Abstract
Background
We aimed to evaluate different fibres of bednets impregnated with various pyrethroids. The stability of insecticide on the bednet was measured using different methods of washings as well as local made detergents.
Methods:
The entire test was carried out according to the WHO-recommended methods. In addition, the impact of the numbers of washes on the stability of the insecticides was determined. Permethrin 10% (EC), deltamethrin 10% (SC), lambdacyhalothrin 2.5% (CS) and cyfluthrin 5% (EW) were used at the recommended dosages. Three different local detergents were used. Two kinds of washing methods (shaking, no shaking) were used and in each method four kinds of washings, i.e. no wash, one wash, two washes and three washes was done. The main malaria vectors, Anopheles stephensi, which is susceptible to all insecticides (BEECH strain), was tested with impregnated bednets in 3 minutes exposure time and the mortality was measured after 24 hours recovery period. Knock-down was measured as well using appropriate statistical methods.
Results:
Lambdacyhalothrin has saved its insecticidal impact after being washed, whereas, deltamethrin has lost its activity faster than other insecticides. Tow other insecticides had moderate effect. Golnar soap detergent has least effect on the durability of insecticides, but the Shoma had the most. Whit increasing the times of washing, insecticidal effects was decreased, but shaking had no influence on the decreasing of the quality of insecticidal impact.
Conclusion:
Results will be useful for local people who wish to use pyrethroid-impregnated bednets with their own local made detergent and bednets.
Keywords: pyrethroid, malaria vector, bednet. Anopheles stephensi
Introduction
Different methods for mosquito control have been proposed by investigators. An important innovation during the past decade is the widespread introduction of insecticidal pyrethroid-treated mosquito nets for protection against malaria transmission. Pyrethroids are today the only insecticides recommended for the treatment of mosquito nets. This is due to the rapid knock-down effects and high insecticidal potency of pyrethroids at low dosages combined with relative safety for human contact and domestic handling. For the treatment of net, WHO lists the insecticide products which have been passed the WHOPES including alpha-cypermethrin 10% SC, cyfluthrin 5% EW, deltamethrin 1% SC and WT 25%, etofenprox 10% EW, lambdacyhalothrin 2.5% CS, permethrin 10% EC (WHO 1997, 2002, Zaim et al. 2000).
The concentration, which is recommended, depends on texture of net, but the different dosages have been proposed for their effectiveness against malaria vectors. Due to operational use of ITNs for malaria control is now advocated as a component of Roll Back Malaria initiative led by the WHO. Iran has been classified into four different strata for malaria epidemiology and vector control (Raeisi et al. 2004). The disease is a major health problem in south-east of Iran. It is unstable with two seasonal peaks mainly in spring and autumn. Outbreaks usually occur after rainy season. Southeastern of Iran includes the provinces of Sistan and Baluchistan, Hormozgan and the tropical areas of Kerman provinces are characterized by “refractory malaria” (Manouchehri et al. 1999). In this part of the country six anopheline mosquitoes including Anopheles culicifacies, An. stephensi, An. dthali, An. fluviatilis, An. superpictus, and An. pulcherrimus are known to be the malaria vectors (Zahirnia et al. 1998, Vatandoost 2001, Zahirnia et al. 2001, Naddaf et al. 2003, Enayati et al. 2003, Vatandoost and Moinvaziri 2004, Vatandoost and Borhani 2004, Vatandoost et al. 2004, Vatandoost et al. 2005, Vatandoost et al. 2005, Hanafi-Bojad and Vatandoost 2006, Vatandoost et al. 2006 a, b, Oshaghi et al. 2006 a, b, Davari et al. 2006). According to the national strategic plan the use of impregnated bed net and recently Long Lasting Impregnated Nets (LLITN) is the one important objective (Ministry of Health, unpublished data).
In this study, we aimed to evaluate different fibres of bednets impregnated with various pyrethroids. The stability of insecticide on the bednet was measured using different methods of washings as well as local made detergents.
Materials and Methods
The supply/material requirements for insecticide treatment of nets
The supply were water, insecticide formulation, measuring device for insecticide (micropipette) and water (measuring cylinder), container to dip the net (s) and to collect insecticide drips after dipping, rubber gloves and protective clothing including a face-mask, spray equipment when nets are sprayed.
