Abstract
Background:
Biting habit of mosquitoes plays an important role in the epidemiology of mosquito-borne diseases. Mosquitoes use a set of elaborate sensory modalities to find their preferred hosts by exploiting cues emanating from a nearby host. It has been suggested that the chemical profile of skin can provide further support for anthropophilic mosquito species to find their suitable hosts. This study aimed at revealing the value of skin emanation for a zoophilic species like Anopheles stephensi as a model.
Methods:
Skin emanations of a man, a cow and a Guinea pig were collected by ethanol soaked cottons. Upwind responses of mosquitoes to 100 and 200 μl of filtered skin materials were non-competitively explored in a dual-choice olfactometer. L-lactic acid and other chemical content of the skin samples were identified by an enzymatic kit and GC-MS, respectively.
Results:
Unexpectedly, only human skin emanation was resulted in the statistically significant activation and attraction responses of An. stephensi in the wind tunnel. L-lactic acid content of this skin sample was 10 and 29 times more than the cow and the Guinea pig, respectively. The possible role of lactic acid and a few other identified compounds have been discussed here.
Conclusion:
Anopheles stephensi showed higher and more specific upwind responses to human skin emanation in the olfactometer. Undoubtedly, the thorough explanation of this unexpected finding needs further investigation. But, if new data verify this result, then, it may be necessary to reconsider the role of skin emanation and thence the human blood index and vectorial capacity of this zoophilic mosquito.
Keywords: Mosquito, Host preference, Host-seeking, An. stephensi, Skin emanation, Olfactometer
Introduction
Malaria remains a major public health problem in southern part of the country which comprises about 80% of all malaria cases in the country. In this part of the country six anopheline mosquitoes including An. culicifacies, An. stephensi, An. dthali, An. fluviatilis, An. superpictus, and An. pulcherrimus are known to be the malaria vectors (Zahirnia et al. 1998,Vatandoost, 2001, Zahirnia et al. 2001, Naddaf et al. 2003, Enayati et al. 2003, Vatandoost and Moinvaziri, 2004, Vatandoost and Borhani 2004, Vatandoost et al. 2004, Vatandoost et al. 2005a, 2005b, Hanafi-Bojad and Vatandoost 2006, Vatandoost et al. 2006a, 2006b, Oshaghi et al. 2006a, 2006b, 2006c, Davari et al. 2006, Abai et al. 2008, Vatandoost et al. 2009. Anopheles sahacrovi and An. maculipennis are considered as malaria vector in northern part of the country (Salari Lak et al. 2002, Sedaghat et al. 2003a, 2003b, Sedaghat et al. 2005, Sedaghat and Harbach 2005, Oshagi et al. 2005, Doosti et al. 2006, Doosti et al 2007, Vatandoost and Zahir Nia 2010). Most of literature on the host-seeking behavior of mosquitoes is allocated to anthropophilic species. In an effort to understand more on the role of skin emanation in the host discrimination of zoophilic mosquitoes this study has been conducted on An. stephensi as a model. This mosquito species which is an important malaria vector in the Middle East and Indian subcontinent (Krishnan 1961, Manouchehri et al. 1976) has a remarkable zoophilic propensity (Kiszewski et al. 2004).
Biting habit of mosquitoes in terms of host preference and frequency of taking blood meal plays a double role in vectorial capacity, a basic entomological concept in the epidemiology of mosquito-borne diseases (Mc Donald 1957). Whereas the former is fundamentally governed by mosquito genetics the latter is under the influence of environmental factors (Takken and Knols 1999). If a female mosquito has no primary problem with the availability and accessibility of the potential host, then she must locate it properly by applying her elaborate sensory modalities (Bowen 1991). However, mosquitoes preferentially select their victims rather than bite in a random manner (Burkot 1988). It is suggested that in addition to other host cues, skin emanation provides further support in olfactory guidance of the female mosquito towards her preferred host (Price et al. 1979, Takken 1991, Braks et al. 1999, Clements 1999). Furthermore, it is proposed that the chemical profile of skin emanation also takes part in the intraspecific variation of the attractiveness of hosts to mosquitoes (Knols et al. 1995, Brady et al. 1997, Qiu et al. 2006).
Over 300 chemical compounds have been identified in human skin emanation (Sastry et al. 1980, Bernier et al. 1999, Curran et al. 2005). It is well demonstrated that a few of these compounds are involved in mosquito-host interaction. L-lactic acid (hereafter called as lactic acid) (Acree et al. 1968, Geier and Boeckh 1999, Braks et al. 2001, Steib et al. 2001, Dekker et al. 2002), ammonia (Geier et al. 1999, Braks et al. 2001, Smallegange et al. 2005) and 1-octen-3-ol (Takken and Kline 1989) are among the most important ones. Electrophysiological and behavioral studies have also shown that a number of carboxylic acids individually or in a blend can be detected and attract mosquitoes in the presence of lactic acid and/or ammonia (Bosch et al. 2000, Smallegange et al. 2005, Smallegange et al. 2009). By contrast, a number of naturally occurring fatty acids on the skin of the human beings elicit negative electrophysiological and/or behavioral effects in mosquitoes (Skinner and Tong 1965, Bernier et al. 2007). Above all, whereas certain dose of a given chemical compound may elicit attraction, a lower or a higher dose may result in a repulsive reaction or nothing at all (Smallegange et al. 2009). Therefore, it is conceivable that the odor-mediated attraction response of a physiologically-competent mosquito to a potential host is too far from simple. This study aimed at revealing the value of skin emanation for a zoophilic species like An. stephensi as a model.
Materials and Methods
Mosquitoes
Anopheles stephensi originated from Iranshahr, Iran was maintained in the insectary of the School of Public Health, Tehran University of Medical Sciences, Tehran, Iran under 29±1° C, 80±5% RH, LD 12:12 h conditions. The stock culture of adults took their blood meals from a Guinea pig once a week. Eggs hatched in a water bowl and first instar larvae were distributed in water filled plastic trays (one per milliliter) on the next day. Elder instar larvae were fed with Tetramin® fish food. Daily collected pupae were placed in populations of 1000 to 1500 in 30×30×30 cm gauze-covered cages. The emerged adults accessed only to 10% glucose solution. All experiments were performed on 4–5-day-old 8–10-h sugar deprived female mosquitoes during the middle third of the dark period. These mosquitoes were selected from their rearing cages by means of attracting to a small warm water filled bottle. They were put into five small cages in population of 10 and transferred to the laboratory in an opaque plastic box matted with wet tissues.
Olfactometer
For this study a modified Giere et al. (1999) type dual-port olfactometer was used (Omrani et al., 2010). In brief, charcoal-filtered, humidified (50±2%) and warm air (27±0.1 and 29±0.1° C) was led via PVC pipelines to the olfactometer arms. Wind speed was constantly kept on 0.2 m/s at the cylindrical wind tunnel outlet. Light intensity of two 25 W incandescent bulbs reduced to 11 lux and scattered over the olfactometer from 80 cm height to provide a relatively homogeneous ambient dim light in all experiments. Two white plastic sheets at the either side of the wind tunnel prevented undesirable optical stimulation of mosquitoes.
