Abstract
Background:
Chemical control method using different acaricides as spray, dipping solution or pour-on is routinely used for controlling ticks. Biological control agents are favorable due to their safety for animals and environment. Entomopathogenic fungi such as Beauveria bassiana are well known for controlling ticks. In this study, two Iranian indigenous strains of B. bassiana (B. bassiana 5197 and B. bassiana Evin) were selected and grown on specific media. The pathogenic effects of these strains were evaluated on adult stages of two Iranian Ixodidae members (H. anatolicum anatolicum Koch 1844, and H. marginatum Koch 1844) by dipping method.
Methods:
Two Iranian strains of Beauveria bassiana (Beauveria bassiana 5197 and Beauveria bassiana Evin) were selected and were grown successfully on specific media. The pathogenic effects of these strains were evaluated on adult stages of Iranian Ixodidae members such as, Hyalomma anatolicum anatolicum and H. marginatum by dipping method (these ticks were grown up at laboratory conditions during 2002 up to 2003 and still it is continued) .
Results:
There was no effect of strain 5197 on mortality or fecundity rates for ticks. There was acute phase sign of paralysis in test group after dipping ticks in suspension made from Evin strain of B. bassiana. In addition, the test groups were totally died after four months, but the control groups survived for six months.
Conclusion:
High concentration of fungal spores is needed for inducing fungal infection. Additional study using different strains and fungi on Iranian ticks is proposed.
Keywords: Biological control, fungi, Beauveria bassiana, ticks, Ixodidae, Iran
Introduction
Hard ticks (Acari: Ixodidae) are important for transmission of different viral, bacterial and protozoan agents to human and animals. At present, different chemical products are going to be used for control of ticks. These chemicals are expensive; some of them are not friendly used for the environment, and may be considered as persistent organic pollutants.
Samish and Rehaacek (1999) have summarized literature about pathogens and predators of ticks and their potential use as biocontrol agents published since the beginning of the 20th century. In nature, many bacteria, fungi, spiders, ants, beetles, rodents, birds, and other living things contribute significantly toward limiting tick populations. Experiments with the most promising potential tick biocontrol agents, especially fungi of the genera Beauveria Vuill. 1912 and Metarhizium Sorokin are described. Frolov (1974) reported that three biological products were available in the former Soviet Union controlling ectoparasites on that time (Frolov 1974). Two of them (Entobakterin and Dendrobacillin were preparations of spores and endotoxin of Bacillus thuringiensis Berliner 1915 and the third one (Boverin) was conidiospores of the fungus Beauveria bassiana (Bals.-Criv.) Vuill.
Bittencourt et al. (1997) evaluated the in vitro efficacy of two isolates of the entomopathogenic fungus B. bassiana in engorged females of Boophilus microplus (Canestrini, 1887) (Acari: Ixodidae) (Bittencourt et al. 1997).
The pathogenicity of two isolates of B. bassiana (747 and 986) against engorged females of B. microplus was investigated in vitro. Twelve groups of 30 ticks were exposed to conidial concentrations of 0, 104, 105, 106, 107 and 108 for each isolate. The weight of females before oviposition, weight of egg masses and percentage of eclosion were recorded for each group. The percentage of eclosion decreased with increasing conidial concentration and concentration of 107 and 108 were considered minimal for field trials (Bittencourt et al. 1997).
The efficacy of various entomopathogenic fungi as biological control agents of B. microplus was tested in the laboratory. Verticillium lecanii (Zimmerman) Viegas (Lecanicillium lecanii) (approved name) strain LBV-2 was the most effective and reduced tick egg viability by 76% followed by V. lecanii strain LBV-1 (68% reduction), Beauveria bassiana strain LBBb-14 (31% reduction) and B. bassiana strain LBBb-(28% reduction). Tick infestations were reduced by up to 93.5% when fungal preparations were repeatedly sprayed onto calves (Rijo 1994).
The effectiveness of V. lecanii LBV-2 strain was determined against the parasitic stage of B. microplus. The biological product was sprayed on cattle, one set in a yard, and the other one in grazing areas, the latter was compared with cattle treated with a mixture of chlorfenvinphos plus cimiazol. The biopreparation showed that since the 1st treatment was applied to the stabled animals, the effectiveness varied between 47.5% and 78.7%. After the 4th bath with the biopreparation the regulation of the ectoparasite was between 93.5 and 98.7%, after which further treatment was unnecessary. The effectiveness of the biological product sprayed on animals in grazing areas did not significantly differ from that obtained by spraying chemical acaricides (Camacho 1998).