Pyrethroid insecticides
Suspension concentrate formulation (SC) of deltamethrin (%10) from Aventis Company at the dosage of (25 mg/m2), emulsifiable concentrate formulation (EC) of permethrin (10%) from Aventis Company at the dosage of (500 mg/m2), suspension concentrate formulation (SC) of lamabdacyhalothrin (2.5%) from Zeneca Company, at the dosage of (20 mg/m2), emulsifiable oil in water formulation (EW) of cyfluthrin (5%) from Bayer Company at the dosage of (50 mg/m2) were used at the recommended dosages.
Detergents
Local made detergents such as wash machine powder named SHOMA, handle clothes wash powder named RAKHT and handle clothes wash soap named GOLNAR were used through the study.
Nets
Local made, nylon net with mesh size of 156 holes per square inch were used and impregnated with recommended dosage of pyrethroids. There was no information about ISO and stability on the local made net.
Bioassay tests
For determination of biological efficacy of pyrethroids on treated mosquito nets there are three methods recommended by WHO as follows: test using WHO holding and exposure tubes as used for adult susceptibility test, test using WHO cones, test using the netting apparatus with wire frame. In this test, we followed the bioassay test with cones methods as described below (WHO 2005).
Composition of test kit: Conical chambers of transparent plastic,8.5 cm in diameter at the bas and 5.5 cm high, two glass (or plastic) aspirator tubes of 12 mm internal diameter, together with 60 cm of tubing, and mouthpiece, one role of self-adhesive plastic tape or one sheet of label tag, Instruction sheet, 3 sheets of log-probit papers for plotting regression line for calculating LT50, using variable times with constant concentration, counter, to count the mosquito while releasing in the cones or calculating the knock-down).
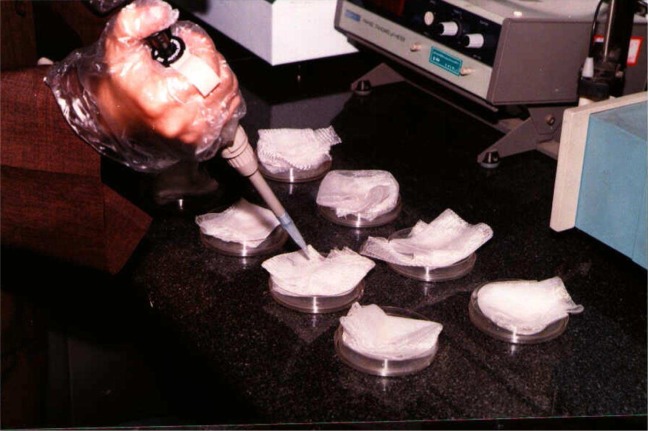
Treatment of netting: Pieces of 25 × 25 cm of netting material will be fold and put in a disposable plastic Petri dish and a pipette on the net will drop carefully and homogeneously required diluted formulation. Netting will be then soaked carefully for few seconds with fingers, protected by plastic gloves, so that all insecticide solution will be absorbed and nothing left in the Petri dish. Net sample will be left in the same dish to dry. Ideally the treated nets shall be used in 1 to 3 d, but not later than 1 wk after treatment. Separate Petri dishes are used for treatment of each net, and later disposed off properly. Net samples will be treated the day before bio-assay and stored at the refrigerator (WHO 2005) (Fig. 1).
Fig. 1.
Treatment of netting (original)
Bioassay
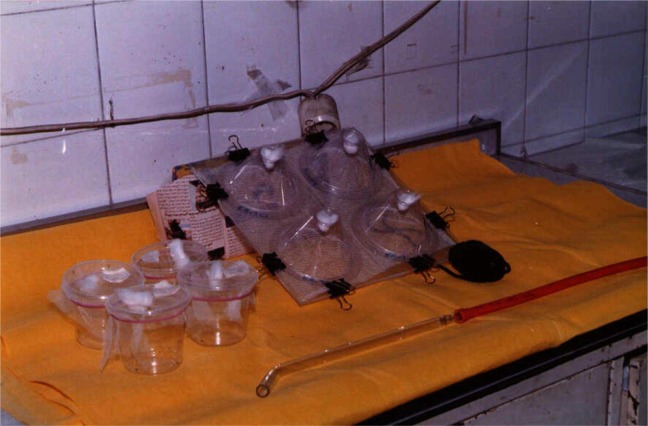
One to 3 d after treatment of the netting materials and 24 h after each washing, as well as after dipping insecticide treated nets, the netting sample was subjected to standard WHO bioassays (3 minute exposure under WHO cones, using 1 to 3 d old, non blood fed, standard susceptible Anopheles females (BEECH strain). Four cones gently fitted on the net. Five female mosquitoes introduced at a time in each cone with 8 replicates per net sample (40 mosquitoes tested). Time interval between each set of a “4 cone” was as brief as possible. Mosquitoes from the first 4 cones, tested and were grouped in one plastic cup (n=20). Knock Down (KD) was recorded at regular interval of time during the 20 to 30 min, following exposure, starting once the fourth cone (in each set) was transferred to the cup and ending when about 80% of mosquitoes were KD, stopping in any case after 60 min. Sucrose provided to each cup, added on a cotton plug. In addition to KD rate at 60 min post-exposure, mortality recorded after 24 h (Fig. 2).
