Abstract
Background:
The aim was to evaluate the relapse risk of vivax malaria in patients who received radical treatment in Hormozgan Province, a malarious area located on southeast of Iran.
Methods:
A total of 95 symptomatic vivax malaria infected patients were enrolled in urban health centers of Bandar-Abbas, Minab, Bandar-Jask and Bashagard districts of Hormozgan Province, southeast of Iran from January 2008 to March 2009 for consideration as a case- series study. DNA was extracted from parasite infected whole blood samples. A polymorphic region of Plasmodium vivax merozoite surface protein 1 (pvMSP1) was selected and a PCR method was employed for all the samples to amplify the specific variable gene fragment. The obtained fragments in primary and secondary samples were sequenced. Both nucleotide and amino acid sequences of the samples were investigated for returned patients.
Results:
3.2% of the patients experienced a second attack between 83–199 days after the initial episode of infection. Alignment of nucleotide and their deduced amino acid sequences between pair sequences of primary and secondary isolates revealed 8 and 6 dissimilarities respectively for the first case, and 9 and 7 dissimilarities for the second case. Although microscopical examination of recurrent thick blood smear of the third patient confirmed new P. vivax infection, the venous blood sample was accidentally missed. Sequencing results of primary and returned isolates 1P, 1S, 2P, 2S and 3P in this study showed an identity with BP13, T117, BP13, TC28 and Chesson genotypes respectively.
Conclusion:
The returned (secondary) isolates may account to be for the sake of reinfection.
Keywords: P. vivax, Relapse, Re-infection, Recrudescence, pvMSP1, Iran
Introduction
Among the four species of human plasmodia, Plasmodium vivax and P. ovale are associated with relapse episodes. Precise discrimination between such phenomenon and re-infection or treatment failure cases in malarious areas particularly in transmission seasons cannot be easily performed unless employing new and advanced bio-molecular techniques that are accounted to be relatively helpful.
Although P. vivax is known as the cause of benign tertian malaria increasing evidences suggest that life-threatening complications of the parasite are more common than previously thought. P. vivax is the major agent of malaria morbidity in Asia and the second most prevalent plasmodium in the most of malarious regions (Wernsdorfer 1988, Mendis et al. 2001, Hay et al. 2004). Widely distribution of P. vivax and existence of relapse behavior in the parasite emphasize the importance of considering the relapse phenomenon particularly after radical treatment. A number of studies have been carried out to explore the nature of relapse using various methods. It has been known that manifestation of relapse in P. vivax depends on variation of the parasite strains (Collins et al. 1996). The Chesson strain an example of tropical zone strains relapses with short intervals almost one month during the year (Coatney et al. 1950). The St. Elizabeth strain of P. vivax with 6 to 14 mo latent period belongs to temperate zone strains that are the second pattern of relapse activity (Manson 1975, Krotoski 1985). The manifestation of relapse in this pattern renewed during long intervals (Collins and Sullivan 1996). In addition to the two main mentioned patterns of relapse activity, an intermediate form also has been reported (Adak et al. 1998).
Beside the relapse activity in P. vivax, renewed attack is also suspected as a re-infection or treatment failure in malarious areas such as Iran located in Middle East region. In 2008, approximately 11460 Malaria cases were microscopically diagnosed in Iran, which 90% of them were detected as P. vivax (CDC Iran, 2008). A retrospective epidemiological study based on the biodata surveillance forms including radical treatment data at a limited part of southeastern Iran showed that the relapse risk of P. vivax were 16.8% and 24.5% depending on one or two years after primary attack respectively (Haghdoost et al. 2006).
Since radical treatment of vivax malaria is precisely employed on the malarious areas of Iran results of the study stimulated us to evaluate the relapse risk of vivax malaria in Hormozgan Province, a malarious area of southeastern Iran using P. vivax merozoite surface protein 1 (pvMSP1), as a useful genetic marker for genotyping- P. vivax isolates (Craig and Kain 1996, Zakeri et al. 2006).
Materials and Methods
Study sites and sample collection
This study was conducted in urban health centers of Bandar-Abbas, Minab, Bandar-Jask and Bashagard districts of Hormozgan Province, southeast of Iran from January 2008 to March 2009 for consideration as a case- series study.
