Abstract
Background:
A molecular survey was conducted to investigate the presence of pathogenic Borrelia persica species causing the tick borne relapsing fever (TBRF) in Takistan district Qazvin Province, western Iran.
Methods:
A number of 1021 soft ticks were collected from 31 villages including previously reported infected and none-infected TBRF cases and individually examined for the presence of B. persica DNA by conventional PCR targeting the 16S rRNA.
Results:
A total of 1021 soft ticks of three species of Ornithodouros tholozani (120: 11.75%), O. lahorensis (461: 45.15%) and Argas persicus (440: 43.1%) were collected and tested against Borrelia infection. Soft ticks were more prevalent (67%) in infected areas than none infected areas. The rate O. tholozani in infected areas was much greater (29 times) than none infected areas. Ninety seven percent of soft ticks in none infected areas were of O. tholozani. Sixteen (16.7%) ticks of tested (n=95) O. tholozani were infected with B. persica. Three (1.3%) out of 205 soft ticks of O. lahorensis were positive for Borrelia sp., and no infection was observed in A. persicus. TaqI RFLP analysis and sequence analysis of the positive PCR products showed the presence of B. persica. The RFLP analysis showed that the positive ticks of O. lahorensis were infected with unknown Borrelia species.
Conclusion:
This study showed that although there were no TBRF cases in Takisan, but still infected O. tholozani, the known vector of TBRF, presented in the region. Control measures needs to be fulfilled in Thakisan.
Keywords: Borrelia persica, tick borne relapsing fever (TBRF), PCR-RFLP, Takistan, Iran
Introduction
The tick borne relapsing fever (TBRF) is an acute infectious disease and one of its basic properties is the recurring attacks of fever and chill. This disease is one of the important hygiene problems in Middle East and Central Asia (Karimi et al. 1979, Karimi 1980, Barbour and Hayes 1986, Arshi et al. 2002). The disease is reported from North and South America, Africa, Asia and Europe. The epidemiology of the disease in each region depends on the relationships between the tick and Borrelia species as well as environmental condition in its distribution area. Reservoir hosts are usually wild rodents (Rebaudet and Parola 2006). Feeding of a tick on an infected vertebrate is the natural route of tick infection. The pathogen is transmitted to another vertebrate host during tick feeding in the next life stage.
The development of the PCR has offered a new dimension in the diagnosis of infectious diseases. Capable of amplifying minute amounts of DNA into billions of copies in just a few hours, PCR facilitates the sensitive and specific detection of DNA or RNA of pathogenic organisms. PCR for the diagnosis of infectious diseases has been directed primarily toward the detection of pathogens for which conventional diagnostic techniques are either too insensitive or too slow such as Borrelia spp. There are many research studies involving PCR in the diagnosis of Borrelia spp. particularly B. burgdorferi infections in clinical samples (Schwartz et al. 1997, Cerar et al. 2008). The PCR method have been used successfully for identification of B. burgdorferi in hard ticks of Ixodes ricinus (Horka et al. 2008) and B. persica in soft ticks of O. tholozani (Oshaghi et al. 2010a, b).
Tick-borne relapsing fever is one of the endemic diseases in Iran and its prevalence was considerable in the past, but the incidence rate of it decreased to 0.06/100,000 in 2006 (Asl et al. 2009). The cause of this disease in Iran is mainly Borrelia persica Dschunkowski 1912, that conveyed by the bite of the Ornithodoros tholozani soft tick (Karimi 1980, Masoumi Asl et al. 2009). However, other species such as B. latshywii, B. microtti and B. balthazardi are responsible for the spread of the disease in certain areas of the country (Karimi 1980).
Qazvin Province is amongst the most important foci of TBRF in Iran (Asl et al. 2009). The province covers 15821 km2 between 45–48 to 50–50 east of Greenwich Meridian of longitude and 35–37 to 36–45 north latitude of the equator (Aghighi et al. 2007). Takistan district with almost 180,000 populations, is located in west of the province. In Takistan district there are no data regarding B. persica circulation in enzootic sites, nor the presence of these bacteria in ticks, even if there were human case records (MHME). On average, five cases of TBRF had been reported from Takistan, however, since 2007, no report of TBRF has been evidenced in the district. Since the risk of infection to humans with Borrelia depends on outdoor recreational activity, on the density of tick populations, and on the infection of the ticks with Borrelia. Therefore, data describing the prevalence of Borrelia in ticks can be used to assess the risk of TBRF for public health (Rauter and Hartung 2005). In this study we tried to assess the potential risk of TBRF by testing the presence of B. persica in soft ticks, particularly O. tholozani, from Takistan that had previously witnessed some outbreaks of TBRF transmitted by this tick species.
Materials and Methods
Study area
Surveys were performed in Takistan district of Qazvin Province in north-west Iran. It covers 2430 km2 and the terrain of the survey areas consists of 4 zones with 133 villages (Vs) distributed in different mountainous, hilly, and plain regions. It has a temperate to dry climate with annual precipitation of about 232 mm. The temperature ranges from −20 to 40° C, with an average of 14° C. The study was performed in all previously infected (ten) and 21 none infected villages. These 21 villages were randomly selected from mountainous (8), hilly (6), and plain (5) areas.
Specimen collection
This survey was conducted from August 2007 to July 2008. Ticks were actively searched directly by eye and help of torch light and collected from cracks, crevices, ceiling, and floor of human dwelling, poultry and animal shelters or rodent burrows. Process of collection took 30 min each time and the specimens transferred into the holding tubes. All ticks were handled with forceps. All the specimens were identified on the basic of their morphological characteristics (Zaim and Shayeghi 1989, Estrada-Peña et al. 2004).