Fig. 2.
Bioassay test in conical (original)
Study the physical removal of the insecticide during washing
Washing with no shaking: Insecticide-treated net samples individually introduced in 1 L beakers containing 0.5 litter deionised water, with 2 gram/litter detergent added just before and fully dissolved and left for 20 min without any shaking. Water maintained at 30 °C during exposure to detergent and rinsing. Then, samples were removed and rinsed twice for 10 min in clean still water (without shaking). Solution changed and beaker decontaminated after every wash (WHO 2005, Fig. 3).
Fig. 3.
Washing of nets with different detergents (original)
Wash with shaking: Net samples individually introduced in 1l glass bottles containing 500 ml deionised water, with 2 g/l detergent added just before and fully dissolved. Bottle immediately introduced into a water bath at 30 °C and shacked for 20 min at 155 movements per minute. They immediately rinsed twice during 10 min at the same agitation speed (Fig. 4). In both cases, pH of the washing bath accurately measured. Comparisons made between no wash, 1-, 2-, and 3-times washed nets.
Fig. 4.
Washing of nets with shaking (original)
Test condition
Tests carried out ideally at 25±2 °C and 70–80% relative humidity (RH); never at temperature higher than 30 °C.
Interpretation of the test results
Percentage mortality was recorded after 24 h recovery period on the report form. If the control mortality was between 5–20%, the percentage mortality was corrected by Abbott’s correction.
For example if the mortality in the control was 10% and mortality at discriminating concentration was 80%, then the corrected mortality would be 78%. If control mortalities exceed 20%, the results should be recorded and test should be repeated again. For calculating KD50 the probit analysis as described by Finney (1971) was used. Statistical methods were used for data analysing using SPSS programme. There were significant difference between variables when P< 0.05%.
Results
Results of tests with permethrin, deltamethrin, lambdacyhalothrin and cyfluthrin are tabulated in Tables 1–4. Results of permethrin exhibited that there was significant difference between mortalities washed by Rakht detergent, by shaking and no shaking methods and two type of washing (P< 0.01). The times of washing also affect on the stability of permethrin. Washing impregnated bednets whit deltamethrin by Rakht detergent, exhibited that no shaking method was similar in mortality rate with other washing method, whereas, in no shaking method, a significant difference was observed between no wash and other washing times (P< 0.01). Washing procedure using lambdacyhalothrin with Rakht detergent, exhibited that all washing stages had significant distinction. Additionally, in no shaking method, a significant difference was observed between no wash and three washes. In shaking method, a significant difference was observed between different washes (P< 0.01).
Table 1.
Mortality rate of An. stephensi in contact to impregnated bednets with permethrin (500 mg/m2) (EC) (%10) in process of washing whit three detergents in different stages of washing with two different kinds of washing
| Detergent |
Mortality ± SE
|
|||
|---|---|---|---|---|
| Kind of wash | Rakht | Soap | Shoma | |
| No Shaking | No wash | 72.4 ± 5.9 | 59.32 ± 6.4 | 50 ± 6.8 |
| One wash | 72.2 ± 6.1 | 91.93 ± 3.5 | 19.23 ± 5.5 | |
| Two washes | 81.66 ± 5 | 96.77 ± 2.2 | 15.25 ± 4.7 | |
| Three washes | 82.45 ± 5 | 93.33 ± 3.2 | 13.33 ± 4.4 | |
| Shaking | No wash | 51.72 ± 6.6 | 67.27 ± 6.5 | 61.67 ± 6.3 |
| One wash | 49.1 ± 6.7 | 94.83 ± 2.9 | 38.33 ± 6.3 | |
| Two washes | 67.85 ± 6.2 | 89.83 ± 3.9 | 36.67 ± 6.2 | |
| Three washes | 68.42 ± 6.2 | 78.69 ± 5.2 | 21.67 ± 5.7 | |
Table 4.