Skin emanations
In 3 consecutive days, a total of 90 skin emanation samples (10 sample per individual per day) were collected from a human being (43 yr old, male), a cow (Holstein, 3–4 yr old, female) and a Guinea Pig (4 mo old, female) by rubbing ethanol soaked (absolute, Merck, Germany) clean dental roll cottons (0.48±0.05 g) over different areas of around 6 square centimeter during a 15 to 20 seconds period. Sampling was distributed all over the body to entrap most of chemicals emanated from the skin. Then, alcohol wet cottons were squeezed simply between fingers to extract trapped chemicals. They were pooled in the last day to get 15 to 20 ml semiturbid liquid from each specimen. The experimenter used sterile surgical gloves throughout the procedure to prevent any contamination risk. Every day samples were transferred to the laboratory in an ice box to minimize loss of volatile compounds. The final specimens were filtered by number 2 Whatman filter paper and stored under −20° C till the time of behavioral bioassays or chemical analysis.
Behavioral bioassays
Skin emanations were tested noncompetitively in two doses of 100 and 200 μl in four and eight replicates, respectively. Every four-replicate set supplemented with a trial of no stimulus at the start up as control. Tests were alternated between right and left arms to prevent the risk of systematic bias. For each trial a small cage containing 10 fresh mosquitoes was connected to the downwind end of the wind tunnel. After one to three minutes mosquito acclimatization a small filter paper (around 6 cm2) containing relevant dose of under test material was placed inside the stimulus chamber. 15 to 20 seconds slow shaking of this filter paper in the experimental room was sufficient to evaporate alcohol solvent before it is inserted in the olfactometer. Then, mosquitoes were allowed to freely choose olfactometer arms during one minute experimentation time. At the end of each trial, mosquitoes were removed from the wind tunnel by an electrical vacuum cleaner. Through-out all experiments the experimenter used cotton gloves and carefully avoided touching the inner surfaces of the wind tunnel.
Proportion of mosquitoes went out of the small release cage and the proportion visited or trapped in either arms of the olfactometer during one minute experimentation time comprised activation (%) and attraction (%) to the either treatment or control arms, respectively.
Chemical analysis of the skin emanation samples
This procedure involved both enzymatic measurement of lactic acid content and GC-MS analysis of the skin emanation specimens. In the course of this study, two further samples from two generally similar cows and one sample of 0.001 dilution of technical DEET (98.8%) were supplemented to primary skin emanation samples for GC-MS analysis.
Lactic acid content of each specimen was measured with the aid of an enzymatic kit (K-late, Megazyme, Ireland). This quantification process involves two reaction steps in which the amount of NADH formed is correspondent with the amount of primary lactic acid. The NADH can be measured thereafter by the increase in light absorbance at 340 nm. For this procedure 100 μl of available specimens was tested and light absorbance was measured with a Diode Array Spectrophotometer (Cecil, Model 1010, England).
Chromatographic separation was performed using an Agilent Technologies 6890N Chromatograph equipped with HP-5 capillary column (5% phenyl 95% methyl poly siloxane) with 30 m length, 320 μm internal diameter and 1μm film thickness. The oven program started at 35° C for 3 min and ramped at 12° C/min to 180° C followed by 25° C/min to 270° C with a final hold time of 5 min. The gas chromatograph was operated at a constant flow of 1.3 ml/min of Helium as the carrier gas with 99.999% purity and the injection port and transfer line temperature was set at 230 and 270° C, respectively. The mass selective detector was Agilent 5973N which scanned a range from 35 to 350 m/z with 70 eV in EI mode. Chemical compounds were tentatively identified by comparison of the obtained mass spectrums with the internal library of the system.
Statistical analysis
Data entered in SPSS 13.0 statistical software and paired comparison of activation responses to each dual skin emanations performed by non parametric Mann-Whitney test (α= 0.05). The same statistical test was used for comparison of attraction responses of mosquitoes between treatment and control arms or different quantities of test materials.
Results
Behavioral bioassays
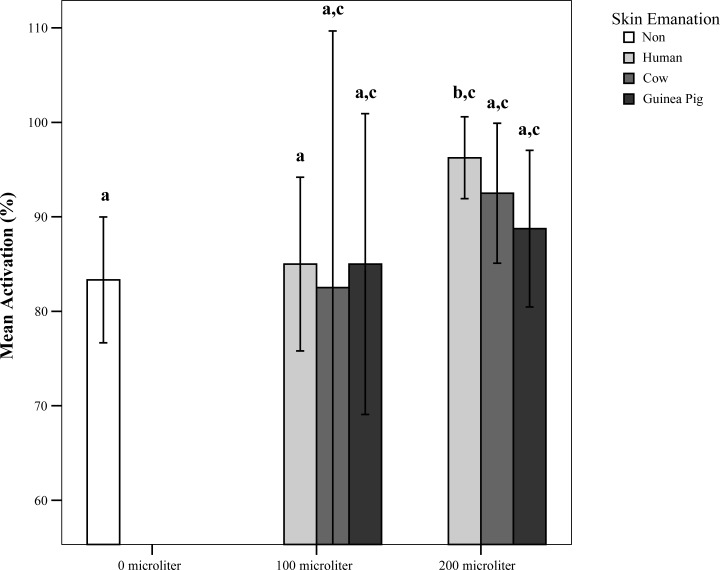
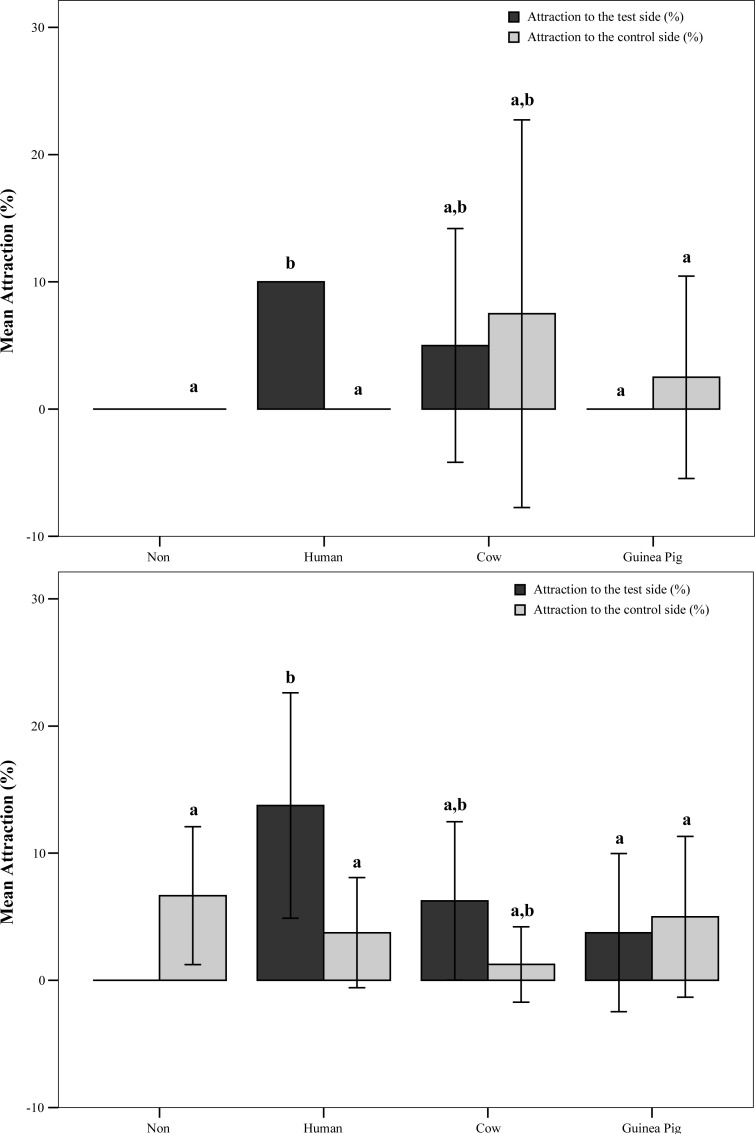
One hundred μl of any skin emanations did not significantly change the primary activation of An. stephensi in the olfactometer. However, when the amount of test material was doubled, the activation of mosquitoes to human skin emanation increased significantly (Z= −2.386, P= 0.017) (Fig. 1). This increase was more than either no stimulus control situation or two other emanations. On the other hand, attraction to human skin emanation was statistically higher than Guinea Pig in both applied quantities (Z= −2.646, P= 0.008 and Z= −1.987, P= 0.047), but not for cow (Fig. 2). The differential attraction of An. stephensi was also significant between olfactometer arms in both 100 (Z= −2.646, P= 0.008) and 200 μl (Z= −2.012, P= 0.044) doses.