The pathogenic action of Metarhizium anisopliae (Metchnikoff) Sorokin on engorged females was clearly demonstrated. Isolates E9 and AM were more effective, causing high tick mortality as well as reduced oviposition. The concentration of 7.5×108 conidia/ml was the most effective with the fungus sporulating on 91.1% of the ticks. Mean percent oviposition was highest in the control treatment and lowest in the treatment with 7.5× 108 conidia/ml (Monteoiro 1998).
Metarhizium anisopliae isolates were passaged through female tick and grown up on rice. Fifteen bulls were infested with B. microplus at 7, 14, and 21 days prior to the application of increasing concentrations of M. anisopliae conidia. Female ticks larger than 4.0 mm were counted at 0, 1, 7, and 16 days after treatment application. There was no effect of M. anisopliae on numbers of ticks parasitizing the bulls; however, 91.7% of ticks had fungal growth and sporulation 1 day after fungal treatment (Correia et al. 1998).
Lopez et al. showed that strain Ma-z4 of M. anisopliae and strain Bb-1 of Beauveria bassiana reduced eclusion of larvae of B. microplus when applied to animals affected by the tick, s initial stages thus showing promise as biological control agents (Lopez et al. 1998).
Two strains of B. bassiana were found to be pathogenic to all stages of Rhipicephalus appendiculatus Neumann, 1901 in the laboratory. A mortality of up to 73% of unfed adults was recorded. M. anisopliae was only slightly pathogenic, killing only 35% of unfed adults. Unfed ticks immersed in suspensions of B. bassiana spores engorged normally on rabbits, but 74% of them failed to lay eggs. The fecundity of those, which laid eggs, was reduced to 10% compared to controls in natural infections (Mwangi et al. 1995).
In this study, two Iranian indigenous strains of B. bassiana (B. bassiana 5197 and B. bassiana Evin) were selected and grown on specific media. The pathogenic effects of these strains were evaluated on adult stages of two Iranian Ixodidae members (H. anatolicum anatolicum Koch 1844, and H. marginatum Koch 1844) by dipping method.
Materials and Methods
Tick rearing
H. anatolicum anatolicum, individuals (origin, Boushehr Province) is routinely being reared at the laboratory. Female engorged ticks were collected from infested hosts in the field. Females were kept at 28 °C and 80% relative humidity. Larvae and nymphs were blood fed on white rabbit and adults blood feeding was took place on sheep (two-host strategy of life cycle at laboratory conditions).
Fungi rearing
Two indigenous strains of B. bassiana, B. bassiana (PTCC), 5197 obtained from Iranian Research Organization for Science and Technology (Persian Type Culture Collection) and cultured according to the manual from the producer and the second strain was B. bassiana st. Evin, (obtained from College of Agriculture and Natural Resources of University of Tehran). It was cultured on SDYA medium under sterile condition according to producer’s manual.
There is a summary chart of the type of treated ticks and different conditions of the study in Table 1.
Table 1.
Summary chart of tested groups using two strains of Beauveria bassiana against Hyalomma species
| No. | Fungal strain | Tick(host) | Groups | Spores’ Conc., (d.time)1, suspen | Results |
|---|---|---|---|---|---|
| 1 | B.b 5197 | H.anatolicum an. (Engorged F2) | 5 group of 5 | 200,500,1000/ml,1 min, ph.saline | Record in 48 hr.intervals(-) |
| 2 | B.b 5197 | H.marginatum (Engorged F) | 2 group of 2 | 1000 / ml, 2 mins, tween 80 | Record in 48 hr.intervals(-) |
| 3 | B.b 5197 | H.marginatum (Starved adults) | 1 group of 7 | 2000,000/ml, 1min,ph.saline | Record in 48 hr.intervals(-) |
| 4 | B.b 5197 | H.marginatum (Starved adults) | 1 group of 7 | 1000,000/ml, 1min,ph.saline | Record in 48 hr.intervals(-) |
| 5 | B.b 5197 | H.marginatum (Engorged nymphs) | 1 group of 10 | 1000,000/ml, 1min,ph.saline | Record in 48 hr.intervals(-) |
| 6 | B.b Evin | H.dromedarii (Starved adults) | 2 groups of 5 | 1500,000/ml, 15 mins ,PBS | Acute phase effects (+) died after 4 months |
| 7 | B.b Evin | H.dromedarii (Starved adults) | 2 groups of 5 | 1500,000/ml,PBS/EDTA,15 mins | Acute phase effects (+) died after 4 months |
-d.time is deeping time for tested ticks in this study.