Mortality rate of An. stephensi in contact to impregnated bednets with cyfluthrin (50 mg/m2) (EC) (%5) in process of washing detergents in different stages of washing with two different kinds of washing
| Detergent |
Mortality ± SE
|
|||
|---|---|---|---|---|
| Kind of wash | Rakht | Soap | Shoma | |
| No Shaking | No wash | 89.65 ± 4 | 80 ± 5.2 | 96.49 ± 2.4 |
| One wash | 87.27 ± 4.5 | 63.33 ± 6.2 | 86.67 ± 4.4 | |
| Two washes | 81.35 ± 5.1 | 51.66 ± 6.4 | 83.93 ± 4.9 | |
| Three washes | 82.14 ± 5.1 | 53.33 ± 6.4 | 60.34 ± 6.4 | |
| Shaking | No wash | 89.83 ± 3.9 | 76.67 ± 5.4 | 83.87 ± 4.7 |
| One wash | 85.96 ± 4.6 | 75.86 ± 5.6 | 33.33 ± 6.1 | |
| Two washes | 84.74 ± 4.7 | 67.24 ± 6.2 | 15 ± 4.6 | |
| Three washes | 82.54 ± 4.8 | 63.33 ± 6.2 | 16.67 ± 4.8 | |
Cyflutrhin impregnated nets whit Rakht detergent, showed significant difference in the mortality rate, either in different stages of washing or in both two methods. Washing the same nets whit soap detergent, only washing in no shaking method, had a significant difference in mortality rate between no wash and other stages of washing (P< 0.01).
Discussion
To determine the resistance of impregnated bed nets with different pyrethroids using washing, shaking and no shaking procedure, the malaria vectors, An. stephensi (BEECH strain) was used. Four pyrethroids i.e.; permethrin, deltamethrin, lambdacyhalothrin and cyfluthrin were employed. Local made bednet was impregnated with pyrethroids as mentioned above. Three main important detergents, which are used frequently in the country, were used for washing.
Results showed that there was significant difference between mortalities of mosquito when using permethrin and washed by Rakht detergent, shaking and no shaking methods and two type of washing (P< 0.01).
This difference has been observed especially in one wash which shaking method; the insecticide removed from the nylon nets.
As it is shown in tables there was no significant difference in mortality among different stages of washing in no shaking method, but there was significant differences between one wash whit two wash or three wash in shaking method (P< 0.05).
While washing the impregnated bednets with permethrin using soap, there had been a significant reduction only between two washes in shaking and no shaking methods (P< 0.05).
There is a significant difference between washing and no washing stages in no shaking method (P< 0.01). The results also observed in shaking method with increasing in mortality rate (P< 0.01).
A difference has been observed between one wash and two wash using Shoma (P< 0.01).
It is observed the permethrin stability on nylon bednets using Shoma is maintained and shaking method had no effect on its reduction, however the number of washing up to three times was effective. The stability of permethrin is reduced whit two washes using soap, although shaking had no effect.
Impregnated bednets washed with Rakht using deltamethrin caused no significant difference in mortality rate, whereas, a significant difference has been observed between no wash and other washing times (P< 0.01).
Rakht detergent reduced deltamethrin efficacy, A significant decrease has been observed in mortality in two washes and three washes compared whit no wash stage (P<0.01). In shaking method, a significant decrease in all stages of washing was met in comparison to no wash stage. (P< 0.01). Therefore, soap was able to decrease the mortality ratio in impregnated bednets with deltamethrin. When deltamethrin impregnated bednets was washed by Shoma detergent, an obvious reduction was seen in each stages (P< 0.01). More reduction in mortality also observed using shaking.
Shaking method whit Shoma decreased the mortality. It was clear that use of soap decreases the stability of this insecticide, however shaking had no effect.
Washing the lambdacyhalothrin impregnated bednets with Rakht exhibited all washing stages had significant effect. In shaking method, a significant difference has been observed between no wash, two and three washes (P< 0.01). Soap and Shoma detergents resulted an increase up to 100% in mortality. The stability of lamdacyhalothrin insecticide has been reduced by the use of Rakht detergent. The number of washing and shaking method was also effective.
Cyflutrhin impregnated nets which was washed with Rakht, no significant difference was observed in the mortality rate, in any time of washing and methods. Washing the same nets whit soap, only washing in no shaking method, had a significant difference in mortality rate (P< 0.01). Shoma, exhibited a significant difference between one wash, wo washes and three washes (P< 0.01). A significant difference in mortality ratio in no wash, two and three wash stages in no shaking method was observed (P< 0.01).
In conclusion, washing by Shoma detergent decreased the mortality rate in An. stephensi and more intensive decreases were observed in shaking method.