The first three districts have hot and humid weather with year round transmission of malaria parasites, but two main peaks are on May to August and October to November. Bashagard district has temperate climate in winter with average temperature of 13.5° C (Min.5° C and Max.22° C) and warm weather in summer with average temperature of 36° C (Min.32° C and Max.40° C).Transmission pattern of malaria in Bashagrd is, more or less, the same as above-mentioned districts. Plasmodium vivax and Anopheles stephensi are the predominant species of parasite and vector in those areas respectively. A total of 95 symptomatic vivax malaria infected patients after preparing an informed consent from each of them or their parents, were enrolled for the consideration. Pregnant women, patients below 4 yr of age and those who were unavailable for the follow up duration (9 months) were excluded from the study. Ethical permission for the study was approved by Tehran University of Medical Sciences Ethical Committee. A 2-ml venous blood from each patient was sampled into an EDTA contained tube. Prior to sampling, all isolates were diagnosed as P. vivax by light microscopical examination of Geimsa-stained thick blood smears. All samples were frozen and stored at −20° C until DNA purification. Following blood sampling the patients received 25 mg/kg chloroquine divided over three days and 0.75 mg/kg primaquine from day 3 onwards weekly for 8 consecutive weeks as a standard radical treatment of vivax malaria. The enrolled patients were followed up by microscopical examination of their blood thick smears bimonthly during next nine months for probable renewed manifestation. Moreover, the registered patients were asked to contact us if they felt fever or any other symptoms at any time. Parasite density for each sample was measured as the number of parasite per microlitre of blood in thick blood smear.
Genomic DNA purification
DNA was extracted from P. vivax infected whole blood samples using a commercial genomic DNA purification kit (QIAGEN) according to the manufacturer's instructions.
Polymerase chain reaction (PCR) amplification
A polymorphic region of pvMSP1, where widely has been used for molecular analysis of relapse cases (Craig and Kain 1996, Kirchgatter and del Portillo 1998, Chen et al. 2007, Imwong et al. 2007) was selected and a PCR method was employed for all the samples to amplify a specific variable gene fragment. This gene fragment is located between two interspecies conserved blocks named: ICB5 and ICB6. The mentioned blocks are usually used as genetic marker for differentiation of P. vivax species. One pair of oligonucleotid primers, designed previously (Craig and Kain 1996, Kirchgatter and del Portillo 1998), were synthesized according to the mentioned conserved sequences as follows: 5′- TACTACTTGATGGTCCTC-3′; 5′- CCTT-CTGGTACAGCTCAATG-3′. PCR amplification were performed in 20μl of reaction mixture containing 2 μl premix (Titan Hot Tag Bioatlas), 20 pmol of each primer, 5 μl DNA template and 9 μl distilled water. The amplification was composed of an initial denaturation for 5 min at 94° C, which followed with 35 cycles of 94° C for 30 seconds, 58° C for 1 min and 72° C for 1 min and 30 seconds. The reaction was stopped after a final extension process for 10 min at 72° C. PCR products were analyzed by performing gel electrophoresis on a 1% agarose gel using 100 bp DNA marker (Fermentase). After visualization of the ethidium bromide- stained gels under UV light, the products were estimated to be approximately 680 bp. The obtained fragments were sequenced by SEQLAB in Germany (www.SEQLAB.de). The resultant sequences of PCR fragments were analyzed in NCBI (NCBI, NIH, USA). Both nucleotide and amino acid sequences for each PCR product of primary and secondary infections have been considered for returned patients.
Nucleotid sequences of the pvMSP1 have been submitted to the National Centre of Biotechnology Information Gene Bank and are available for public access under accession numbers GQ403477, GQ403478, GQ403480 and GQ403481 for the Bandar- ask isolates and GQ403479 for the Bashagard isolate.
Results
During the nine months follow up three cases (3.2%) of 95 P. vivax infected patients experienced a second attack on 83–199 days after the initial episode of infection respectively (Table 1).
Table 1:
Characteristics of returned patients and their paired primary/ secondary samples
| Patient isolate | Day/month/year | Age (year) | Area of aquisition | Parasitemia/μL of blood |
|---|---|---|---|---|
| 1P | 01/07/2008 | 11 | Bandar - jask | 13848 |
| 1R | 05/11/2008 | 15467 | ||
| 2P | 25/08/2008 | 9 | Bandar- jask | 308 |
| 2R | 16/11/2008 | 231 | ||
| 3P | 14/06/2008 | 11 | Bashagard | * |
| 3R | 01/01/2009 | 27 |
The rate of parasitemia was not known.
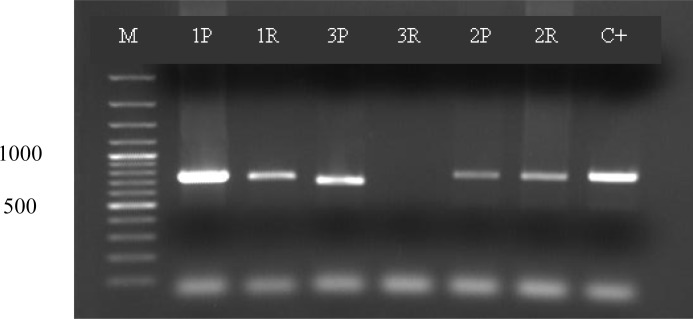
The amplified segment of pvMSP1 block 5 gene in returned patients displayed identical size approximately 680 basepairs for both primary and secondary isolates (Fig. 1).
