; p = 037).
2.2.5. Experiment 2
To determine if the findings in Exp 1 reflected a shift in the concentration–effect curve for appetitive taste stimuli, 3 different concentrations of sucrose solutions as well as a water control were examined in this experiment. Taste reactivity to a quinine solution was also assessed to determine whether age-related differences in affective responses observed in Exp 1 would also be evident in response to aversive stimuli.
A 2 age (adolescent and adult) × 5 solution (water, 0.34%, 3.4% or 34.0% sucrose, and quinine) within subjects design was used. The procedure was similar to Exp 1 with the exception that animals were given a 1-day recovery period following surgery. On Exp Day 2 (P 30 for adolescents; P 72 for adults), all animals received a water trial. Exp Days 3–5 consisted of 1 sucrose trial per day, with the order of concentration presentation varied randomly among animals. A quinine (3.0 × l0−4 M) trial was conducted on the last day of testing for all animals. Each infusion was given over 45 s and was infused at a rate of 1 ml/min. Oral responses to each tastant were examined during the 45 s infusion period and for 30 s thereafter.
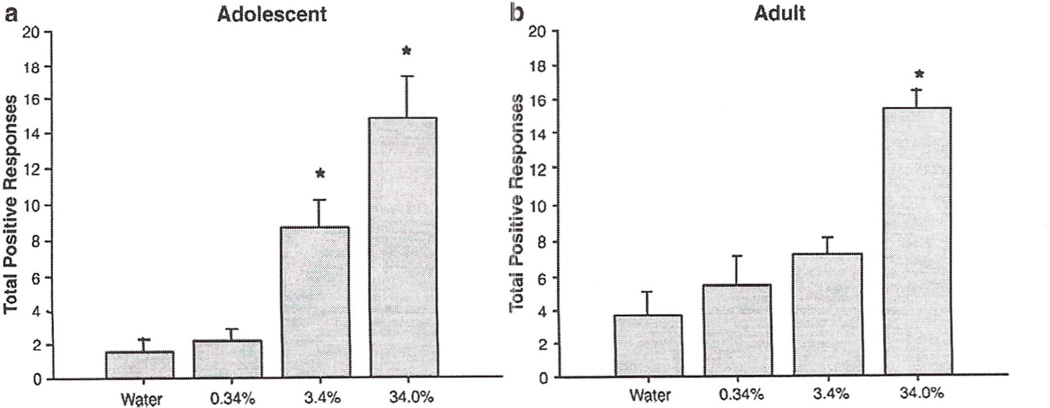
Adult animals exhibited significantly greater baseline responding (i.e., more positive taste responses to water) during the infusion period than did adolescents (adults: 4.25 ± 1.2; adolescents: 1.5 ± 1.1). Given this age difference in responding to the baseline test stimulus, adolescent and adult data were analyzed separately. In these analyses, positive taste responses to water and sucrose (0.34, 3.4 & 34.0%) were examined at each age via a repeated measures ANOVA (n = 10). with responses to quinine versus water examined separately (n = 8). Repeated measures ANOVA of positive taste responses observed during the 45-s infusion period again revealed a significant effect of solution in both adolescents [F(3,27) = 17.58, p<0.05] and adults [F(3,24) = 14.32, p<0.05]. Post hoc tests showed that the 3.4 and 34.0% sucrose concentrations produced significantly elevated levels of appetitive taste reactions in adolescent animals, whereas 34.0% was the only concentration that produced an increase in responding among adults. These data are shown in Fig. 2.
Fig. 2.
Positive responses to water. 0.34, 3.4 & 34.0% sucrose during the 45-s infusion period of Exp 2 in adolescent (a) and adult (b) rats. Asterisks (*) indicate significant difference from water (p<0.05). Bars reflect standard error of the mean.
The repeated measures ANOVAs of positive responses observed during the 30-s post-infusion period again revealed a main effect of concentration in both adolescents [F(3,24) = 5.24, p<0.05] and adults [F(3,24) = 12.7, p<0.05]. Post hoc tests in both the adolescents' and adults' analyses indicated that positive taste reactions in response to 34% sucrose were significantly greater than those exhibited to water (Fig. 3).
Fig. 3.
Positive responses to water, 0.34. 3.4 & 34.0% sucrose during the 30-s post-infusion period of Exp 2 in adolescent (a) and adult (b) rats. Asterisks (*) indicate significant difference from water (p<0.05). Bars reflect standard error of the mean.