A decrease in the stability of cyfluthrin was observed using Shoma. In addition, both shaking and number of washing has more effect. Rakht was not able to decreased the stability of cyfluthrin.
Residual effect of pyrethroid impregnated bednets reduced due to UV light, dust, fogging, weather condition, washing method, type of insecticide (Rozendaal 1989). Kayedi et al (2008) found that washing is more effective than UV light for degradation of insecticide on net.
Many fields research exhibited that if pyrethroid impregnated bednets was not exposed to washing, the residual effect of insecticide will be remained up to 6 to 12 months. This residual effect depends on type and quantities of insecticide used as well as type and characteristics of bed net. In practice, washing methods, type of detergents, the numbers and method of washing, are not as the same as in different parts of the world. It is determined that remaining soap on nets will demolish the molecular structure of pyrethroids (WHO 1989). For instance, the number of washing in Surinam is weekly (Rozendaal, 1989), once every two weeks in Gambia (Snow 1987) and annually in China (Zuzi 1987) was documented. Results of bioassay in different parts of the world are vary. This difference was due to formulation of insecticide, species of Anopheles, susceptibility level of mosquito, the time of exposure, and texture of bednet, and type of test. (Yaghoobi et al. 2006, Kazemi et al. 2007).
In this study, results showed that stability of permethrin impregnated bednets using Shoma decreased and the numbers of washings up to three times caused more instability. It should be noted that the method of washing (shaking and no shaking had no significant effect on this phenomenon.
The studies for measuring the washing effects on the stability of permethrin showed that washing with washing-machine powder and soap detergent in 50 ˚C, significantly removed the effectiveness of permethrin. The results of bioassay test using WHO holding tubes in 30 second exposure, the mortality rate in no wash, one wash, two washes, and three washes were 100%, 95%, 10% and 0%, respectively. The results of washing in cold water were significantly different. The net samples had been impregnated with permethrin at 63% g/m2, showed even 100% mortality after 10 times of washing with soap and cold water (Schreck et al. 1978). During the tests of nylon bednets impregnated with permethrin after seven weeks of impregnation against An. sinensis, the KD50, and KD 95% were estimated, 13′ 51″ and 21′ 1″ respectively, and knock-down starting time was seven minute after exposure (Jinijiang et al. 1988).
The next step of this study was focused on washing of impregnated nets with deltamethrin. Results showed that stability of deltamethrin on nets, decreased significantly by using soap an method of washing had not effect, but increasing the numbers of washing up to three times had significant effect. Washing of deltamethin impregnated bed net with soap increased the KD50.
Miller et al (1991) showed that net impregnated with deltamethrin washed three times, indicated no signs of the insecticide residues at the end of the processing.
Our study revealed that washing of lambdacyhalothrin impregnated bednets by Rakht reduced the effectiveness of insecticide. The number and type of washing had significant effect on mortality.
Millers et al. (1991) showed that washing impregnated bednets with lambdacyhalothrin for 3 times decreased the effectives of insecticide.
Rafinejd et al. (2008) used three methods of bioassay tests against An. stephensi. They found that Permanet® was more efficient than Olysetnet® net. Results on ITNs showed that deltamethirn and permethin were more effective than bifenthrin and etofenptorx
Result of this study showed that a decrease in the stability of cyfluthrin by using Shoma, also both shaking and number of washing has been effective, while using Rakht detergent are not able to decrease the stability of cyfluthrin.
Finally statistical analysis to test the interaction of four pyrethroids, three detergents and 2 types of washing showed that the stability of permethrin using Shoma and deltemethrin using soap with two type of washing decreased. The stability of lambdacyhalothrin with Rakht and shaking method reduced. The stability of cyfluthrin using Shoma has significantly reduced. It should be emphasized that the number of washing had negative effect.
Results of tests revealed that lambdacyhalothrin more than other insecticides has saved its insecticidal effect after being washed, on the contrary, deltamethrin lost its effectiveness faster than other insecticides. Permethrin and cyfluthrin had moderate effect.
Washing by soap had no more effect on the residue of insecticides than other two detergents. Increasing in the numbers of washing had significant effect on insecticidal activity. With increasing the number of washing these figures was more significant. Shaking has no overall influence on the decreasing of the quality of insecticidal effect. Monitoring and evaluation of impregnated bednet in the field condition for adverse effect of washing using different detergents is vital for implementation of any impregnated bednet.
Table 2.