Fig. 1.
Activation response of Anopheles stephensi to 100 μl and 200 μl doses of various skin emanations in the olfactometer. Bars that share no letter above are significantly different at P< 0.05.
Fig. 2.
Attraction responses of Anopheles stephensi to different skin emanations in the olfactometer. (A) 100 μl (B) 200 μl. Bars that share no letter above are significantly different at P<0.05.
Lactic acid measurement
Forty three, 4.4 and 1.5 μg lactic acid were detected in a 100 μl sample of human, cow and Guinea Pig skin emanations, respectively.
GC-MS chemical analysis
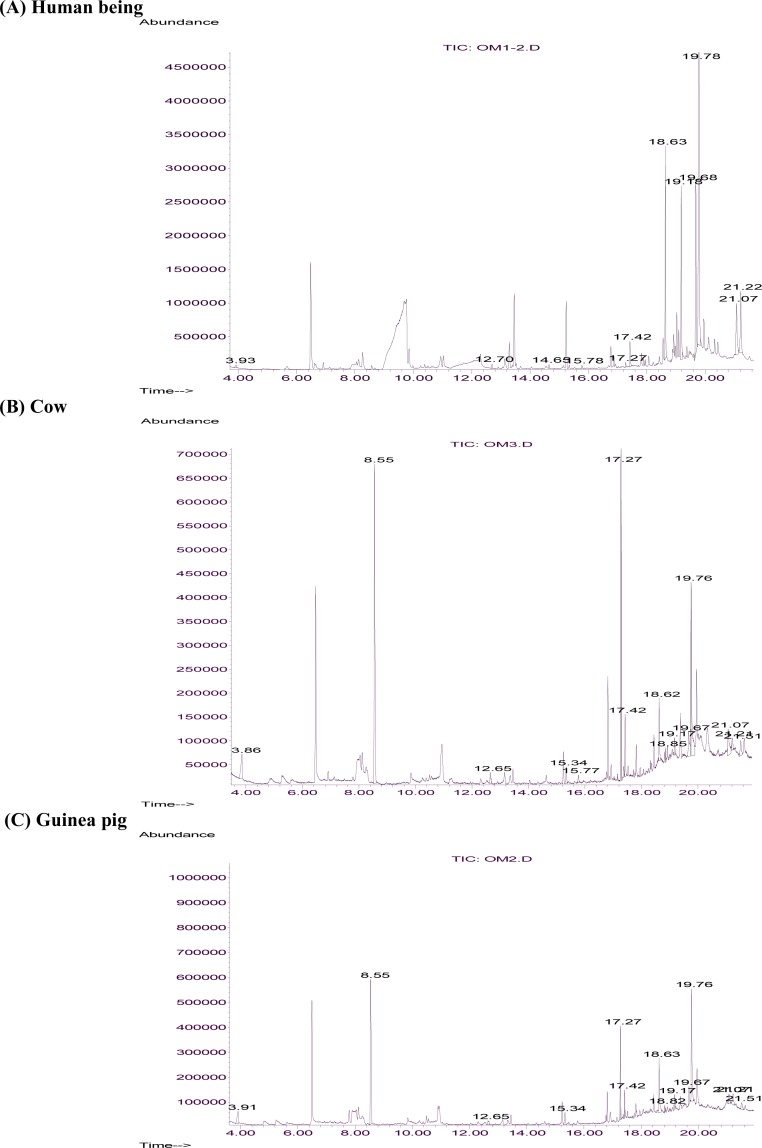
In general, 21, 30 and 34 chemical compounds were identified in human, cow and Guinea Pig skin emanations, respectively (Fig. 3, Table 1). Chromatograms revealed the presence of some more compounds. However, they were either unidentifiable by the available chemical library or irrelevant for being extracted from a living thing. Ten compounds namely ethanoic acid, 2-ethyl hexanoic acid, butylated hydroxytoluene, dodecanoic acid, tetradecanoic acid, pentadecanoic acid, 9-hexadecenoic acid, hexadecanoic acid, 9-octadecenoic acid, octadecanoic acid were found in all three specimens. While four further compounds i.e. 4-hydroxy-4-methylpentan-2-one; N, N-dibutyl-formamide; tetradecanoic acid, methyl ester and 9-octadecanamide were common between cow and Guinea Pig, nonanoic acid and decanoic acid were identified in both human and Guinea Pig skin emanations.
Fig. 3.
Reconstructed ion chromatogram from the electron ionization of compounds thermally desorbed from skin emanation samples on to a HP-5 capillary column. Labeled peaks correspond to those listed in table 1. (A) Human being (B) Cow (C) Guinea pig.
Table 1.