Fig. 1.
Size polymorphism of both paired isolates. M fermentase marker, P as primary infection and R as recurrent infection. C+ as positive control. For 3R we could not extract DNA from prepheral blood smear.
All five sequenced isolates in both directions revealed four distinct genotypes for pvMSP1. The rate of homology between primary and secondary isolates in each case at nucleotide and amino acid level is tabulated in Table 2. Sequencing results of 1P, 1S, 2P, 2S and 3P isolates, which were named pv-jask-1 to pv-jask-4 and pv-bashagard respectively in Genebank, showed an identity with BP13, T117, BP13, TC28 and Chesson genotypes respectively in checking with Genebank (NCBI, NIH, USA). Four isolates pv-jask-1 to pv-jask-4 showed close similarity to Salvador I (Sal-1) allelic type.
Table 2.
| No | patient | Homology at nt level | Homology at a.a level |
|---|---|---|---|
| 1 | 1P (pv-jask-1) | 98% | 97% |
| 1S(pv-jask-2) | |||
| 2 | 2P(pv-jask-3) | 98% | 96% |
| 2S(pv-jask-4) |
nt : nucleotide,
aa : amino acid
Alignment of nucleotide and their deduced amino acid between paired sequences of primary and secondary isolates revealed 8 and 6 dissimilarities for the first case, and also 9 and 7 for the second case in sequential characters respectively. Two nucleotide insertions encoding Q (CAA) occurred in pv-jask-1 and pv-jask-3 alleles at the beginning of the P-type part of sal-1 like sequence. Moreover, in both alleles a P to Q (CCA to CAA) and a H to Q (CAC to CAA) substitutions was observed in amino acid sequence. In each of pv-jask-2 and pv-jask-4 alleles a single nucleotide polymorphism (SNPs) has occurred in P-type part of sal-1 like sequence, V to A (GTA to GCA). In pv-jask-1, pv-jask-2, pv-jask-3 and pv-jask-4 alleles I to T (ATC to ACC), A to V (GCC to GTC) and E to Q (GAA to CAA) single nucleotide polymorphisms emerged at the same region of the gene.
Although microscopical examination of secondary thick blood smear of the third returned patient confirmed new P. vivax infection, the venous blood sample was accidentally missed.
Discussion
Understanding the type of reappearing P. vivax, the main causative plasmodium for malaria outside Africa, at the same patient is a crucial recognition for combating vivax malaria at the endemic areas. Such phenomenon will be more important when we encounter with the new infection after a course of radical treatment. Relapses are a feature of P. vivax with at least one episode of recurrence because of activating dormant liver parasites (hypnozoites). They usually cause new clinical manifestations with a blood schizogonic pattern that are needed to be treated. Beside of the relapse, reinfection (resulting from a new infected mosquito biting) and recrudescence (originating from drug resistant asexual blood-stage parasites) subjects attract a number of relevant studies. Reports released from some malarious areas indicate many relapses with different features of recurrence (Craig and Kain 1996, Rowland and Durrani 1999, Gogtay et al. 2000, Leslie et al. 2004, Haghdoost et al. 2006, Chen et al. 2007).
This study was designed to perform a comparative molecular analysis of paired primary and secondary P. vivax isolates that appear in the same patient following an 8 wk radical treatment. Three out of 95 patients experienced second episode of vivax malaria within 83–199 days after primary attack. DNA sequences analysis of two paired primary and secondary P. vivax isolates collected from two patients displayed that they were not identical. With referring to the results obtained in this study we encounter with four generic explanations for these results:
The first probability is the recrudescence phenomenon. The new manifestation of P. vivax after primary attack in returned patients occurred on day 83 that usually is beyond the range of returning time for drug resistance at asexual blood-stage as defined (Pukrittayakamee et al., 2004), unless we imagine the emergence of some primaquine-resistant hypnozoites, but owing to lack of reasonable facts the imagination remains unclear. On the other hand, the patients were living in the malarious endemic areas where transmission of malaria potentially may occur over the year. Therefore, differentiation between recrudescence and reinfection may not be possible without some complementary considerations.