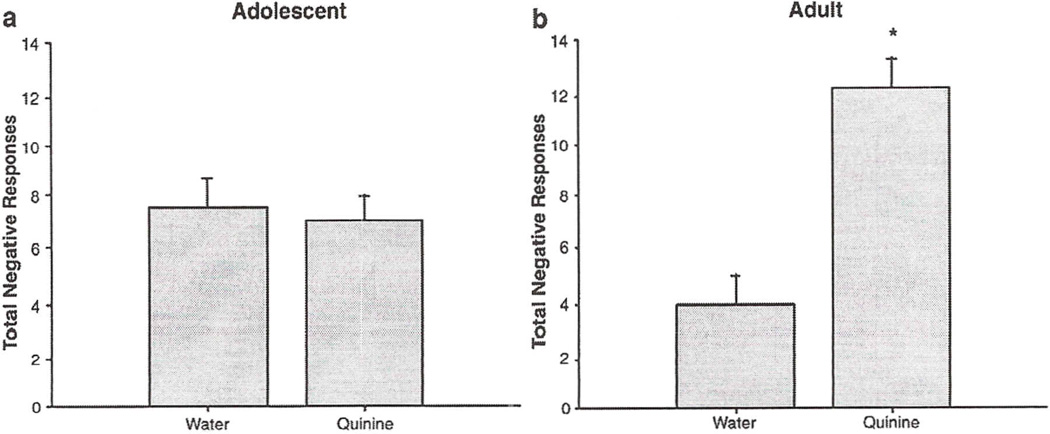
An age difference in negative taste responses to water was also observed, with adult animals exhibiting significantly fewer negative responses to water during the infusion period than adolescents (adult 4.4 ± 0.9; adolescent 7.3 ± 1.0) (t = 2.1; df = 14; p<0.05). Given this age difference in baseline responding, adolescent and adult data were analyzed separately. In these analyses, negative taste responses to water and quinine were examined at each age via repeated measures ANOVAs. Adults exhibited significantly higher negative taste responses to quinine relative to their water baseline during the 45-s infusion period [F(l,6) = 19.3, p<0.05], whereas negative responding to quinine among adolescents did not differ from their water baseline [F(l,8) = 0.4, p>0.05]. A similar pattern was seen, during the 30-s post-infusion period, with adults exhibiting higher negative responses to quinine than water [F(1,6) = 16.1, p<0.05], while the adolescents' TR to quinine did not differ from their response to water [F(l,8) = 1.0, p>0.05] (see Fig. 4).
Fig. 4.
Negative responses to water and quinine during a 45-s infusion period in adolescent (a) and adult (b) rats. Total negative responses = gapes + chin rubs + face washing + forelimb flails + head shake. Asterisks (*) indicate significant difference from water control (p<0.05). Bars reflect standard error of the mean.
2.2.6. Experiment 3
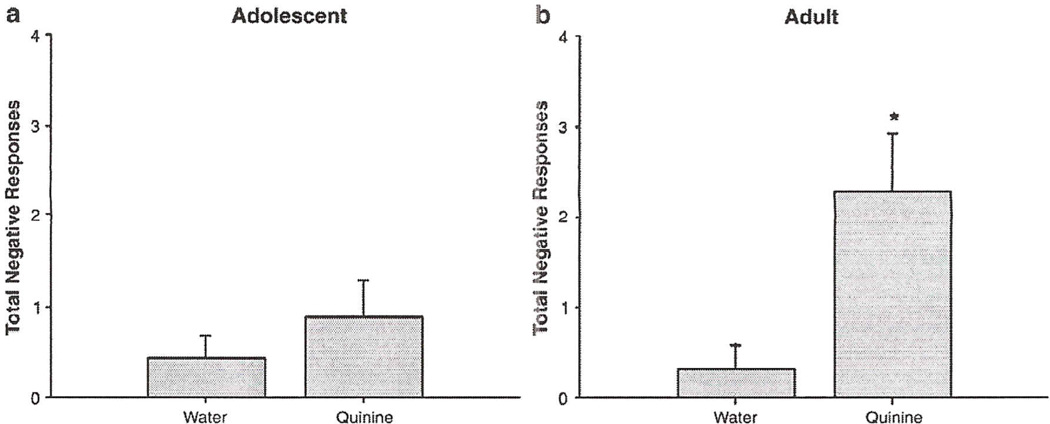
Previous studies have suggested that under certain experimental conditions post-infusion responding can be more sensitive to manipulation than responding during the infusion period (Grill et al., 1996). Unfortunately, in Exp 1 and 2, levels of responding during the post-infusion period were generally low, and hence possible floor effects constrain interpretation of these data. Given that shorter infusion periods have previously been associated with greater post-infusion responding (Grill et al., 1996), in this experiment the duration of each infusion period was shortened to 30 s (Fig. 5).
Fig. 5.
Negative responses to water and quinine during a 30-s post-infusion period in adolescent (a) and adult (b) rats. Total negative responses = gapes + chin rubs + face washing + forelimb flails + head shake. Asterisks (*) indicate significant difference from age-matched water control (p<0.05). Bars reflect standard error of the mean.
The design of this study was a 2 age (adolescent and adult) × 3 solution (water, 10% and 34% sucrose) factorial (n = 8), with the last factor consisting of a repeated measure. To reduce the number of animals lost to cannula occlusion, animals were implanted with bilateral cheek cannulas. As in Exp 2, animals were given a 1-day recovery period following surgery prior to testing. Animals received one trial with each tastant, with all trials conducted on the same day. A 2 h interval separated each trial, during which animals were returned to their home cage. Water was presented first, followed by the sucrose solutions in ascending concentration. For each test, animals were placed into the test chamber and given a 15 min habituation period, followed by a 30 s continuous infusion of the solution presented at a rate of 1 ml/min. Behaviors were initially scored as described in General Methods, and then were rescored using criteria designed to minimize differential weighing of behaviors to the composite positive TR score. With this rescoring, behaviors previously analyzed as counts per 2 or 5 s bins were rescored into 1 s bins.