Chemical compounds identified by GC-MS analysis in the skin emanation specimens from a human, a cow and a Guinea pig. H= Human being, C= Cow, GP= Guinea pig.
| Compound | Retention Time | Skin Emanation Specimen | Compound | Retention Time | Skin Emanation Specimen |
|---|---|---|---|---|---|
| Ethanoic acid | 3.93 | H, C, GP | 4-phenyl, 5-formyl, 1,2,3-H-triazole | 17.37 | GP |
| Propanoic acid | 5.6 | C | Dodecanoic acid | 17.42 | H, C, GP |
| Benzeneacetic acid, alpha-oxo, methyl ester | 6.4 | C | N,N-dibutyl thiourea | 17.52 | GP |
| Benzenecarbothioic acid | 6.46 | GP | Hexadecane | 17.71 | GP |
| Benzeneacetonitrile, 2,5-difluoro | 6.9 | GP | N,N-diethyl-4-toluamide | 17.77 | C |
| Hexanal | 7.79 | C | Tridecanoic acid | 18.06 | H |
| Carbonic acid | 8.26 | H | Heptadecane | 18.31 | GP |
| 4-hydroxy-4-methyl-pentan-2-one | 8.54 | C, GP | Hexadecane ,2,6,10,14 tetra methyl | 18.35 | GP |
| 2-pentanone | 8.57 | H | Tetradecanol | 18.44 | C |
| 4-methoxy benzaldehyde,oxime | 9.84 | C | Hexadecanal | 18.46 | GP |
| 1-ethyl-phosphindoline | 9.84 | GP | Tetradecanoic acid | 18.64 | H, C, GP |
| 1,2,3-propanetriol | 11.02 | H | Tetradecanoic acid methyl ester | 18.82 | C, GP |
| 1-butanamine, N-butyl | 11.28 | GP | Octadecane | 18.85 | GP |
| Urea | 12.2 | H | Nonadecane | 18.85 | C |
| Cis-4-ethoxy methyl nitrostyrene | 12.31 | GP | Eicosane | 18.91 | GP |
| 2-ethyl hexanoic acid | 12.72 | H, C, GP | Tetradecanoic acid,12 methyl | 19.02 | H |
| Benzoic acid | 13.35 | GP | 14-pentadecenoic acid | 19.09 | H |
| 2,5-dimethylfuran | 14.04 | C | 2-heptanone | 19.14 | C |
| (1R,6S)-6-methyl-bicyclo-(4,2,0)-octa-2-one | 14.04 | GP | Pentadecanoic acid | 19.18 | H, C, GP |
| Carbamothioic acid, butyl ethyl ester | 14.33 | GP | 9-hexadecanoic acid, methyl ester | 19.66 | GP |
| Nonanoic acid | 14.65 | H, GP | 9-hexadecenoic acid | 19.67 | H, C, GP |
| N,N-dibuthyl formamide | 15.33 | C, GP | Hexadecanoic acid | 19.78 | H, C, GP |
| 2,3-dimethyl-(1,4-pentadiene) | 15.47 | C | Heptadecanoic acid | 20.11 | H |
| Decanoic acid | 15.77 | H, GP | 9-octadecenoic acid | 21.07 | H, C, GP |
| Hexadecanoic acid, 2-propyl methyl ester | 16.67 | H | Octadecanoic acid | 21.22 | H, C, GP |
| 4-amino ,1-methyl uracil | 16.81 | GP | 9-octadecenamide | 21.5 | C, GP |
| Pyrimidine,4-fluoro2-dimethyl amino | 16.81 | C | Furfuryl alcohol | 21.63 | C |
| Phenol-(1,1-dimethyl ethyl), 4-methoxy | 16.92 | C | 5-octadecane | 21.63 | GP |
| 2-bromoethanol | 17.15 | C | Squalene | 22.39 | C |
| Butylated hydroxytoluene | 17.27 | H, C, GP | |||
| 8-hydroxy-2-quinoline carboxyaldehyde | 17.37 | C |
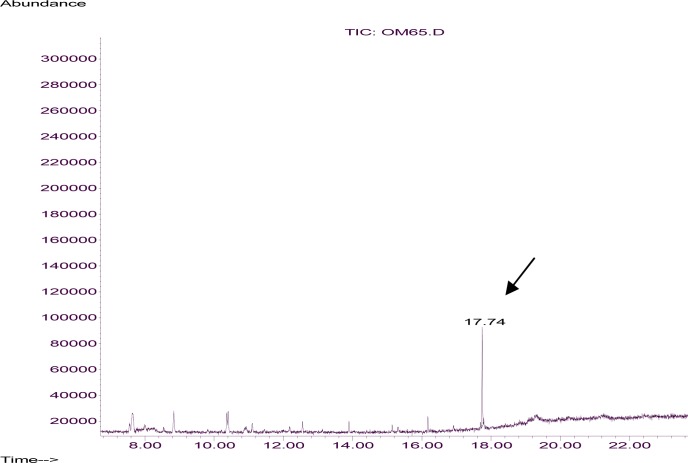
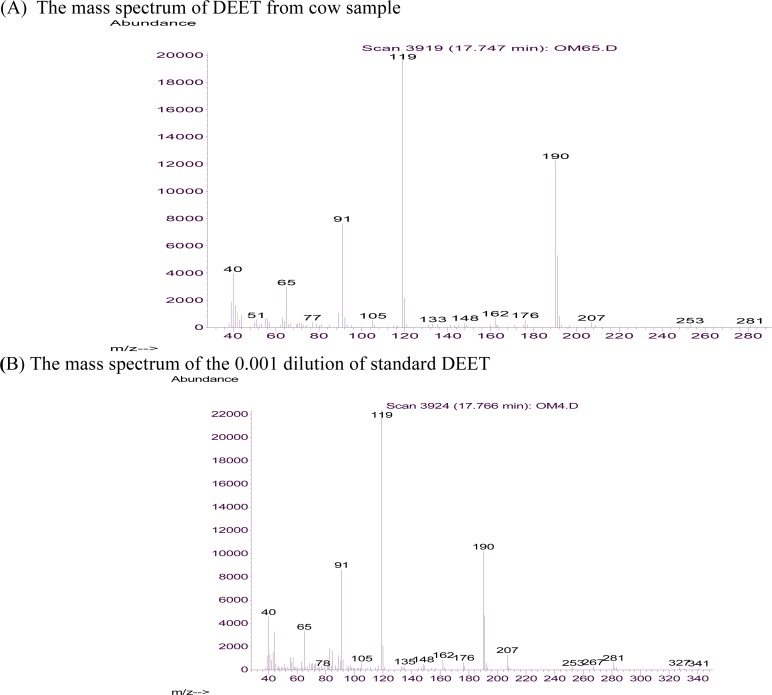
Hexadecanoic acid (45.21%), 4-hydroxy-4-methylpentan-2-one (4.7%) and again this compound (22.56%) were the most relatively abundant chemicals found in the skin emanations of the human being, cow and Guinea pig, respectively (Table 2). Interestingly, a small quantity of N, N-diethyl-4-toluamide (0.12%) was identified in the cow skin emanation sample. Repeating the analysis of the same sample as well as two further samples from similar cows (data not shown here) and comparing these results with reconstructed chromatogram (Fig. 4) and mass spectrum of the 0.001 dilution of technical DEET (Fig. 5) verified that this repellent is present in the cow skin emanation.
Table 2.