Long incubation period, this phenomenon is one of the complicated propositions in P. vivax studies. Inoculation of mixed genotypes of P. vivax (multiple clone infections) with different incubation periods by vector(s) or multiple bites happening over a long period can confuse among real relapse, successive infections released from liver because of different incubation periods and reinfection. Besides the reason of endemicity, effective radical treatment of patients in this study seriously decreases the implementation of emergence of P. vivax with different incubation periods.
Relapse phenomenon is another cause to explain the obtained results. Although relapse is put usually at the first line attribution to new infections of P. vivax particularly if a similar genotype would be considered between primary and secondary infections (Craig and Kain 1996), some authors believe that parasites involved in relapse infection often bear a genotype different from those present at primary infection (Imwong et al. 2007, Koepfli et al. 2009). Results obtained in this study may at the first view indicate to relapses, but employing precise radical treatment against P. vivax and also endemicity of malaria in the studied areas weaken such indication.
Reinfection is the fourth conception that draws a line of explanation about the reappearance of P. vivax in this study. In spite of either 14-day or 8-week radical treatment of vivax malaria at the endemic malarious areas those recurrences occur after day 30 or day 74 can be assumed mostly to be reinfection unless reasonable facts refuse the assumption.
According to our results, the nearest and latest recurrences occurred on 83 and 199 days after primary attack and other reasons mentioned above we adopt the reinfection of P. vivax as a resultant for both returned patients in this study. Leslie et al. in a comparative study between 14-day and 8-week P. vivax malaria treatment using primaquine as an antirelapse drug showed 1.8% and 5.4% treatment failure respectively (Leslie et al. 2008). The latter failure is comparable with the results obtained in our study. The authors did not document how they could distinguish treatment failures from those that are new infections. Indeed, it is difficult to differentiate between either relapses and new infections or recrudescence by using current genotyping methods without including a number of aspects such as mixed genotyping markers and epidemiological features.
Although in this study recurrent venous blood sample of the third patient was lost accidentally, DNA sequencing of the primary sample surprisingly revealed a Chesson genotype P. vivax from Bashagard area with warm-dry and temperate climates in summer and winter respectively. This is the first report to our knowledge of Chesson type P. vivax from such climate.
Some authors, as cited above, believe that the hypnozoites induce relapses are similar to those parasites cause primary attack (Craig and Kain 1996), but the question is that how we can differentiate between real relapses and reinfections or recrudescences. On the other hand, if the parasites in primary infections and relapses are genotypically identical why they follow different patterns of emergence particularly if genotype-specific immunity phenomenon that develops against primary blood- stage parasites (Imwong et al. 2007) would be accounted.
More combined studies including epidemiological and genotyping considerations are needed to clarify the questions that have been released in this field.
Acknowledgments
We would like to thank Mr. GH Mohseni, Mrs. E Torabi, Mr. A Parekar, Mrs. M Shahrokhi, Mr. A Ramezanpour, Mr. A Ahmadi and Mr. M Safari for their technical cooperation and specific thanks to Dr. H. Hajjaran, Mrs. A. Motevalli, Dr. R. Safari and Mrs. L. Farivar for their usefull contribution in the study.
This study was supported financially by center of Infectious Disease Management in Iran and Tehran university of Medical Sciences. The authors declare that they have no conflicts of interest.
References
- Adak T, Sharma VP, Orlov VS. Studies on the Plasmodium vivax relapse pattern in Delhi, India. Am J Trop Med Hyg. 1998;59:175–179. doi: 10.4269/ajtmh.1998.59.175. [DOI] [PubMed] [Google Scholar]
- CDC of Ministry of Health & Medical Education Annual report of malaria cases. 2008. I.R.Iran.