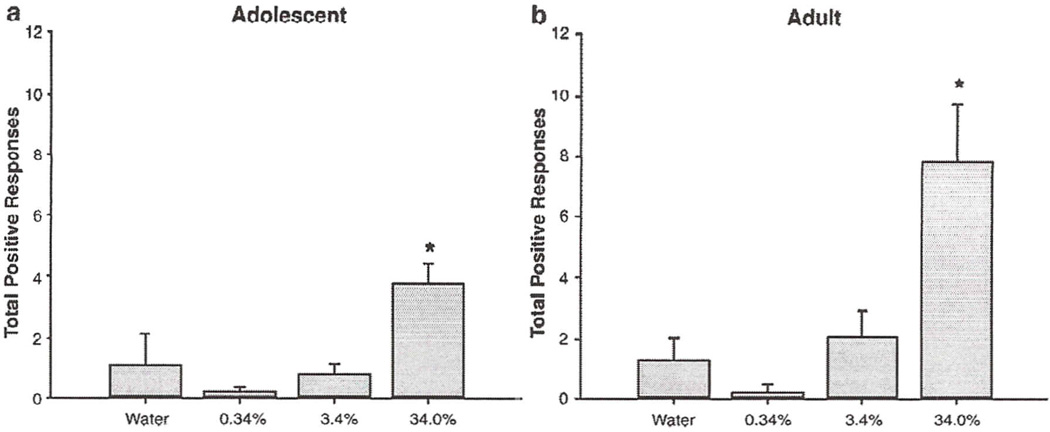
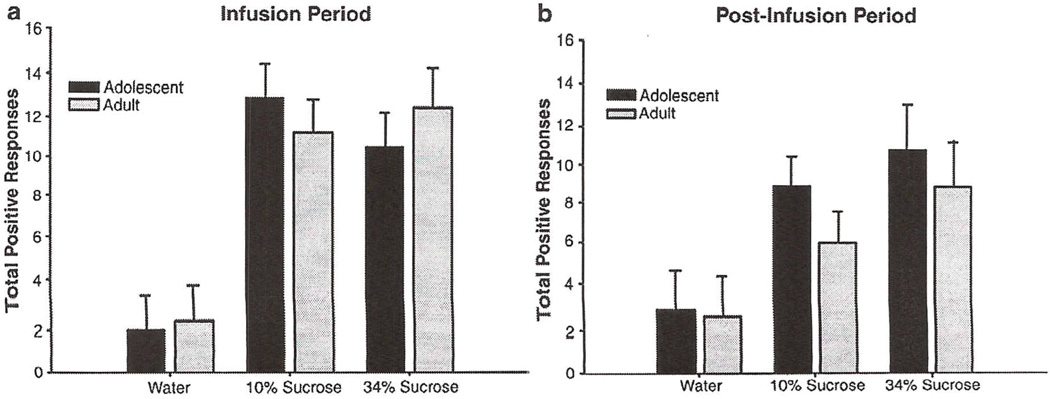
When the data were analyzed using the original scoring criteria, no age differences emerged during the water infusion period. The ANOVA of adolescent and adult total positive responses during the 30-s infusion period revealed a main effect of solution, with both concentrations of sucrose producing a higher number of responses than to water [F(2, 492) = 24, p<0.05] (see Fig. 6a). There was no significant main effect or interaction involving age. A main effect of solution also was evident in the analysis of the 30-s post-infusion period, with both concentrations of sucrose producing significantly more responses than water [F(2. 206) = 8, p<0.05] (see Fig. 6b). Again, no age effects were evident.
Fig 6.
Total positive responses to water and sucrose (10 &, 34%) in adolescents and adults during a 30-s infusion (a) and 30-s post-infusion period (b). Total positive responses = paw licks + lateral tongue protrusion + rhythmic tongue protrusions + lateral tongue movements. Bars reflect standard error of the mean.