Mortality rate of An. stephensi in contact to impregnated bednets with deltamethrin (25 mg/m2) (SC) (%10) in process of washing whit three detergents in different stages of washing with two different kinds of washing
| Detergent |
Mortality ± SE
|
|||
|---|---|---|---|---|
| Kind of wash | Rakht | Soap | Shoma | |
| No Shaking | No wash | 48.27 ± 6.6 | 54.24 ± 6.5 | 57.63 ± 6.4 |
| One wash | 16.7 ± 4.8 | 52.54 ± 6.5 | 62.07 ± 6.4 | |
| Two washes | 16.1 ± 4.9 | 20.69 ± 5.3 | 42.37 ± 6.4 | |
| Three washes | 11.86 ± 4.2 | 5 ± 2.8 | 36.06 ± 6.1 | |
| Shaking | No wash | 47.27 ± 6.7 | 54.17 ± 7.2 | 88.33 ± 4.1 |
| One wash | 16.67 ± 4.8 | 25.45 ± 5.9 | 17.24 ± 5 | |
| Two washes | 15 ± 4.6 | 23.73 ± 5.5 | 8.93 ± 3.8 | |
| Three washes | 13.33 ± 4.4 | 6.78 ± 3.3 | 8.33 ± 3.6 | |
Table 3.
Mortality rate of An. stephensi in contact to impregnated bednets with lambdacyhalothrin (20 mg/m2) (EC) (%2.5) in process of washing detergents in different stages of washing with two different kinds of washing
| Detergent |
Mortality ± SE
|
|||
|---|---|---|---|---|
| Kind of wash | Rakht | Soap | Shoma | |
| No Shaking | No wash | 79.31 ± 5.3 | 91.67 ± 3.6 | 91.80 ± 3.5 |
| One wash | 78.9 ± 5.4 | 100 ± 0 | 100 ± 0 | |
| Two washes | 78.69 ± 5.2 | 100 ± 0 | 100 ± 0 | |
| Three washes | 56.36 ± 6.7 | 100 ± 0 | 100 ± 0 | |
| Shaking | No wash | 83.05 ± 4.9 | 89.47 ± 4.1 | 95 ± 2.8 |
| One wash | 71.19 ± 5.9 | 100 ± 0 | 100 ± 0 | |
| Two washes | 62.59 ± 6.3 | 100 ± 0 | 100 ± 0 | |
| Three washes | 37.29 ± 6.3 | 100 ± 0 | 100 ± 0 | |
Acknowledgments
The authors would like to appreciate very much for kind collaboration of all staff of department of Medical Entomology and Vector Control, School of Public Health and Institute of Health Research, Tehran University of Medical Sciences. This project is financially supported by WHO and Tehran University of Medical Sciences.
References
- Abbott WS. A method of comparing the effectiveness of an insecticide. J Eco Entomol. 1965;18:265–267. [Google Scholar]
- Davarai B, Vatandoost H, Ladonni H, Shaeghi M, Oshaghi MA, Basseri HR, Enayati AA, Rassi Y, Abai MR, Hanafi-Bojd AA, Akbarzadeh K. Comparative efficacy of different imagicides against different strains of Anopheles stephensi in the malarious areas of Iran, 2004–2005. Pakistan J Biological Sci. 2006;9(5):885–892. [Google Scholar]
- Enayati AA, Vatandoost H, Ladonni H, Townson H, Hemingway J. Molecular evidence for a Kdr-like pyrethroid resistance mechanism in the malaria vector mosquito Anopheles stephensi. Med Vet Entomol. 2003;17(2):138–144. doi: 10.1046/j.1365-2915.2003.00418.x. [DOI] [PubMed] [Google Scholar]
- Jinjiang XU, Meiluao Z, Xintu L, Rangen G, Shi Xian P, Shuyou L. 1988. Evaluation of permethrin-impregnated mosquito nets against mosquito in China. WHO/VBC/88-96.
- Hanafi-Bojd AA, Vatandoost H, Jafari R. Susceptibility status of An. dthali and An. fluviatilis to commonly used larvicides in an endemic focus of malaria, southern Iran. J Vector Borne Dis. 2006;43:34–38. [PubMed] [Google Scholar]
- Kayedi MH, Lines JD, Haghdoost AA, Vatandoost MH, Rassi Y, Khamisabady K. Evaluation of the effects of repeated hand washing, sunlight, smoke and dirt on the persistence of deltamethrin on insecticide-treated nets. Trans R Soc Trop Med Hyg. 2008;102:811–816. doi: 10.1016/j.trstmh.2008.05.025. [DOI] [PubMed] [Google Scholar]
- Li Z, Jingjiang X, Banquan L, Taihua Z, Mingxin L. 1987. Mosquito nets impregnated whit deltamethrin against malaria vectors in China. WHO/VBC/87-93.