- Chen N, Aullif A, Rieckmann K, Gatton M, Cheng Q. Relapses of Plasmodium vivax infection result from clonal hypnozoites activated at predetermined intervals. J Infect Dis. 2007;195:934–941. doi: 10.1086/512242. [DOI] [PubMed] [Google Scholar]
- Coatney GR, Cooper WC, Young MD. Studies in human malaria.xxx. A summary of 204 sporozoite- induced infection with the Chesson strain of Plasmodium vivax. Journal of National Malaria Society. 1950;9:381–396. [PubMed] [Google Scholar]
- Collins WE, Jeffery GM. Primaquine resistance in Plasmodium vivax. Am J Trop Med Hyg. 1996;55:243–249. doi: 10.4269/ajtmh.1996.55.243. [DOI] [PubMed] [Google Scholar]
- Collins WE, Sullivan JS, Morris CL, Galland GG, Richardson B. Observations on biological nature of Plasmodium vivax sporozoites. J Parasitol. 1996;82:216–219. [PubMed] [Google Scholar]
- Craig AA, Kain KC. Molecular analysis of strains of Plasmodium vivax from paired primary and relapse infection. J Infect Dis. 1996;174:373–379. doi: 10.1093/infdis/174.2.373. [DOI] [PubMed] [Google Scholar]
- Gogtay NJ, Desai S, Kadam VS, Kamtekar KD, Dalvi SS, Kshirsagar NA. Relapse pattern of Plasmodium vivax in Mumbai: a study of 283 cases of vivax malaria. J Assoc Phys India. 2000;48:1085–1086. [PubMed] [Google Scholar]
- Haghdoost A, Mazhari SH, Bahaadini K. Estimating the relapse risk of Plasmodium vivax in Iran under national chemotherapy scheme using a novel method. J Vect Borne Dis. 2006;43:168–172. [PubMed] [Google Scholar]
- Hay SI, Guerra CA, Tatem AJ, Noor AM, Snow RW. The global distribution and population at risk of malaria: past, present, and future. Lancet Infect Dis. 2004;4:327–336. doi: 10.1016/S1473-3099(04)01043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imwong M, Snounou G, Pukrittayakamee S, Tanomsing N, Kim JR, Nandy A, et al. Relapse of Plasmodium vivax infection usually result from activation of heterologous hypnozoites. J Infect Dis. 2007;195:927–933. doi: 10.1086/512241. [DOI] [PubMed] [Google Scholar]
- Kirchgatter K, del Portillo HA. Molecular analysis of Plasmodium vivax relapses using the MSP1 molecule as a genetic marker. J Infect Dis. 1998;177:511–515. doi: 10.1086/517389. [DOI] [PubMed] [Google Scholar]
- Koepfli C, Mueller I, Marfurt J, Goroti M, Sie A, Oa O, Genton B, Beck HP, Fleger I. Evaluation of Plasmodium vivax Genotyping Markers for Molecular Monitoring in Clinical Trials. J Infect Dis. 2009;199:1074–1080. doi: 10.1086/597303. [DOI] [PubMed] [Google Scholar]
- Krotoski WA. Discovery of the hypnozoite and a new theory of malarial relapse. Trans R Soc Trop Med Hyg. 1985;79:1–11. [PubMed] [Google Scholar]
- Leslie T, Rab MA, Ahmadzai H, Durrani N, Fayaz M, Kolaczinski J, Rowland M. Compliance with 14-day primaquine therapy for radical cure of vivax malaria-a randomized placebo-controlled trial comparing unsupervised with supervised treatment. Trans R Soc Trop Med Hyg. 2004;98:168–173. doi: 10.1016/s0035-9203(03)00041-5. [DOI] [PubMed] [Google Scholar]
- Leslie T, Mayan I, Mohammed N, Erasmus P, Kolackzinski J, Whitty CJM, Rowland M. A Randomized Trial of an Eight-Week, Once Weekly Primaquine Regimen to Prevent Relapse of Plasmodium vivax in Northern Frontie Province, Pakistan. 2008. PLoS ONE www.plosone.org 8. [DOI] [PMC free article] [PubMed]
- Manson J. Patterns of Plasmodium vivax recurrence in a high-incidence coastal area of El Salvador, C.A. Am J Trop Med Hyg. 1975;24:581–585. doi: 10.4269/ajtmh.1975.24.581. [DOI] [PubMed] [Google Scholar]
- Mendis K, Sina BJ, Marchesini P, Carter R. The neglected burden of Plasmodium vivax malaria. Am J Trop Med Hyg. 2001;64:97–106. doi: 10.4269/ajtmh.2001.64.97. [DOI] [PubMed] [Google Scholar]
- Pukrittayakamee S, Imwong M, Looareesuwan S, White NJ. Therapeutic responses to antimalarial and antibacterial drugs in vivax malaria. Acta Tropica. 2004;89:351–356. doi: 10.1016/j.actatropica.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Rowland M, Durrani N. Randomized controlled trials of 5- and 14-days primaquine therapy against relapses of vivax malaria in an Afghan refugee settlement in Pakistan. Trans R Soc Trop Med Hyg. 1999;93:641–643. doi: 10.1016/s0035-9203(99)90081-0. [DOI] [PubMed] [Google Scholar]
- Wernsdorfer WH. Principles and practice of malariology. Churchill Livingstone; London: 1988. [Google Scholar]
- Zakeri S, Barjesteh H, Djadid N. Merozoite surface protein-3α is a reliable marker for population genetic of Plasmodium vivax. Malaria J. 2006;5:53–58. doi: 10.1186/1475-2875-5-53. [DOI] [PMC free article] [PubMed] [Google Scholar]