-F stands for adult female ticks.
Instant signs of acute phase effects of fungi on ticks, such as tremor or paralytic effects were recorded, and then fecundity of engorged female ticks and hatchability of their eggs or mortality rate for adults were studied up to 1 month after the beginning of the study or later. The ticks were kept in 28 °C and 80% relative humidity in separate vessels. There were enough non-treated cases as control groups whenever a test was applied.
Results
Two different strains of B. bassiana were cultured successfully (Fig.1 and 2). Engorged female ticks were weighed and suspended in different concentrations of spores of B. bassiana after spore counting, using a haemocytometer slide and microscopically studies. Spores were suspended in different media according to Table 1. Different data after treating the ticks could be categorized in four parts.
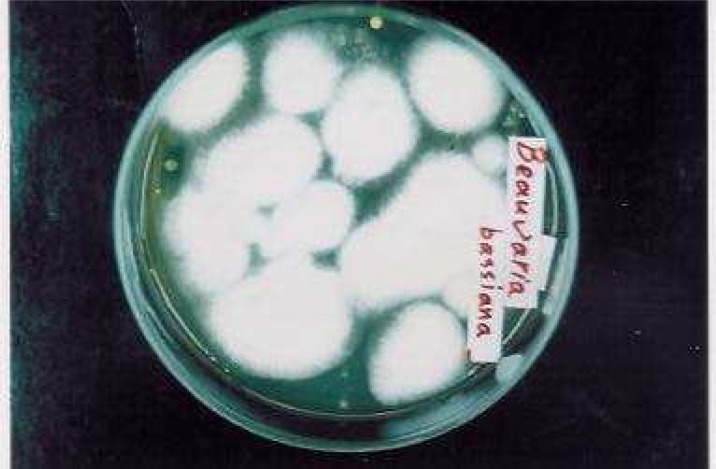
Fig. 1:
White colonies of Beauveria bassiana (strain 5197) are seen after 15 days were grown successfully on special medium according to producer’s manual
Fig. 2:
White colonies of Beauveria bassiana (Evin strain) are seen after 15 days were grown successfully on SDYA medium
A-H. anatolicum anatolicum and H. marginatum unfed adult ticks in 5 groups of 5 ticks (at the same generation and almost equal size, weight and starvation time) (siblings from one female tick) were suspended in different concentrations of spores of B. bassiana st. 5197 for 1 min. They were kept in standard laboratory conditions and inspected every 48 h. There were no differences in mortality rate between tested and none tested groups. There were also no differences in oviposition rate or time and hatchability decrease after engorgement of female ticks.
B-Two groups of two engorged female H. marginatum ticks were suspended in 1×103 spores/ml of B. bassiana st. 5197 for two min (suspension medium was Tween 80 in physiological saline). They were kept in standard laboratory conditions and inspected every 48 hours. There were no differences in mortality rates between tested and none tested groups. There were also no differences in oviposition rate or time and hatchability decrease.
C-Two groups of seven unfed adult H. marginatum ticks were suspended in 1×106 and 2×106 spores/ml of B. bassiana st. 5197 for one minute, respectively.
Additional group of 10 engorged nymphs of H. marginatum were suspended in 1×106 spores/ml of B. bassiana st. 5197 for one minute. There were no differences in mortality rate between tested and control groups even after increasing the concentration of spores to 2×106 spores/ml. There were no differences in mortality rate between tested and control groups.
D-Another strain of B. bassiana, (Evin strain) was provided from College of Agriculture and Natural Resources of University of Tehran. This strain was grown on SDYA medium successfully (Fig. 3, 4). Four groups of five unfed adult ticks (H. dromedarii Koch 1844) (Lab-reared individuals) (two tested groups and two as control groups) were selected. Tested groups were suspended in fungal spores (1,500,000 spores/ml) in PBS and PBS/EDTA for fifteen min. Acute phase signs of toxic effect of fungi such as tremor and paralysis were observed in ticks immediately after the experiment. The ticks were incubated separately from the control group. Daily inspection of ticks showed that paralytic effects were extended up to 1 week. After 1 week, this sign was disappeared and there were no other apparent recordable differences in appearance between tested and control groups of ticks. Tested ticks were died after four months, but the control groups were active and viable even after six months.