- Manouchehri AV, Zaim M, Emadi AM. A review of malaria in Iran, 1957–1990. J Am Mosq Control Assoc. 1992;8(4):381–385. [PubMed] [Google Scholar]
- Miller JE, Lindsay SW, Armestrong JRM. Experimental hut trials of bednets impregnated with synthetic pyrethroid or organophosphate insecticide for mosquito control in the Gambia. Med Vet Entomol. 1991;5:465–476. doi: 10.1111/j.1365-2915.1991.tb00575.x. [DOI] [PubMed] [Google Scholar]
- Moosa-Kazemi SH, Yaghoobi-Ershadir MR, Akhavan AA, Abdoli H, Zahraei-Ramazani AR, Jafari R, Houshmand B, Nadim A, Hosseini M. Deltamethrin impregnated bed nets and curtains in an anthroponotic cutaneaous leishmaniasis control programme in north-eastern Iran. Ann Saudi Med. 2007;27(1):6–12. doi: 10.5144/0256-4947.2007.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naddaf SR, Oshaghi MA, Vatandoost H, Asmar M. Molecular characterization of the Anopheles fluviatilis species complex in Iran. WHO Eastern Mediterr Hlth J. 2003;9(3):257–265. [PubMed] [Google Scholar]
- Oshaghi MA, Moradi MT, Taghilo B. Specific detection of malaria parasites using nested-PCR in individual mosquitoes and infected bloods in Chabahar and Iranshar, Iran. Hakim. 2004;7(3):24–31. [Google Scholar]
- Oshaghi MA, Taghilo B, Moradi MT, Vatandoost H. Mosquitoes of Anopheles culicifacies complex, species A and B in Baluchistan using mtDNA PCR-RFLP assay: the first report of species B from Iran. Hakim. 2004;7(1):35–41. [Google Scholar]
- Oshaghi MA, Chavshin AR, Vatandoost H, Yaaghoobi F, Mohtarami F, Noorjah N. Effects of postingestion and physical conditions on PCR amplification of host blood meal DNA in mosquitoes. Exp Parasitol. 2006a;112:232–236. doi: 10.1016/j.exppara.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Oshaghi MA, Yaghoobi F, Vatandoost H, Abai MR, Akbar Zadeh K. Anopheles stephensi biological forms, geographical distribution, and malaria transmission in malarious regions in Iran. Pakistan J Biological Sci. 2006b;9(2):294–298. [Google Scholar]
- Raeisi A, Shahbazi A, Ranjbar M, Shoghli A, Vatandoost H, Faraji L. Ministry of Health and Medical Education of Iran Publication; Tehran, Iran: 2004. National Strategy Plan for Malaria Control (I.R. Iran, 2004–2008) [Google Scholar]
- Rafinejad J, Vatandoost H, Nikpoor F, Abai MR, Shaeghi M, Duchen S, Rafi F. Effect of washing on the bioefficacy of insecticide-treated nets (ITNs) and long-lasting insecticidal nets (LLINs) against main malaria vector Anopheles stephensi by three bioassay methods. J Vector Borne Dis. 2008;45:143–150. [PubMed] [Google Scholar]
- Rozendaal JA, Curtis CF. Recent research on impregnated mosquito nets. J Am Mosq Control Assoc. 1989;5:500–506. [PubMed] [Google Scholar]
- Schreck CF, Posey K, Smith D. Durability of permethrin as a potential clothing treatment to protect against blood feeding arthropods. Med Vet Entomol. 1978;71(3):397–400. doi: 10.1093/jee/71.3.397. [DOI] [PubMed] [Google Scholar]
- Snow RW, Jawara M, Curtis CF. Observation on Anopheles gambiae during a trial of permethrin treated bednets in Gambia. Bull Entomol Res. 1987;77:279–286. [Google Scholar]
- Vatandoost H, Borhani N. Susceptibility and Irritability levels of main malaria vectors to synthetic pyrethroids in the endemic areas of Iran. Acta Med Iran. 2004;42(4):240–247. [Google Scholar]
- Vatandoost H. Irritability level of Anopheles sephensi in Iran (2001) Iranian J Pub Hlth. 2001;30(1–4):27–30. [Google Scholar]
- Vatandoost H, Gholizadeh MR, Abai MR, Djavadian E. Laboratory efficacy of protection rate of torn nets treated with pyrethroids, cufluthrin, deltamethrin and permethrin against Anopheles stephensi (Diptera: Culicidae) J Biological Sci. 2006a;6(2):331–336. [Google Scholar]