Relative abundance of the indentified compounds by GC-MS analysis. Percentages are calculated by dividing the peak area of a compound by the total area of compounds identified in the same skin emanation sample. H = Human being, C = Cow, GP = Guinea Pig
| Compound | Human (%) | Cow (%) | Guinea pig (%) |
|---|---|---|---|
| Ethanoic acid | 0.55 | 0.45 | 3.02 |
| 4-hydroxy-4-methyl-2-pentanone | 0 | 4.70 | 22.56 |
| 2-ethyl hexanoic acid | 0.44 | 0.25 | 1.16 |
| Nonanoic acid | 0.29 | 0 | 0.55 |
| N,N dibuthyl formamide | 0 | 0.45 | 1.39 |
| Decanoic acid | 0.17 | 0 | 0.59 |
| Butylated hydroxytoluene | 0.23 | 2.14 | 16.47 |
| Dodecanoic acid | 1.37 | 0.59 | 3.47 |
| Tetradecanoic acid | 11.38 | 1.23 | 3.11 |
| Tetradecanoic acid, methyl ester | 0 | 0.10 | 0.31 |
| Pentadecanoic acid | 10.26 | 0.17 | 1.06 |
| 9-hexadecenoic acid | 11.89 | 0.45 | 1.37 |
| Hexadecanoic acid | 45.21 | 3.31 | 10.37 |
| 9-octadecenoic acid | 0.09 | 0.43 | 1.62 |
| Octadecanoic acid | 6.33 | 0.45 | 1.22 |
| 9-Octadecenamide | 0 | 0.38 | 1.61 |
Fig. 4.
Reconstructed ion chromatogram from the electron ionization of the 0.001 dilution of technical DEET on to a HP-5 capillary column.
Fig. 5.
(A) Negative ion product mass spectrum of DEET observed from the sample of cow skin emanation. (B) Negative ion mass spectrum of the 0.001 dilution of the standard DEET in ethanol.
Discussion
This study illustrates non-competitive dominance of the human skin sample over cow or Guinea pig skin emanations in a dual-choice olfactometer for the zoophilic malaria mosquito An. stephensi.
At first glance, this result appears to be against the hypothesis that the olfactory signature of the skin of a preferred host provides specific supportive signal to a host-searching female mosquito. However, a few points are needed to be taken into consideration, here. First of all, labeling a mosquito as a zoophilic or an anthropophilic species is primarily based on field studies and it is well known that such data is under the influence of environmental factors (Garrett-Jones et al. 1980). So, at times it is possible that an anthropophilic mosquito feeds on a non-human host or a zoophilic mosquito bites a human being, on the contrary. On the other hand, there are a few evidences that a highly zoophilic mosquito species may show degrees of anthropophily under controlled conditions. For example, An. quadriannulatus and An. stephensi were attracted equally to human and cow odor in a dual-choice olfactometer (Pates et al. 2001, Waka et al. 2006). The former species also showed unexpected anthropophagy under semi-field conditions (Pates et al. 2001). Collection of the skin emanations from individuals of a population with extreme attractive potential, inadequacy and insufficiency of skin sampling and/or the chemical analysis procedure, and above all, ignoring the indispensable role of other host cues are other sources of the misinterpretation of the current result. None the less, the chemical profile of tested emanations might still provide some useful clues on the observed selective attractiveness of An. stephensi in this study. The increased upwind activity of mosquitoes in response to doubling the amount of skin materials witnesses that this is a logic approach.
The lactic acid content of the human skin emanation (43 μg) was measured to be around 10 and 29 times more than the cow (4.4 μg) and the Guinea pig (1.5 μg) samples, respectively. This finding is similar to the result of the experiments of Dekker et al. (2002) who found that lactic acid content of human skin emanation was 15 times more than the cow specimen. They did not detect any lactic acid in skin samples of a rat or a rabbit, as well. By adding or removal of lactic acid to the cow or the human skin emanations they were able to demonstrate that the lower attraction responses of An. gambiae or Aedes aegypti in the olfactometer were mainly due to the specific level of lactic acid concentration in these skin samples (Steib et al. 2001, Dekker et al. 2002). Based on these evidences it is likely that the higher attractive responses of An. stephensi to human skin emanation in the olfactometer might be related to the higher lactic acid content of this specimen.
Gas chromatography coupled with mass spectrometry revealed that there is very little amount of DEET in the cow skin emanation. Because DEET is a synthetic material (Acree and Beroza 1962) it is obvious that this chemical compound is exogenous, but we were not able to determine the source of this contamination. Whereas some workers have reported that the DEET is an effective spatial repellent (Wirtz et al. 1980, Hoffman and Miller 2002), others have considered that it is a weak one (Mc Govern et al. 1967, Bernier et al. 2005). It has been explained that in the latter situations the concentration of this material in the vapor phase had been very low (Bernier et al. 2007). According to these evidences, it is suggested that whatever the source of DEET in the cow skin sample it can not justify well the inferiority of this skin emanation for An. stephensi in the olfactometer.
Ethanoic acid, a short-chain aliphatic carboxylic acid was identified in all three skin samples. Electrophysiological recordings from sensilla trichodea of the zoophilic species An. quadriannulatus and An. atroparvus have shown that the responses of the housed sensory neurons are inhibitory (Van den Broek and den Otter 1999). Therefore, the lower activation and attraction responses of An. stephensi to Guinea pig skin emanation in the wind tunnel may has some correlation with higher relative abundance of this chemical compound in this sample.
Nonanoic, decanoic and dodecanoic acid are three other carboxylic acids which have been reported to repel anthropophilic mosquito species individually at certain doses (Reifenrath 2005). Despite of the lower relative abundance of these fatty acids, their comparative level in each skin emanation sample and/or their combination with other chemical compounds therein might have particular contribution in the observed upwind responses of An. stephensi in the olfactometer.
Tetradecanoic acid was also common in all tested skin emanations. There are contradictory reports on the effect of this carboxylic acid on the upwind responses of mosquitoes. While this fatty acid alone or in combination with lactic acid and ammonia were attractive for Culex quinquefasciatus and An. gambiae, respectively (Cork and Park 1996, Smallegange et al. 2009), its addition to the blend of lactic acid plus a set of other carboxylic acids decreased attraction responses of Ae. aegypti in the wind tunnel (Bosch et al. 2000). If tetradecanoic acid assumed an attractive agent, then, it can be mentioned in line with lactic acid to explain the higher attractive responses of An. stephensi to human skin emanation. But, if it is considered as an attraction inhibitor, since its relative abundance in the human skin sample (11.38%) was 10 times more than in the cow skin emanation (1.23%), it may provide further support that the very small quantity of identified DEET can not justify inferior responses of An. stephensi to this skin sample.
Although hexadecanoic acid was the most relatively abundant chemical compound found in the human skin emanation (45.21%), there is no evidence that it can play a role in attractive responses of mosquitoes (Knols and Meijerink 1997, Bosch et al. 2000, Puri et al. 2006).
The relative abundance of butylated hydroxytoluene was much higher in the Guinea pig (16.47%) than the human (0.23%) or the cow (2.14%) skin emanations. This chemical agent which is a synthetic antioxidant is commonly used in the preservation of food materials (Koltover 2009). Although, the frequent consumption of nutritional supplements may explain the origin and higher relative abundance of butylated hydroxytoluene in the skin emanation of the Guinea pig, it is not know whether it could take any part in the observed responses of An. stephensi in the olfactometer.
