Abstract
Background
Many microorganisms in midgut of mosquito challenge with their host and also other pathogens present in midgut. The aim of this study was presence of non-pathogens microorganisms like fungal flora which may be crucial on interaction between vectors and pathogens.
Methods:
Different populations of Anopheles stephensi were reared in insectary and objected to determine fungal flora in their midguts. The midgut paunch of mosquito adults and larvae as well as breading water and larval food samples transferred on Subaru-dextrose agar, in order to detect the environment fungus.
Results:
Although four fungi, Aspergillus, Rhizopus, Geotrichum and Sacharomyces were found in the food and water, but only Aspiragilus observed in the midgut of larvae. No fungus was found in the midgut of adults. This is the first report on fungal flora in the midgut of the adults and larvae of An. stephensi and possible stadial transmission of fungi from immature stages to adults.
Conclusion:
The midgut environment of adults is not compatible for survivorship of fungi but the larval midgut may contain few fungi as a host or even pathogen.
Keywords: Anopheles stephensi, fungal flora, mosquito midgut, stadial transmission
Introduction
There are more hesitant points about biology of parasite in the body of vector and so interaction each other. Therefore, the identification of flora existed in the midgut of mosquito is necessary that might play important role in transporting of parasites (Robert et al. 2001).
Generally, fungi, as the case with other major groups of eukaryotes, symbiotic microbes, have a major impact on the biology of insects. At one end of the symbiotic continuum, entomopathogenic microbes, the most intensively studied group of insect symbionts, are a significant source of mortality in insects and have been explored for their potential application as biological control agents (Pedigo et al. 2006, Douglas 2007, Thomas 2008).
All insects can be almost infected by fungi, but there is limited information about fungal/insect symbiosis in most mycological textbooks. Trichomycetes are the most striking example of commensalisms among the fungi. They are associated with living arthropods such as insects, millipedes, and crustaceans, growing extensively in the hindgut (Lichtwardt 1986). Some of the trichomycetes, such as Smittium spp. that occur on mosquitoes, have received considerable attention. High populations of Smittium spp. on mosquitoes have been shown to have a negative impact on mosquito colonies and thus might serve as a type of biological control (Moss 1979).
Many trichomycetes appear to be communalistic, but under particular circumstances the gut fungi may provide their hosts with some essential organic nutrients. At least one species, Smittium morbosum, is lethal to mosquito larvae, and fungal species in blackflies and other insects are known to invade the ovary and produce cysts which are “oviposited” by females in lieu of eggs, thus reducing the fertility in populations of those kinds of aquatic insects (Lichtwardt 1986, 1996, Misra 1998, Lichtwardt et al. 2001, 2003).
In this study, the presence of fungi in alimentary canal of different populations of Anopheles stpehnsi and possible transfer of fungi flora from immature stage to adult stage were studied.
Materials and Methods
Mosquito rearing
Four geographical populations of Anopheles stephensi from Bandar-Abbas, Iranshahr, Kazeroon area and Beech strain (collected from India) were colonized as follows. All development stages of the larvae of each population were maintained in 25×45 cm with and 5 cm depth trays. The larvae were fed with fish food and corn shuck and kept in insectary at 27±2 °C. All adult mosquitoes were held separately in 30×30×30 cm cloth cages and kept in insectary maintained at temperature 27±2 °C and humidity of 70%±5 relative humidity with photoperiod of 14 h light and 10 h dark. Adult mosquitoes were offered water contain 10% sugar soaked cotton pads as a source of energy and fed on blood of pig for maintenance of colonies.
Dissection of mosquito and sample preparation
All dissections of the midguts were carried out in sterile saline. Each group consisted of cohorts of 10 larvae (last instar) and adults (fed and/or unfed) from each geographical population were randomly chosen and their midguts simultaneously isolated from individually dissected larvae or adults. The midguts were washed separately three times with sterile saline and subsequently they were opened by a longitudinal incision and subsequently the contents under sterile condition and close to flame were transferred on medium culture pallet.
Culturing of Fungi
In order to identify fungi species, present in larval food, water and comparing with midgut content of larvae and/or adult stages, a fungal medium culture was prepared as follow. In order to preparing Subaru dextrose agar, a mixture of 10 gm peptone, 20 gm dextrose, 20 gm agars in was dissolved in 950 ml dH2O in sterile condition and then the medium aliquot to culture palates and kept at temperature 4 °C in refrigerator until used. The midgut contents, larval food and water were separately transferred on medium separately as explain above and incubated at temperature 27–30 °C for 3–7 d until the fungus growth.