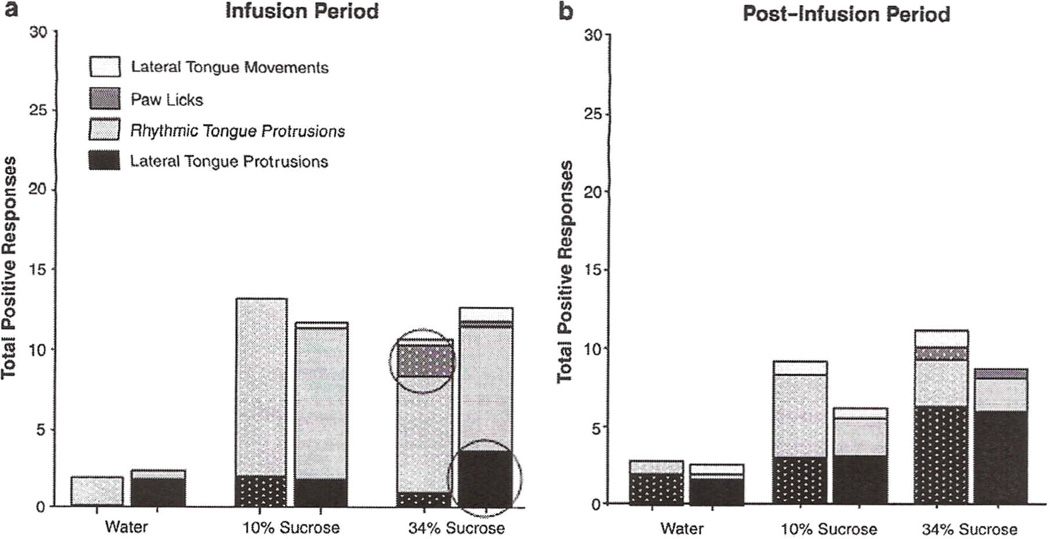
Examination of individual TR responses revealed age differences in some of the component behaviors to specific taste stimuli (see Fig. 7). For instance, adolescents exhibited primarily rhythmic tongue protrusions during water infusion (1.9 ± 0.9), whereas the adults' response consisted mainly of lateral tongue protrusions (1.8 ± 1.8). The pattern of behavioral responses to 10% sucrose was relatively similar between adolescents and adults, with rhythmic tongue protrusions comprising the major component responses at both ages at this concentration (adolescent: 10.9 ± 1.6; adult: 9.3 ± 1.8) and in response to the 34% sucrose concentration (adolescent: 7.4 ± 2.1; adult 7.8 ± 2.0). During the infusion of 34% sucrose, however, paw licking emerged as a prominent component of adolescents' TR behavior (2.0 ± 1.0), whereas this behavior was rare in adults (0.3 ± 0.3). In contrast, lateral tongue protrusions continue to be a notable component of adults' response to 34% sucrose (3.6 ± 0.8), as it was also in response to water and 10% sucrose.
Fig. 7.
Individual positive responses to water and sucrose (10 & 34%) as calculated by the traditional scoring method during a 30-s infusion (a) and 30-s post-infusion (b) period. Textured bars represent adolescents, while solid bars represent adults. Circled portions of the bars represent notable age differences in expression of component TR behaviors between adolescents and adults.
Given the different patterns of specific TR responses observed across age, composite data summed over differentially weighted responses are difficult to interpret. Consequently, data from Experiment 3 were rescored using criteria designed to minimize differential weighing of behaviors to the composite positive taste reactivity score. Specifically, all behaviors were scored either as single occurrences or in 1 s bins.
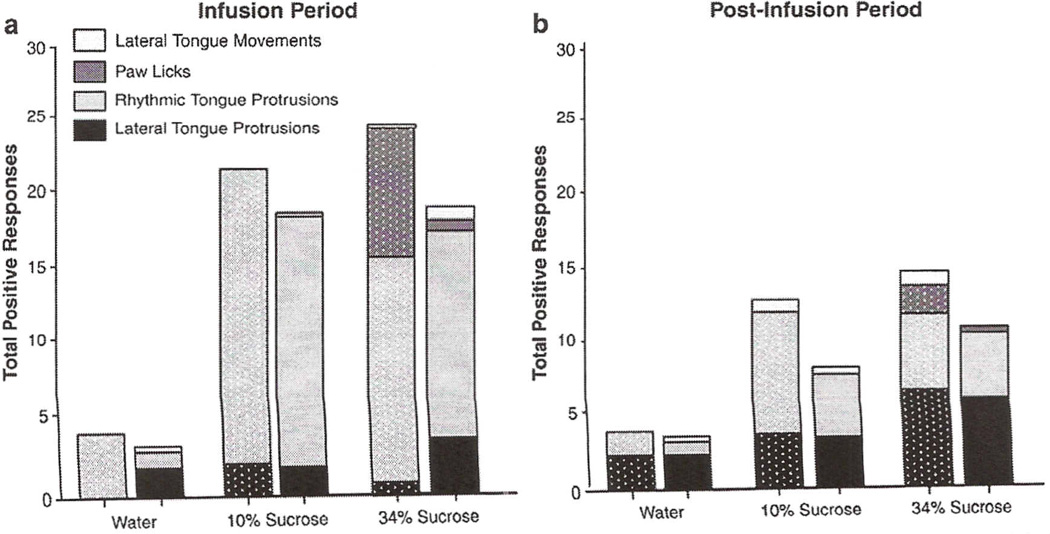
The repeated measures ANOVA of total positive responses as scored by the revised scoring method during the 30-s infusion period revealed a main effect of solution, with both concentrations of sucrose eliciting greater total positive TR than water [F(2,28) = 36.07, p<0.05] (see Fig. 8). There was no significant main effect or interaction involving age, despite a trend for adolescents to exhibit greater positive TR in response to both concentrations of sucrose than adults [F(2, 28) = 0.65, p>0.05]. A main effect of solution was again observed in the analysis of the 30-s post-infusion period, with both concentrations of sucrose producing higher responding than water [F(2,28) = 11.26, p<0.05], and adolescents again tended to show greater overall positive responding than adults, although the age effect did not reach significance [F(l,14) = 0.82, p>0.05].