4-hydroxy-4-methyl-pentan-2-one is a ketonic material which was found with a high relative abundance in the skin emanation of Guinea pig (22.56%). It was not detected in the human skin sample. This chemical compound is derived from 2-pentanone which is reported to be attractive for Ae. aegypti (Bernier et al. 2004). However, we do not know the effect of 4-hydroxy-4-methyl-pentan-2-one on mosquitoes.
In conclusion, although it is likely that the dominance of human skin emanation for An. stephensi in the olfactometer results from the higher content of lactic acid and contribution of a few carboxylic acids, more investigation is needed to make a reasonable judgment on the role of skin emanations in host-seeking behavior of this zoophilic mosquito. However, if semi-field and field data verify the unexpected findings of this study, it will be necessary to rethink on the current values of human blood index and vectorial capacity for this malaria mosquito species.
Acknowledgments
This article is a part of the results of the first author’s dissertation for fulfillment of a Ph.D. degree in Medical Entomology and Vector Control from Department of Medical Entomology and Vector Control, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran. The authors are grateful to Dr. Seyed Mahmood Reza Nikbakhtzadeh, Department of Parasitology and Medical Entomology, Tarbiat Modarres University, Tehran, Iran, Dr. Ali Mehdinia and Mrs. Sahar Farzadnia, National Institute of Oceanology, Tehran, Iran for their kind contribution in GC-MS analysis of the skin emanation samples. We also thank Mrs. Fatemeh Mohtarami, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran for her supervision on the meticulous measurement of the lactic acid content of the skin emanation samples. This study was financially supported by School of Public Health, Academic Pivot for Education and Research, Tehran University of Medical Sciences, project No. 85-01-63-3687. The authors declare that there is no conflict of interest.
References
- Abai MR, Mehravaran A, Vatandoost H, Oshaghi MA, Javadian E, Mashayekhi M, et al. Comparative performance of imagicides on Anopheles stephensi, main malaria vector in a malarious area, southern Iran. J Vector Borne Dis. 2008;45(4):307–312. [PubMed] [Google Scholar]
- Acree F, Turner RB, Gouck HK, Beroza M, Smith N. L-Lactic acid: a mosquito attractant isolated from humans. Science. 1968;161:1346–1347. doi: 10.1126/science.161.3848.1346. [DOI] [PubMed] [Google Scholar]
- Acree FJ, Beroza M. Quantiative gas chromatography of isomers of insect repellent N, N-diethyltoluamide. J Econ Entomol. 1962;55:619–622. [Google Scholar]
- Bernier UR, Booth MM, Yost RA. Analysis of human skin emanations by gas chromatography/mass spectrometry. 1. Thermal desorption of attractants for the yellow fever mosquito (Aedes aegypti) from handled glass beads. Anal Chem. 1999;71:1–7. doi: 10.1021/ac980990v. [DOI] [PubMed] [Google Scholar]
- Bernier UR, Kline DL, Posey HP. Human emanations and related natural compounds that inhibit mosquito host finding abilities. In: Debboun M, Frances SP, Strickman D, editors. Insect Repellents: Principles, Methods and Uses. CRC Press Taylor and Francis Group; New York: 2007. pp. 77–100. [Google Scholar]
- Bernier UR, Furman KD, Kline DL, Allan SA. Comparison of contact and spatial repellency of catnip oil and N, N-diethyl-3-methylbenzamide (Deet) against mosquitoes. J Med Entomol. 2005;42:306–311. doi: 10.1603/0022-2585(2005)042[0306:cocasr]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Bernier UR, Barnard DR, Booth MM, Kline DL, Posey KH, et al. Chemical composition that attract arthropods. 2004. Oct 4, 2004. US Patent 6,800,279 B2.
- Bosch OJ, Geier M, Boeckh J. Contribution of fatty acids to olfactory host finding of female Aedes aegypti. Chem Senses. 2000;25:323–330. doi: 10.1093/oxfordjournals.chemse.a014042. [DOI] [PubMed] [Google Scholar]
- Bowen MF. The sensory physiology of host-seeking behaviour in mosquitoes. Annu Rev Entomol. 1991;36:139–158. doi: 10.1146/annurev.en.36.010191.001035. [DOI] [PubMed] [Google Scholar]
- Brady J, Costantini C, Sagnon N, Gibson G, Coluzzi M. The role of body odours in the relative attractiveness of different men to malarial vectors in Burkina Faso. Ann Trop Med Parasitol. 1997;91:S121–S122. [Google Scholar]
- Braks MAH, Anderson RA, Knols BGJ. Infochemicals in mosquito host selection: Human skin micrflora and Plasmodium parasites. Parasitol Today. 1999;15:409–513. doi: 10.1016/s0169-4758(99)01514-8. [DOI] [PubMed] [Google Scholar]
- Braks MAH, Meijerink J, Takken W. The response of the malaria mosquito, Anopheles gambiae, to two components of human sweat, ammonia and L-lactic acid, in an olfactometer. Physiol Entomol. 2001;26:142–148. [Google Scholar]
- Burkot TR. Non-random host selection by Anopheline mosquitoes. Parasitol Today. 1988;4:156–162. doi: 10.1016/0169-4758(88)90151-2. [DOI] [PubMed] [Google Scholar]
- Clements AN. The biology of mosquitoes. Vol. 2. New York: Cornel University; 1999. p. 130. CABI Publishing. [Google Scholar]
- Cork A, Park KC. Identification of electrophysiologically-active compounds for the malaria mosquito, Anopheles gambiae, in human sweat extracts. Med Vet Entomol. 1996;10:269–276. doi: 10.1111/j.1365-2915.1996.tb00742.x. [DOI] [PubMed] [Google Scholar]
- Curran AM, Rabin SI, Prada PA, Furton KG. Comparison of the volatile organic compounds present in human odor using SPME-GC/MS. J Chem Ecol. 2005;31:1607–1619. doi: 10.1007/s10886-005-5801-4. [DOI] [PubMed] [Google Scholar]
- Davari B, Vatandoost H, Oshaghi MA, Ladonni H, Enayati AA, Shaeghi M, et al. Selection of Anopheles stephensi with DDT and dieldrin and cross-resistance spectrum to pyrethroids and fipronil. Pestic Biochem Physiol. 2007;89(2):97–103. [Google Scholar]
- Dekker T, Steib B, Carde RT, Geier M. L-Lactic acid: a human-signifying host cue for the anthropophilic mosquito Anopheles gambiae. Med Vet Entomol. 2002;16:91–98. doi: 10.1046/j.0269-283x.2002.00345.x. [DOI] [PubMed] [Google Scholar]