Fungi identification
The fungus was identified based on direct observation of fungus colonies or microscopic observation of fungi cells. Primarily, each colony of fungi was observed by naked eyes or under a stereomicroscope and then characterized based on its size or color. Secondly, a smear of fungus war prepared by picking up small piece of fungi colony and fixed on slide by mild flame. Then the fixed fungus stained with methylene blue for 30 to 60 sec and washed with water. The fungi were surveyed perpendicularly under 100X eyepiece of microscope.
Results
In this study, Rhizopus spp., Geotrichum spp. and Saccharomyces were commonly present in water trays containing larvae and food supply for larvae growth (Table 1). By transferring the water on medium Subaru dextrose agar, colonies of Rhizopus spp., Geotrichum spp. Saccharomyces and Aspiragilus spp. were grown indicating these fungi are presence in water. Although, all above fungi were existed in the water, only Aspiragilus spp. was found in alimentary canal of the larvae of Bandar-Abbas, India (beech) and Iranshahr population (Fig. 1) indicating this fungus can survive in midgut of the larvae and is compatible to enzymatic environment of larvae digestive system. As it has been shown in Table 1, no fungus was found in the alimentary canal of adult mosquitoes both females and males, indicating the environment of alimentary canal of adult mosquitoes was not suitable for the fungal growth.
Table 1.
Presence of fungi in the alimentary canal of the larvae and adults of the different populations of Anopheles stephensi
| Fungus species | |||||
|---|---|---|---|---|---|
| Aspergillus sp. | Geotrichum sp. | Rhizopus sp. | Sacharomyces sp. | ||
| An. staphensi | |||||
| Iranshar | Larva | + | - | - | - |
| Adult | - | - | - | - | |
| Bandar-Abbas | Larva | + | - | - | - |
| Adult | - | - | - | - | |
| Kazeroon | Larva | - | - | - | - |
| Adult | - | - | - | - | |
| Beech | Larva | + | - | - | - |
| Adult | - | - | - | - | |
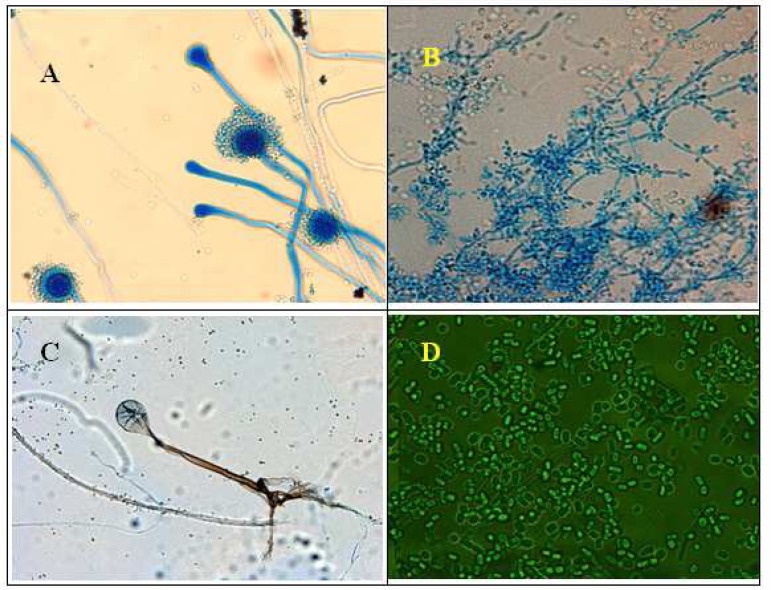
Fig. 1.
Microscopic demonstration of fungi found in larval food, larvae trey water and alimentary canal of larvae. Aspergillus spp (A), Sacharomyces sp (B), Rhizopus sp (C), Geotrichum sp (D) was found in dry food supply of mosquito larvae. A sample of dry food was cultured on medium Subaru dextrose agar after incubated at temperature 27–30°C for 3–7 days until the fungus growth
Discussion
In this study, Aspiragilus spp., Rhizopus spp. Geotrichum spp. and Saccharomyces were found in the water of breeding trays and larval food. This indicated that all mosquito larvae were exposed to fungal infection either oral or dermal contamination.
However, among above fungi only Aspiragilus spp. was found in the midgut larvae of different strains of An. stephensi except Kazeroun population. The results show that there may be a competency between Aspiragilus spp. as fungus flora and the midgut environment to at least allow the fungus to survive. Generally, some fungus live in alimentary canal of insects as symbiotic microorganism (Hati et al. 1961) but many fungi are pathogen of insects and majority of them are belong to geniuses of Lagenidium, Coelomomyces and Culicinomyces which are crucially mortal for mosquitoes (Roberts et al. 1987).