Fig. 8.
Individual positive responses to water and sucrose (10 & 34%) as calculated by the revised scoring method during a 30-s infusion (a) and 30-s post-infusion (b) period.
Chi square tests comparing component behaviors across age with each sucrose solution and test period revealed that individual responses composing the total positive TR to the infusion of 34% sucrose differed significantly between adolescents and adults [X2(3, n = 16) = 8.32, p<0.05]. As in the analysis of the data from the original scoring method, adolescents given 34% sucrose exhibited significantly more paw licks than adults (adolescent: 8.9 ± 4.2; adult: 0.8 ± 0.8), whereas lateral tongue protrusions were exhibited with significantly higher frequency among adults than adolescents (adolescent: 0.9 ± 0.6; adult: 3.6±0.8). There was no difference in individual responses to 10% sucrose during the infusion or post-infusion period or the 34% sucrose post-infusion period.
2.3. Voluntary sucrose intake test
2.3.1. Experiment 4
All animals were single housed (Exp Day 0) and given a 24 h acclimation period prior to initiation of testing (n = 8). On the following 14 days (Exp Day 1–14 — P 28–42 for adolescents; P 65–79 for adults) animals were given 24 h access to one bottle containing water and one bottle containing 1.0% sucrose solutions, with the location of the two bottles alternated daily. Food was continuously available ad libitum. Consumption of food, water and sucrose solution was recorded daily.
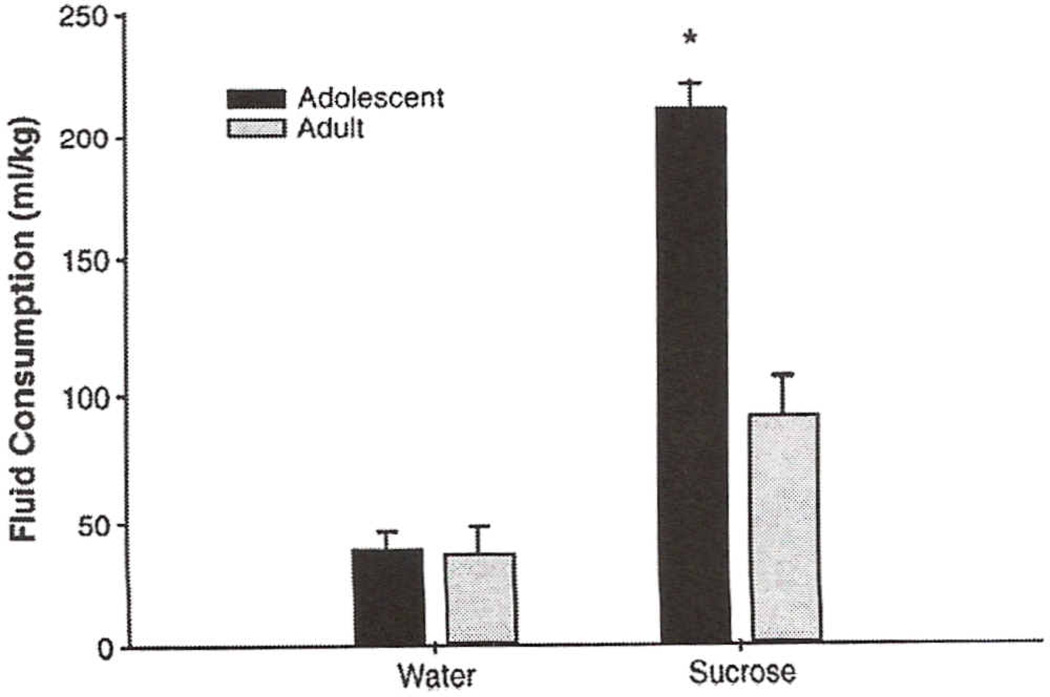
As expected, a t-test revealed that adolescents' average daily food consumption (g/kg) across Exp Day 1–14 was significantly greater than their adult counterparts [t = 1.5; p<0.05] (see Table 1). A repeated measures ANOVA of solution (ml/kg) consumed revealed a significant solution by age interaction [F(l, 14) = 14.9, p<0.05]. Post hoc tests indicated that adolescents consumed significantly more sucrose solution than adults did and when compared with their own intake of water, whereas adult sucrose intake did not differ from water (Fig. 9). Despite adolescents' elevated sucrose intake, no significant age difference emerged in the analysis of the sucrose preference scores (sucrose intake / (sucrose intake + water intake) × 100) across Exp Day 1–14 [t = 1.32; df = 14; p>0.05].
Table 1.
Mean value of food, water and sucrose consumption and sucrose preference scores across Exp Day 1–14.
| Adolescent | Adult | |
|---|---|---|
| Food consumption (g/kg) | 161.7* (± 10.5)** | 84.6* (±4.8)** |
| Water consumption (ml/kg) | 39.4 (±7.7) | 37.4 (± 11.2) |
| Sucrose consumption (ml/kg) | 212.5* (±9.7)** | 91.7* (±14.7)** |
| Sucrose preference score | 84.7 (3.6) | 70.6 (10.1) |
Values significantly different across age are presented in bold.