Discussion
Mwangi et al. (1995) found locally isolated strains of B. bassiana to be more virulent to R. appendiculatus than an exotic strain of this fungus. In our study, two indigenous strains of B. bassiana were selected as the strains to be implicated on host ticks. Strain type of B. bassiana was changed after doing experiments in parts, A, B, C and Evin strain was used in part D. Then acute phase effect of fungi on ticks was recorded.
The virulence of a specific strain may be lost if an isolate is maintain in in vitro culture (Tanada and Kaya 1993). The virulence of strain 5197 of B. bassiana in this study might be lost during repetitive passages in its production process, so there was no toxic effect of this strain on ticks.
The formulation of the fungus can have an effect on the performance of the fungus as a management agent. Kaaya and Hassan (2000) found that while both oil based and aqueous formulations were effective in causing mortality, the oil based formulation resulted in greater mortality of ticks. Kaaya (2000) reported similar results. The speed of infection and disease development may be enhanced by adding agents that facilitate spore attachment to the cuticle (Frazzon et al. 2000). In this study, suspension medium was physiological saline in parts, A, B and C. Tween 80 was also used in part B, but there was no effect on the results. Suspension medium was PBS and PBS plus EDTA for two groups of ticks used in part D. There was no difference between the results for these groups.
The method of application of fungi (fungal formulation) on host is important. There are suspension (dipping method), inundation, and spray methods used by different scientists. In this study, dipping method was used that is more trustful than inundation method used by Cradock (2005).
The suspension time for tested ticks regarded as one minute, which is nearly the estimated time when spray or washing is going to be done on animal host. A standard or enough dipping (anti tick bath) is normally inaccessible and expensive in most regions of Iran. Unofficial sources reported that there exist 3% of anti tick baths are routinely used in one reported province. Then animal keepers prefer using spraying, washing methods or simply using a barrel full of diluted toxins and using a piece of sponge soaked in diluted toxins on infested regions of body of animal. Then deeping or suspension time (dtime) (Table 1) was increased from 1–2 min to 15 min in experiment part D for B. bassiana (Evin strain) to be enough to see the pathological effect of the fungi.
The concentration of spores was increased after experiments in parts A, B and in part C from 200 spores/ml to 1×103 and later to 1×106 and 2×106 spores/ml. There was no effect of spores’ concentration for B. bassiana st. 5197.
In another study, Cradock, studied different aspects of using B. bassiana and its interactions with Dermacentor variabilis (Say, 1821) and Amblyomma americanum Linnaeus, 1758 in the laboratory and in the field. He also reported a significant mortality rate for both species of tested ticks at the laboratory (Cradock 2005).
The production of mycotoxins and some enzymes may be associated with the pathogenicity of a fungus. Since there was no signs of fungal growth on the body of ticks (intersegments of cuticle have been observed microscopically), so the observed mortality after using the second strain of the B. bassiana in this study may be due to releasing of a kind of mycotoxins or enzymes (Ferron 1978, McCoy et al. 1988).
A darkening of the tick and immobility after about two days indicated infection in larvae and sporulation from the tick surface was observed after 72 h (Samish et al. 2001). In our study, immobility of adult ticks was seen after one week (part D of this study), that indicated infection and sporulation from the tick surface was not observed.
The species of tick used in a study can influence on the specifics of the interaction (Benjamin et al. 2002). Finally, two indigenous strains of B. bassiana from Iran were grown and were applied on three laboratory-bred species of Iranian Ixodidae (Hyalomma anatolicum anatolicum, H. marginatum and H. dromedarii). The minimum concentration of spores of B. bassiana needed to induce the infection was estimated to be 1,500,000 spores/ml. Additional studies on these two strains of fungi and other biological control agents against ticks should be planned in future to improve the information on biological control of ticks and implementation of these information in integrated control of ticks.
Acknowledgments
The authors wish to thank all colleagues at Department of Parasitology, Razi Vaccine and Serum Research Institute. This study was financially supported by the Ministry of Jehad and Agriculture, devoted to confirmed projects at this institute. The authors declare that they have no conflict of interests.