Larval black flies (Diptera: Simuliidae) are hosts for at least 20 species of trichomycetes (Crosskey 1990). Most species of trichomycetes colonizing black flies inhabit the hindgut. Harpella melusinae, however, is known only from the midgut peritrophic matrix of black flies (Lichtwardt 1986). This was the only trichomycete known to colonize black fly midguts until (Labeyrie et al. 1996) recorded Stachylina spp. in the midguts of Simulium vittatum. Even though several works had been done on fungus flora of aquatic some insects but still interaction of symbiotic fungus in mid-gut of mosquito larvae is unknown. Although trichomycetes are obligate inhabitants of the arthropod gut, the responses of the host to trichomycete colonization are largely unknown. The association often is described as ommensalistic (Lichtwardt 1986). Trichomycete fungi are obligate endobionts of aquatic and terrestrial arthropods and were discovered by Leidy in 1849. More than 200 species of trichomycetes are known from 15 orders of arthropods (Lichtwardt 1986, Misra 1998).
However, we could not found any fungus in midgut of adult mosquitoes. This can be demonstrating lyses enzymes of midgut of An. stephensi inhibited fungi development in midgut of this mosquito. Also it is not clear that Aspiragilus spp. can stadial transmission from larvae and pupa to adult stage so this need further study. Conversely, our study show that fungus cannot be normally partial of flora in digestive system of adult An. stephensi but the larvae alimentary canal can be host for limited fungus.
Acknowledgments
The authors are indebted to the personnel of the Department of Medical Entomology and Vector Control and Department of Parasitology, Section of Mycology, School of Public Health, Tehran University of Medical Sciences. The authors declare that they have no conflicts of interest.
References
- Crosskey RW. The Natural History of Blackflies. John Wiley and Sons; New York: 1990. [Google Scholar]
- Douglas AE. Symbiotic microorganisms: untapped resources for insect pest control. Trends Biotechnol. 2007;25(8):338–42. doi: 10.1016/j.tibtech.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Hati AK, Ghosh SM. Vorticella infestation of mosquito larvae and its effects on their growth and longevity. Calcutta Schl Trop Med Bull. 1961;9(4):155. [Google Scholar]
- Labeyrie ES, Molloy DP, Lichtwardt RW. An investigation of Harpellales (Trichomycetes) in New York State blackflies (Diptera: Simuliidae) J Invert Pathol. 1996;68:293–298. doi: 10.1006/jipa.1996.0099. [DOI] [PubMed] [Google Scholar]
- Lichtwardt RW. The Trichomycetes: Fungal Associates of Arthropods. Springer-Verlag; New York: 1986. [Google Scholar]
- Lichtwardt RW. Trichomycetes and the arthropod gut. In: Howard D, Miller D, editors. The Mycota, Animal and Human Relations. Springer-Verlag; New York: 1996. pp. 315–330. [Google Scholar]
- Lichtwardt RW, Cafaro MJ, White MM. The Trichomycetes: fungal associates of arthropods. 2001. University of Kansas, the Kansas City. Available at: http://www.nhm.ku.edu/
- Lichtwardt RW, White MM, Cafaro MJ. Freshwater Trichomycetes and their arthropod hosts. Fungal Divers Res Ser. 2003;10:81–100. [Google Scholar]
- Misra JK. Trichomycetes-fungi associated with arthropods: review and world literature. Symbiosis. 1998;24(2):179–220. [Google Scholar]
- Moss ST. Commensalism of the Trichomycetes. In: Batra LR, editor. Insect Fungus Symbiosis, Nutrition, Mutualism and Commensalisms. Allanheld and Osmun Co; Montclair: 1979. pp. 175–227. [Google Scholar]
- Pedigo LP, Rice ME. Entomology and Pest Management. Prentice Hall; Englewood Cliffs: 2006. [Google Scholar]
- Robert ES, Billingsley PF. Plasmodium invasion of mosquito cells: hawk or dove. Trends Parasitol. 2001;17(5):209–212. doi: 10.1016/s1471-4922(01)01928-6. [DOI] [PubMed] [Google Scholar]
- Roberts DW, Dunn HM, Ramsay G, Sweeney AW, Dunn NW. A procedure for preservation of the mosquito pathogen Culicinomyces clavisporus. Microbiol Biotechnol. 1987;26:186–188. [Google Scholar]
- Thomas AM. Pest and disease challenges and insect biotechnology solutions. Entomol Res. 2008;38:34–40. [Google Scholar]