Numbers in parentheses represent standard error of the mean for each group.
Fig 9.
Average daily water and sucrose solution (ml/kg) consumed across 14 days in adolescent and adult rats. Asterisks (*) indicate significant difference from water (p<0.05). Bars reflect standard error of the mean.
3. Discussion
The purpose of the present series of experiments was to examine developmental differences in hedonic sensitivity in adolescent and adult rats as measured by both taste reactivity and voluntary consumption. The TR experiments suggest that adolescent rats exhibit a developmental difference in hedonic sensitivity to both appetitive and aversive stimuli. In general adolescents were more sensitive to the hedonic properties of appetitive stimuli than adults, findings that are inconsistent with the hypothesis that adolescence is characterized by an age-related partial anhedonia. Conversely, adolescents were less sensitive to the aversive properties of quinine. This finding was also unexpected, given reports that human adolescents may show a propensity to exhibit greater negative affect to various experiences relative to other aged individuals (e.g. Larson and Asmussen, 1991).
In Exp 1, adolescents demonstrated nearly 3 times more positive TR in response to a 45 s infusion of 10% sucrose than did adult animals, although there was a trend for adults to exhibit more TR during the post-infusion period. Consistent with these findings, adolescent rats in Exp 2 required a lower concentration of sucrose (3.4%) to exhibit an increase in positive TR relative to water compared to adults (34.0%). Although an age effect was not observed during the post-infusion period, adults again exhibited a trend for more pronounced positive TR in response to 34.0% sucrose when compared with their adolescent counterparts. The shorter infusion period used in Exp 3 resulted in similar levels of total positive responding to 10 and 34% sucrose solutions across age during both the infusion and post-infusion periods. An examination of the individual positive responses in this later experiment, however revealed some age differences in the component behaviors contributing to the overall positive TR responses. When the results were rescored using a revised scoring method designed to minimize differential weighing of component behaviors, adolescents showed a trend for greater total responding to sucrose at both concentrations tested.
It was only during the infusion of 34% sucrose that paw licks emerged, with the incidence of this response to the most concentrated sweet solution more prominent in adolescents than adults. As can be seen in Fig. 8, both ages exhibited different patterns of responding across the two observation periods, with rhythmic tongue protrusion being the primary response during the infusion period, while lateral tongue protrusions occurred more frequently during the post-infusion period.
In Exp 4, when sucrose and water intake were examined in two bottle choice tests, adolescents consumed significantly more sucrose than adults and than their own intake of water, while adult intake did not differ across solutions. However, no significant age difference in sucrose preference ratios emerged. Adolescents' elevated consumption of sucrose solution is reminiscent of the findings of Exp 1–3, where adolescents were observed to be more sensitive to appetitive properties of sucrose. Unlike the TR test, the two-bottle choice test does not discriminate wanting from liking, given that voluntary consumption tests inherently contain an element of instrumental behavior. Traditionally, hedonic aspects of reward have been inferred from tests that do not differentiate between liking and wanting aspects of reward (i.e. preference (choice), consumption (intake), or instrumental behavior (bar pressing or approach), based on the assumption that the two processes are mutually inclusive. In direct contradiction of this assumption are the findings that pharmacological manipulations of the dopamine system decrease both food intake and instrumental measures without altering liking as measured by TR (Peciña et al., 1997; Treit and Berridge 1990). Additionally, 6-hydroxydopamine lesions are known to produce aphagia and adipsia, but do not alter hedonic or aversive taste reactions (Berridge et al., 1989).
A hierarchical system involving opioid, cannabinoid, and to some extent GABA systems is thought to mediate hedonic properties of rewarding stimuli (Peciña et al., 2006), although little is known about these systems during adolescence. There is some evidence for ontogenetic difference in expression of type 1 endocannabinoid receptors (CB1), with numbers peaking during adolescence in striatum. limbic forebrain, and ventral mesencephalon. and lower levels in other brain areas including the NAc, hippocampus and cortex as compared to adults (Rodríguez de Fonseca et al., 1993; Romero et al., 1997). Likewise, the limited behavioral evidence available suggests that adolescent rats may be more sensitive to CB agonists under some conditions than adults (Cha et al., 2007; Cha et al., 2006), an increased sensitivity postulated to reflect compensatory upregulation of CB1 receptors in response to lower endogenous CB “tone” in areas such as the hippocampus of adolescents (Kang-Park et al., 2007). To the extent that adolescence is characterized by low CB tone and compensatory CB receptor upregulation within hedonic hotspots, the endocannabinoid system may partially mediate the increased hedonic sensitivity observed in adolescent animals in the present series of experiments. Opiate receptor systems could contribute to enhanced hedonic sensitivity during adolescence as well, with for instance stimulatory effects of mu opioid receptor agonist DAMGO on social facilitation peaking in early adolescence and declining dramatically thereafter (Varlinskaya and Spear 2008).