References
- Benjamin MA, Zhioua E, Ostfeld RS. Laboratory and field evaluation of the entomopathogenic fungus Metarhizium anisopliae (Deuteromycetes) for controlling questing adult Ixodes scapularis (Acari: ixodidae) J Med Entomol. 2002;(39):723–728. doi: 10.1603/0022-2585-39.5.723. [DOI] [PubMed] [Google Scholar]
- Bittencourt V, Souza EJ, Peralva S, Mascarenhas A. Evaluation of the in vitro efficacy of two isolates of the entomopathogenic fungus Beauveria bassiana (Bals.) vuill. In engorged females of Boophilus microplus (Canestrini, 1887) (Acari: Ixodidae) Revista Brasileira de Parasitologia Veterinaria. 1997;6(1):49–52. [Google Scholar]
- Camacho ER, Navaro G, Rodriguez RM, Marillo EY. Effectiveness of Verticillium lecanii against the parasitic stage of the tick Boophilus microplus (Acari: Metastigmata: Ixodidae) Revista-Colombiana-de-Entomologia. 1998;24(1–2):67–69. [Google Scholar]
- Correia AD, Fiorin A, Monteiro AC, Verissimo C. Effects of Metarhizium anisopliae on the tick Boophilus microplus (Acari: Ixodidae) in stabled cattle. J Inv Pathol. 1998;71(2):189–191. doi: 10.1006/jipa.1997.4719. [DOI] [PubMed] [Google Scholar]
- Cradock KR. Interactions of Beauveria bassiana with the American dog tick, Dermacentor variabilis (Say), And the lone star tick, Amblyomma americanum L [PhD Dissertation] The Ohio State University; USA: 2005. [Google Scholar]
- Ferron P. Biological Control of insect pests by entomopathogenic fungi. Annual Review of Entomology. 1978;23:409–442. [Google Scholar]
- Frazzon AP, Vaz Da Silva, Jr, Masuda A, Schrank A, Vainstein MH. In vitro assessment of Metarhizium anisopliae isolates to control the cattle tick Boophilus microplus Veterinary Parasitology. 2000;94:117–125. doi: 10.1016/s0304-4017(00)00368-x. [DOI] [PubMed] [Google Scholar]
- Frolov BA. Chemical and Biological methods of controlling poultry ectoparasites. Veterinary Moscow. 1974;12:66–68. [PubMed] [Google Scholar]
- Kaaya GP, Hassan S. Entomopathogenous fungi as promising biopesticides for tick control. Experimental and Applied Acarology. 2000;24:913–926. doi: 10.1023/a:1010722914299. [DOI] [PubMed] [Google Scholar]
- Kaaya GP. Laboratory and field evaluation of entomopathogenous fungi for tick control. Annals of the New York Academy of Science. 2000;916:559–564. doi: 10.1111/j.1749-6632.2000.tb05336.x. [DOI] [PubMed] [Google Scholar]
- Lopez G, Marin H, Londono M, Vahos R. Utilization of Metarhizium anisopliae and Beauveria bassiana for the biological control of the tick Boophilus microplus. Noticampo. 1998;10:12–14. [Google Scholar]
- McCoy CW, Samson RA, Boucias DG. Entomogenous fungi. In: Ignoffo CM, editor. CRC Handbook of Natural Pesticides Microbial Insecticides Part A: Entomogenous protozoa and fungi Vol 5. CRC Press; Boca Raton, USA: 1988. pp. 151–236. [Google Scholar]
- Mwangi E, Kaaya G, Essuman S. Experimental infections of the tick Rhipicephalus appendiculatus with entomopathogenic fungi, Beauveria bassiana and Metarhizium anisopliae and natural infections of some ticks with bacteria and fungi. Journal of African Zoology. 1995;109(2):151–160. [Google Scholar]
- Rijo E. Biological control of ticks with entomopathogenic fungi. Rivista Pecuaria de Nicaragua. 1998;22:17–18. [Google Scholar]
- Samish M, Rehaacek J. Pathogens and predators of ticks and their potential in biological control. Annual Review of Entomology. 1999;44:159–182. doi: 10.1146/annurev.ento.44.1.159. [DOI] [PubMed] [Google Scholar]
- Samish M, Gindin G, Alekseev E, Glazer I. Pathogenicity of entomopathogenic fungi to different developmental stages of Rhipicephalus sanguineus (Acari: Ixodidae) J Parasitol. 2001;(87):1355–1359. doi: 10.1645/0022-3395(2001)087[1355:POEFTD]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Tanada Y, Kaya HK. Insect pathology. Academic Press. Inc; San Diego: 1993. [Google Scholar]