- Doosti S, Azari-Hamidian S, Vatandoost H, Oshaghi MA, Hosseini M. Taxonomic differentiation of Anopheles sacharovi and An.maculipennis S.l. (Diptera: Culicidae) larvae by seta 2 (antepalmate hair) Acta Medica Iranica. 2006;44(1):21–27. [Google Scholar]
- Doosti S, Vatandoost H, Oshaghi MA, Hosseini M, Sedaghat MM. Applying Morphometric Variation of Seta 2 (Antepalmate Hair) among the Larvae of the Members of the Maculipennis Sub-group (Diptera: Culicidae) in Iran. Iran J Arthropod-Borne Dis. 2007;1(1):28–37. [Google Scholar]
- Enayati AA, Vatandoost H, Ladonni H, Townson H, Hemingway J. Molecular evidence for a Kdr-like pyrethroid resistance mechanism in the malaria vector mosquito Anopheles stephensi. Med Vet Entomol. 2003;17(2):138–144. doi: 10.1046/j.1365-2915.2003.00418.x. [DOI] [PubMed] [Google Scholar]
- Garrett-Jones C, Boreham PFL, Pant CP. Feeding habits of Anophelines (Diptera: Culicidae) in 1971–1978, with reference to the human blood index: a review. Bull Entomol Res. 1980;70:165–185. [Google Scholar]
- Geier M, Boeckh J. A new Y-tube olfactometer for mosquitoes to measure the attractiveness of host odours. Entomol Exp Appl. 1999;92:9–19. [Google Scholar]
- Geier M, Bosch OJ, Boeckh J. Ammonia as an attractive component of host odour for the yellow fever mosquito, Aedes aegypti. Chem Senses. 1999;24:647–653. doi: 10.1093/chemse/24.6.647. [DOI] [PubMed] [Google Scholar]
- Hanafi-Bojd AA, Vatandoost H, Jafari R. Susceptibility status of An. dthali and An. fluviatilis to commonly used larvicides in an endemic focus of malaria, southern Iran. J Vector Borne Dis. 2006;43:34–38. [PubMed] [Google Scholar]
- Hoffman EJ, Miller JR. Reduction of mosquito (Diptera: Culicidae) attacks on a human subject by combination of wind and vapor-phase deet repellent. J Med Entomol. 2002;39:935–938. doi: 10.1603/0022-2585-39.6.935. [DOI] [PubMed] [Google Scholar]
- Kiszewski A, Mellinger A, Spielman A, Malaney P, Sachs SE, Sachs J. A global index representing the stability of malaria transmission. Am J Trop Med Hyg. 2004;70:486–498. [PubMed] [Google Scholar]
- Knols BGJ, Meijerink J. Odors influence mosquito behavior. Sci Med. 1997;4:56–63. [Google Scholar]
- Knols BGJ, de Jong R, Takken W. Differential attractiveness of isolated humans to mosquitoes in Tanzania. Trans R Soc Trop Med Hyg. 1995;89:604–606. doi: 10.1016/0035-9203(95)90406-9. [DOI] [PubMed] [Google Scholar]
- Koltover VK. Bioantioxidants: the systems reliability standpoint. Toxicol Ind Health. 2009;25:295–299. doi: 10.1177/0748233709103029. [DOI] [PubMed] [Google Scholar]
- Krishnan KS. Vectors of malaria in India. National Society of India for Malaria and Other Mosquito-borne Disease; Delhi: 1961. pp. 27–37. [Google Scholar]
- Manouchehri AV, Javadian E, Eshghi N, Motabar M. Ecology of Anopheles stephensi Liston in southern Iran. Trop Geogr Med. 1976;28:228–232. [PubMed] [Google Scholar]
- Mc Donald G. The epidemiology and control of malaria. Am J Trop Med Hyg. 1958;7(5):577–578. [Google Scholar]
- Mc Govern TP, Beroza M, Gouck HK. Chemicals tested as space repellents against yellow-fever mosquitoes, II. Carbanilates, benzamides, aliphatic amides, and imides. J Econ Entomol. 1967;60:1591–1594. doi: 10.1093/jee/60.6.1591. [DOI] [PubMed] [Google Scholar]
- Naddaf SR, Oshaghi MA, Vatandoost H, Asmar M. Molecular characterization of the Anopheles fluviatilis species complex in Iran. East Mediterr Health J. 2003;9(3):257–265. [PubMed] [Google Scholar]
- Omrani SM, Vatandoost H, Oshaghi MA, Shokri F, Guerin PM, Yaaghoobi-Ershadi MR, et al. Fabrication of an olfactometer for mosquito behavioural studies. J Vector Borne Dis. 2010;47:17–25. [PubMed] [Google Scholar]
- Pates HV, Takken W, Curtis CF, Huisman PWT, Akinpelu O, Gill GS. Unexpected anthropophagic behaviour in Anopheles quadriannulatus. Med Vet Entomol. 2001;15:293–298. doi: 10.1046/j.0269-283x.2001.00310.x. [DOI] [PubMed] [Google Scholar]
- Price GD, Smith N, Carlson DA. The attraction of female mosquitoes (Anopheles quadrimaculatus Say) to stored human emanations in conjunction with adjusted levels of relative humidity, temperature, and carbon dioxide. J Chem Ecol. 1979;5:383–395. [Google Scholar]
- Puri SN, Mendki MJ, Sukumaran D, Ganesan K, Prakash S, Sekhar K. Electroantennogram and behavioral responses of Culex quinquefasciatus (Diptera: Culicidae) females to chemicals found in human skin emanations. J Med Entomol. 2006;43:207–213. doi: 10.1603/0022-2585(2006)043[0207:eabroc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Oshaghi MA, Chavshin AR, Vatandoost H, Yaaghoobi F, Mohtarami F, Noorjah N. Effects of postingestion and physical conditions on PCR amplification of host blood meal DNA in mosquitoes. Exp Parasitol. 2006a;112:232–236. doi: 10.1016/j.exppara.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Oshaghi MA, Chavshin AR, Vatandoost H. Analysis of mosquito bloodmeals using RFLP markers. Exp Parasitol. 2006b;114(4):259–264. doi: 10.1016/j.exppara.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Oshaghi MA, Yaghoobi F, Vatandoost H, Abai MR, Akbar Zadeh K. Anopheles stephensi biological forms, geographical distribution, and malaria transmission in malarious regions in Iran. Pak J Biol Sci. 2006c;9(2):294–298. [Google Scholar]
- Qiu YT, Smallegange RC, van Loon JJA, Ter Braak CJF, Takken W. Interindividual variation in the attractiveness of human odours to the malaria mosquito Anopheles gambiae s. s. Med Vet Entomol. 2006;20:280–287. doi: 10.1111/j.1365-2915.2006.00627.x. [DOI] [PubMed] [Google Scholar]
- Reifenrath WG. Natural insect repellent. In: Office USPaT, editor. Washington D.C.: U.S.; 2005. p. 134. [Google Scholar]
- Sastry SD, Buck KT, Janak J, Dressler M, Preti G. Volatiles emitted by humans. In: Wiley J, editor. Biochemical applications of mass spectrometry. Interscience; New York: 1980. pp. 1085–1129. [Google Scholar]