Although the traditional scoring method was revised to reduce differential weighing of individual behaviors by scoring all behaviors as single occurrences or in 1 s bins, age differences in the expression of component behaviors continue to complicate interpretation of hedonic sensitivity. For example, adolescents primarily exhibit behaviors scored in time bins (e.g., rhythmic tongue protrusions and paw licks) creating an inherent ceiling effect, whereas adults exhibit more behaviors measured in counts (e.g., lateral tongue protrusions), presenting challenges for data interpretation.
For instance, is it reasonable to conclude that adolescents demonstrate a greater hedonic response to sucrose than adults from data showing that a behavior emitted only in response to the sweetest solution (i.e., paw licks) is emitted with greater frequency among adolescents? Additionally, ontogenic differences in the individual behaviors comprising patterns of TR across concentrations of sucrose make it difficult to determine how individual behaviors relate to intensity of the hedonic response.
One possible contributor to the ontogenic differences in taste reactivity seen here is the decision to equate rate and duration of administration across age, hence delivering the same total volume of fluid at each age, despite differences in body size between adolescents and adults. This strategy was chosen based on preliminary work showing no significant effects of variation in volume on positive TR at either age. Ontogenetic differences in the current experiments certainly do not appear to be related in any simple fashion to differences in solution volume to body size ratio, given that adolescents demonstrated greater positive TR than adults in response to sucrose in Exp 1, but diminished negative TR in response to quinine in Exp 2.
The results of the current series of experiments do not support the hypothesis that adolescents exhibit an age-related, partial anhedonia that might contribute to their propensity to “consume” natural and drug reward more avidly (e.g. see Spear, 2000). Indeed, the present experiments suggest adolescents often express enhanced sensitivity to the hedonic properties of appetitive tastants. The possibility remains that taste reactivity data might not extrapolate to other reinforcers. For example, when hedonic affect is measured via ultrasonic vocalizations (USV) during social interactions, adolescent animals emit fewer USVs compared to adults, suggesting adolescents may be less sensitive to the hedonic properties of social interaction (Willey et al., in press). It may be the case that age differences in liking are dependent on the reinforcer examined. Future studies will need to address this possibility by examining a number of natural rewards.
Acknowledgements
The National Institute on Drug Abuse (NIDA) Grant DA 019071 supported this research.
References
- Berridge KC. Food reward: brain substrates of wanting and liking. Neurosci Biobehav Rev. 1996;20(1):1–25. doi: 10.1016/0149-7634(95)00033-b. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Measuring hedonic impact in animals and infants: microstructure of affective taste reactivity patterns. Neurosci Biobehav Rev. 2000;24(2):173–198. doi: 10.1016/s0149-7634(99)00072-x. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Grill HJ. Alternating ingestive and aversive consummatory responses suggest a two-dimensional analysis of palatability in rats. Behav Neurosci. 1983;97(4):563–573. doi: 10.1037//0735-7044.97.4.563. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Rev. 1998;28(3):309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Venier IL, Robinson TE. Taste reactivity analysis of 6-hydroxydopamine-induced aphagia: implications for arousal and anhedonia hypotheses of dopamine function. Behav Neurosci. 1989;103(1):36–45. doi: 10.1037//0735-7044.103.1.36. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. J Neurosci. 2004;24(8):1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha YM, Jones KH, Kuhn CM, Wilson WA, Swartzwelder HS. Sex differences in the effects of delta9-tetrahydrocannabinol on spatial learning in adolescent and adult rats. Behav Pharmacol. 2007;18(5–6):563–569. doi: 10.1097/FBP.0b013e3282ee7b7e. [DOI] [PubMed] [Google Scholar]
- Cha YM, White AM, Kuhn CM, Wilson WA, Swartzwelder HS. Differential effects of delta9-THC on learning in adolescent and adult rats. Pharmacol Biochem Behav. 2006;83(3):448–455. doi: 10.1016/j.pbb.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160(6):1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csikszentmihalyi M, Larson R, Prescott S. The ecology of adolescent activity and experience. J Youth Adolescence. 1977;6:281–294. doi: 10.1007/BF02138940. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Drug addiction as dopamine-dependent associative learning disorder. Eur J Pharmacology. 1999;375(1–3):13–30. doi: 10.1016/s0014-2999(99)00372-6. [DOI] [PubMed] [Google Scholar]
- Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychol Med. 2006;36(3):299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, et al. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J Neurosci. 2006;26(25):6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill HJ, Norgren R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res. 1978;143(2):263–279. doi: 10.1016/0006-8993(78)90568-1. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Roitman MF, Kaplan JM. A new taste reactivity analysis of the integration of taste and physiological state information. Am J Physiol. 1996;271(3):R677–R687. doi: 10.1152/ajpregu.1996.271.3.R677. [DOI] [PubMed] [Google Scholar]
- Kang-Park MH, Wilson WA, Kuhn CM, Moore SD, Swartzwelder HS. Differential sensitivity of GABA A receptor-mediated IPSCs to cannabinoids in hippocampal slices from adolescent and adult rats. J Neurophysiol. 2007;98(3):1223–1230. doi: 10.1152/jn.00091.2007. [DOI] [PubMed] [Google Scholar]
- Larson R, Asmussen L. Anger, worry, and hurt in early adolescence: an enlarging world of negative emotions. In: Colten ME, Gore S, editors. Adolescent stress: causes and consequences. New York, NY: Aldine de Gruyter; 1991. pp. 21–41. [Google Scholar]
- Nance DM. The developmental and neural determinants of the effects of estrogen on feeding behavior in the rat: a theoretical perspective. Neurosci Biobehav Rev. 1983;7(2):189–211. doi: 10.1016/0149-7634(83)90015-5. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. Guide for the care and use of laboratory animals. Washington, DC: U.S. Government Printing Office; 1986. (DHEW Publication No. 86-23) [Google Scholar]
- Peciña S, Berridge KC, Parker LA. Pimozide does not shift palatability: separation of anhedonia from sensorimotor suppression by taste reactivity. Pharmacol Biochem Behav. 1997;58(3):801–811. doi: 10.1016/s0091-3057(97)00044-0. [DOI] [PubMed] [Google Scholar]
- Peciña S, Smith KS, Berridge KC. Hedonic hot spots in the brain. Neuroscientist. 2006;12(6):500–511. doi: 10.1177/1073858406293154. [DOI] [PubMed] [Google Scholar]
- Pellis SM, Pellis VC. Differential rates of attack, defense, and counterattack during the developmental decrease in play fighting by male and female rats. Dev Psychobiol. 1990;23(3):215–231. doi: 10.1002/dev.420230303. [DOI] [PubMed] [Google Scholar]
- Pellis SM, Pellis VC. The prejuvenile onset of play fighting in laboratory rats [Rattus norvegicus) Dev Psychobiol. 1997;31(3):193–205. [PubMed] [Google Scholar]
- Petersen AC, Compas BE, Brooks-Gunn J, Stemmler M, Ey S, Grant KE. Depression in adolescence. Am Psychol. 1993;48(2):155–168. doi: 10.1037//0003-066x.48.2.155. [DOI] [PubMed] [Google Scholar]
- Post GB, Kemper HC. Nutrient intake and biological maturation during adolescence. The Amsterdam growth and health longitudinal study. Eur J Clin Nutr. 1993;47(6):400–408. [PubMed] [Google Scholar]
- Primus RJ, Kellogg CK. Pubertal-related changes influence the development of environment-related social interaction in the male rat. Dev Psychobiol. 1989;22(6):633–643. doi: 10.1002/dev.420220608. [DOI] [PubMed] [Google Scholar]
- Rodríguez de Fonseca F, Ramos JA, Bonnin A, Fernández-Ruiz JJ. Presence of cannabinoid binding sites in the brain from early postnatal ages. Neuroreport. 1993;4(2):135–138. doi: 10.1097/00001756-199302000-00005. [DOI] [PubMed] [Google Scholar]
- Romero J, Garcia-Palomero E, Berrendero F, Garcia-Gil L, Hernandez ML, Ramos JA, et al. Atypical location of cannabinoid receptors in white matter areas during rat brain development. Synapse. 1997;26(3):317–323. doi: 10.1002/(SICI)1098-2396(199707)26:3<317::AID-SYN12>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Steiner JE, Glaser D, Hawilo ME, Berridge KC. Comparative expression of hedonic impact: affective reactions to taste by human infants and other primates. Neurosci Biobehav Rev. 2001;25(1):53–74. doi: 10.1016/s0149-7634(00)00051-8. [DOI] [PubMed] [Google Scholar]
- Treit D, Berridge KC. A comparison of benzodiazepine, serotonin, and dopamine agents in the taste-reactivity paradigm. Pharmacol Biochem Behav. 1990;37(3):451–456. doi: 10.1016/0091-3057(90)90011-6. [DOI] [PubMed] [Google Scholar]
- Trimpop R, Kerr J, Kirkcaldy BD. Comparing personality constructs of risk-taking behaviour. Pers Indiv Differ. 1998;26:237–254. [Google Scholar]
- Varlinskaya EI, Spear LP. Social facilitation induced by pharmacological activation of mu opioid receptors: impact of age, sex and stress. Poster presented at the Society for Neuroscience meeting; Washington, D.C. 2008. [Google Scholar]
- Willey AR, Varlinskaya EI, Spear LP. Hedonic value of social interactions measured by 50 kHz ultrasonic vocalizations in adolescent and adult rats. Behav Brain Res. doi: 10.1016/j.bbr.2009.03.025. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology. 1987;93(3):358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- Wise RA. Opiate reward: sites and substrates. Neurosci Biobehav Rev. 1989;13(2–3):129–133. doi: 10.1016/s0149-7634(89)80021-1. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. Action of drugs of abuse on brain reward systems: an update with specific attention to opiates. Pharmacol Biochem Behav. 1982;17(2):239–243. doi: 10.1016/0091-3057(82)90076-4. [DOI] [PubMed] [Google Scholar]
- Wise RA, Spindler J, deWit H, Gerberg GJ. Science. 4352. Vol. 201. New York, NY: 1978. Neuroleptic-induced “anhedonia” in rats: pimozide blocks reward quality of food; pp. 262–264. [DOI] [PubMed] [Google Scholar]