- Vatandoost H, Oshaghi M, Abaie MR, Shahi M, Yaghoobi F, Baghai M, Hanafi-Bojd AA, Zamain G, Townson H. Bionomics of Anopheles stephensi Liston in the malarious area of Hormozgan province, southern Iran. Acta Trop. 2006b;97(2):196–205. doi: 10.1016/j.actatropica.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Vatandoost H, Mashayekhi M, Abaie MR, Aflatoonian MR, Hanafi-Bojd AA, Sharifi I. Monitoring of insecticides resistance in main malaria vectors in a malarious area of Kahnooj district, Kerman province, southeastern Iran. (2005) J Vector Borne Dis. 2005;42(3):100–108. [PubMed] [Google Scholar]
- Vatandoost H, Moinvaziri VM. Larvicidal activity of neem tree extract (Neemarin) against mosquito larvae in the Islamic Republic of Iran. Eastern Mediterr Hlth J. 2004;10(4):573–578. [PubMed] [Google Scholar]
- Vatandoost H, Shahi H, Abai MR, Hanafi-Bojd AA, Oshaghi MA, Zamani G. Larval habitats of main malaria vectors in Hormozgan province and their susceptibility to different larvicides. Southeast Asian J Trop Med and Public Hlth. 2004;35(2):22–25. [PubMed] [Google Scholar]
- Vatandoost H, Hanafi-Bojd AA. Current resistant status of Anopheles stephensi Liston to different larvicides in Hormozgan province, southeastern Iran. Pakistan J Biological Sci. 2005;8(11):1568–1570. [Google Scholar]
- Yaghoobi-Ershadi MR, Moosa-Kazemi SH, Zahraei-Ramazani AR, Jalai-Zand AR, Akhavan AA, Arandain MH, Abdoli H, Houshmand B, Nadim A, Hosseini M. Evaluation of deltamethrin-impregnated bed nets and curtains for control of zoonotic cutaneous leishmaniasis in a hyperendemic area of Iran. Bull Soc Pathol Exot. 2006;99(1):43–48. doi: 10.3185/pathexo2818. [DOI] [PubMed] [Google Scholar]
- Zaim M, Aitio A, Nakashima N. Safety of pyrethroid-treated mosquito nets. Med Ve Entomol. 2000;14(1):1–5. doi: 10.1046/j.1365-2915.2000.00211.x. [DOI] [PubMed] [Google Scholar]
- Zaim M, Manouchehri AV, Motabar M, Emadi AM, Nazari M, Pakdad K, Kayedi MH, Mowlaii G. Anopheles culicifacies in Baluchistan, Iran. Med Vet Entomol. 1995;9(2):181–186. doi: 10.1111/j.1365-2915.1995.tb00176.x. [DOI] [PubMed] [Google Scholar]
- Zahirnia AH, Vatandoost H, Nateghpour M, Javadian E. Insecticide resistance/susceptibility monitoring in Anopheles pulcherrimus (Diptera: Culicidae) in Ghasreghand district, Sistan and Baluchistan province, Iran, 1997. Hakim. 1998;1(2):97–106. [Google Scholar]
- Zahirnia AH, Taherkhani H, Vatandoost H. Observation of malaria sporozoite in Anopheles culicifacies (Diptera: Culicidae) in Ghasreghand district, Sistan and Baluchistan province. Hakim. 2001;4(2):149–153. [Google Scholar]
- World Health Organization . 1997. Guidelines on the use of insecticide-treated mosquito nets for the prevention and control of malaria in Africa. CTD/MAL/AFRO/97.4. [Google Scholar]
- World Health Organization . 1998. Test procedure for insecticide resistance monitoring in malaria vectors, bio-efficacy and persistence of insecticides on treated surfaces, WHO/CDS/CPC/MAL/98.12. [Google Scholar]
- World Health Organization . 1989. The use of impregnated bednets and other material for vector-borne diseases control. WHO/VBC/89-91. [Google Scholar]
- World Health Organization . 2001. Specifications For Netting Materials, Roll Back Malaria, WHO/CDS/RBM/2001. [Google Scholar]
- World Health Organization . 2002. Malaria vector control, decision making criteria and procedures for judicious use of insecticides, By; D JA Najera and Dr M Zaim. WHO/CDS/WHOPES/2002.5. [Google Scholar]
- World Health Organization . 2005. Guideline for laboratory and field testing of long-lasting insecticidal mosquito nets. WHO/CDS/WHOPES/GCFDPP/2005.11. [Google Scholar]