- Sedaghat MM, Linton Y-M, Nicolescu G, Smith L, Koliopoulos G, Zounos AK, et al. Morphological and molecular characterization of Anopheles (Anopheles) sacharovi Favre, a primary vector of malaria in the Middle East. Systematic Entomol. 2003a;28:241–256. [Google Scholar]
- Sedaghat MM, Linton Y-M, Oshaghi MA, Vatandoost H, Harbach RE, et al. The Anopheles maculipennis complex (Diptera: Culicidae) in Iran: molecular characterisation and recognition of a new species. Bull Entomol Res. 2003b;93:527–535. doi: 10.1079/ber2003272. [DOI] [PubMed] [Google Scholar]
- Sedaghat MM, Harbach RE. An annotated checklist of the Anopheles mosquitoes (Diptera: Culicidae) in Iran. J Vector Ecol. 2005;30:272–276. [PubMed] [Google Scholar]
- Salari Lak SH, Vatandoost H, Entezarmahdi MR, Ashraf H, Abai MR, Nazari M. Monitoring of insecticide resistance in Anopheles sacharovi (Favre, 1903) in borderline of Iran, Armenia, Naxcivan and Turkey, 2001. Iran J Pub Health. 2002;31(3–4):96–99. [Google Scholar]
- Skinner WA, Tong H. Repellency of skin-surface lipids of humans to mosquitoes. Science. 1965;149:395–306. doi: 10.1126/science.149.3681.305. [DOI] [PubMed] [Google Scholar]
- Smallegange RC, Qiu YT, Van Loon JA, Takken W. Synergism between ammonia, lactic acid and carboxylic acids as kiromones in the host-seeking behaviour of the malaria mosquito Anopheles gambiae sensu stricto (Diptera: Culicidae) Chem Senses. 2005;30:145–152. doi: 10.1093/chemse/bji010. [DOI] [PubMed] [Google Scholar]
- Smallegange RC, Qiu YT, Bukovinszkine-Kiss G, Van Loon JJA, Takken W. The effect of aliphatic carboxylic acids on olfaction-based host-seeking of the malaria mosquito Anopheles gambiae sensu stricto. J Chem Ecol. 2009;35:933–943. doi: 10.1007/s10886-009-9668-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steib BM, Geier M, Boeckh J. The effect of lactic acid on odour-related host preference of yellow fever mosquitoes. Chem Senses. 2001;26:523–528. doi: 10.1093/chemse/26.5.523. [DOI] [PubMed] [Google Scholar]
- Takken W. The role of olfaction in host-seeking of mosquitoes: a review. Insect Sci Applic. 1991;12:287–295. [Google Scholar]
- Takken W, Kline DL. Carbon dioxide and 1-octen-3-ol as mosquito attractants. J Am Mosq Cont Assoc. 1989;5:311–316. [PubMed] [Google Scholar]
- Takken W, Knols BGJ. Odor-mediated behaviour of Afrotropical malaria mosquitos. Annu Rev Entomol. 1999;44:57–131. doi: 10.1146/annurev.ento.44.1.131. [DOI] [PubMed] [Google Scholar]
- Van den Broek IVF, den Otter CJ. Olfactory sensitivities of mosquitoes with different host preferences (Anopheles gambiae s.s., An. arabiensis, An. quadriannulatus, An. m. atroparvus) to synthetic host odors. J Insect Physiol. 1999;45:1001–1010. doi: 10.1016/s0022-1910(99)00081-5. [DOI] [PubMed] [Google Scholar]
- Vatandoost H. Irritability level of Anopheles sephensi in Iran. Iran J Public Health. 2001;30(1–4):27–30. [Google Scholar]
- Vatandoost H, Moinvaziri VM. Larvicidal activity of neem tree extract (Neemarin) against mosquito larvae in the Islamic Republic of Iran. East Mediterr Health J. 2004;10:573–578. [PubMed] [Google Scholar]
- Vatandoost H, Borhani N. Susceptibility and Irritability levels of main malaria vectors to synthetic pyrethroids in the endemic areas of Iran. Acta Medica Iranica. 2004;42(4):240–247. [Google Scholar]
- Vatandoost H, Shahi H, Abai MR, Hanafi-Bojd AA, Oshaghi MA, Zamani G. Larval habitats of main malaria vectors in Hormozgan province and their susceptibility to different larvicides. Southeast Asian J Trop Med Pub Hlth. 2004;35:22–25. [PubMed] [Google Scholar]
- Vatandoost H, Mashayekhi M, Abaie MR, Aflatoonian MR, Hanafi-Bojd AA, Sharifi I. Monitoring of insecticides resistance in main malaria vectors in a malarious area of Kahnooj district, Kerman province, southeastern Iran. J Vector Borne Dis. 2005a;42:100–108. [PubMed] [Google Scholar]
- Vatandoost H, Hanafi-Bojd AA. Current resistant status of Anopheles stephensi Liston to different larvicides in Hormozgan province, southeastern Iran. Pakistan J Bio Sci. 2005b;8:1568–1570. [Google Scholar]
- Vatandoost H, Gholizadeh MR, Abai MR, Djavadian E. Laboratory efficacy of protection rate of torn nets treated with pyrethroids, cufluthrin, deltamethrin and permethrin against Anopheles stephensi (Diptera: Culicidae) J Biol Sci. 2006a;6(2):331–336. [Google Scholar]
- Vatandoost H, Oshaghi M, Abaie MR, Shahi M, Yaghoobi F, Baghai M, et al. Bionomics of Anopheles stephensi Liston in the malarious area of Hormozgan province, southern Iran. Acta Trop. 2006b;97(2):196–205. doi: 10.1016/j.actatropica.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Vatandoost H, Ramin E, Rassi Y, Abai MR. Stability and wash resistance of local made mosquito bednets and detergents treated with pyrethroids against Anopheles stephensi. Iran J Arthropod-Borne Dis. 2009;3(1):19–28. [PMC free article] [PubMed] [Google Scholar]
- Vatandoost H, Zahir Nia AH. Responsiveness of Anopheles maculipennis to different imagicides during resurgent malaria. Asia Pac J Trop Med. 2010;3(5):360–363. [Google Scholar]
- Waka M, Hopkins RJ, Glinwood R, Curtis CF. The effect of repellents Ocimum forskolei and deet on the response of Anopheles stephensi to host odours. Med Vet Entomol. 2006;20:373–376. doi: 10.1111/j.1365-2915.2006.00645.x. [DOI] [PubMed] [Google Scholar]
- Wirtz RA, Turrentine JD, Rutledge LC. Mosquito area repellents: laboratory testing of candidate materials against Aedes aegypti. Mosq News. 1980;40:432–439. [Google Scholar]
- Zahirnia AH, Taherkhani H, Vatandoost H. Observation of malaria sporozoite in Anopheles culicifacies (Diptera: Culicidae) in Ghasreghand district, Sistan & Baluchistan province. Hakim. 2001;4(2):149–153. [Google Scholar]
- Zahirnia AH, Vatandoost H, Nateghpour M, Javadian E. Insecticide resistance/ susceptibility monitoring in Anopheles pulcherrimus (Diptera: Culicidae) in Ghasreghand district, Sistan and Baluchistan province, Iran. Hakim. 1998;1:97–106. [Google Scholar]