DNA extraction
DNA extraction from ticks was performed as previously described (Cao et al. 2000). Briefly, each tick was placed into a microtube and mechanically disrupted with sterile scissors in 50 μl DNA extraction buffer (10 mM Tris/HCl pH 8.0, 2 mM EDTA, 0.1 % SDS, and 500 μg proteinase K ml−1). The sample was incubated for 2 h at 56 ° C, and then boiled at 100 °C for 10 min to inactivate proteinase K. After centrifugation, the supernatant was transferred to a fresh sterile microtube and purified by extracting twice with an equal volume of phenol/chloroform before use in PCR.
16S rRNA gene PCR
A conventional PCR was performed with primers designed to amplify the 16S ribosomal RNA (16S rRNA) gene of the Borrelia spp. Primers rec4 (5′-ATG CTA GAA ACT GCA TGA-3′) and rec9 (5′-TCGTCTGAGTCCCC-ATCT-3′) were used as previously described (Ras et al. 1996). Theses primers are expected to yield a 523-bp PCR products regardless of Borrelia species. PCR amplification were performed in a volume of 30 μl containing 3 μl 10xPCR buffer (containing 100 mM Tris/HCl, 500 mM KCl, 15 mM MgCl2), 0.3 μl Taq DNA polymerase (5 U μl−1), 0.3 μl dNTP mix (10 mM) (all from Bioneer, S. Korea), 17.4 μl deionized water, 5 μl DNA template and 2 μl of each primer (10 μM). The cycling conditions for amplification involved 5 min denaturation at 95 °C, followed by 35 cycles of 94 °C for 60 s, 45 °C for 60 s and 72 °C for 120 s, and a final extension at 72 °C for 7 min. In parallel with each amplification, a positive control (previously extracted DNA of standard B. persica from Pasteur Institute of Iran) and two negative controls including previously extracted DNA of standard B. microtti from Pasteur Institute of Iran and distilled water were included.
All the PCR products were separated by 1.5% agarose gel electrophoresis, stained with ethidium bromide, and visualized under UV light. To minimize contamination, DNA extraction, the reagent setup, amplification, and agarose gel electrophoresis were performed in separate rooms.
RFLP analysis
In order to identify the species of Borrelia spp. infecting the ticks, the positive PCR products were analyzed by RFLP as described by Oshaghi et al. (2010). Previously extracted DNA of standard B. persica was used as positive controls. For each positive sample, 10–15 μl amplified DNA was digested at 65 °C overnight with endonuclease TaqI (Fermentas) according to the manufacturer’s recommendations. Electrophoresis was conducted in 2% agarose gel at 100 V for 5 min and then 80 V for 1 h. The gels were stained with ethidium bromide, and visualized under UV light. A 100 bp DNA Ladder Marker (Cinagen, Fermentas) was used as a molecular size marker. RFLP profile of B. persica was identified by digestion of 523-bp into 324- and 199-bp according to Oshaghi et al. (2010). 16S rDNA PCR products of B. microtti was used in parallel to compare the RFLP profiles of both species.
DNA sequencing of PCR product
In order to confirm the species of Borrelia spp. infecting the ticks, a sample from those PCR products that showed unique RFLP profile was selected for PCR-direct sequencing. The nucleotide sequences were determined by a dideoxynucleotide cycle sequencing method with an automated DNA sequencer (Seqlab, Germany). The sequence obtained in the present study was compared with the previously published sequences deposited in GenBank using the BLASt program from the National Center for Biotechnology Information website (http://www.ncbi.nlm.nih.gov/BLAST). The accession number of the 16S rRNA gene sequence obtained in this study is EU914141.
Results
Tick prevalence and distribution
A total of 1021 soft ticks of three species of Ornithodouros tholozani (120: 11.75%), O. lahorensis (461: 45.15%) and Argas persicus (440: 43.1%) were collected and tested against Borrelia infection. Soft ticks were more prevalent (67%) in infected areas than none infected areas. The rate O. tholozani in infected areas was 29 times more than none infected areas. 97% of soft ticks in none infected areas were of O. tholozani. On average, number of females was twice as males. Ornitodorous lahorensis with 45.15% was the most common species in the study area. Among ten previously infected villages, O. tholozani specimens were found only in four villages of Mehin, Meshkin, Andagh, and Ghalat. In none previously infected villages, different species of Ornitodorous spp. were present in ten out of 21 villages, though O. lahorensis with 97.9% was the most prevalent soft ticks.
Borrelia persica prevalence in ticks
DNA of B. persica was detected by a conventional PCR specifically targeting the 16S rRNA gene. A total of 344 soft ticks including 95 O. tholozani, 205 O. lahorensis, and 44 A. persicus were selected based on the collection sites and individually examined by PCR against Borrelia genome. The target region (16S rDNA) was successfully amplified for 5.5% (19/344) of the investigated ticks. Sixteen (16.7%) ticks of O. tholozani were infected with B. persica. Three (1.3%) soft ticks of O. lahorensis were also positive for Borrelia sp., but no infection was found in A. persicus. So, two Borrelia species, B. persica and Borrelia sp. (unknown) were identified by PCR-RFLP: B. persica was the most frequently detected species (16/19, 84.2%). All of the infected ticks with B. persica collected in the sites previously TBRF was reported whereas 100% of ticks infected with Borrelia sp. were found in none previously TBRF reported areas. Infection rates of ticks with respect to the area of sample collection are shown in Table 1.
Table 1.
PCR-based Borrelia spp infection rate in O. tholozani and O. lahoriansis in Takistan
| Area studies | O. tholozani | O. lahoriansis | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| No. tested |
Infected (%)
|
No. tested |
Infected (%)
|
||||
| B. pesrica | B. sp | B. pesrica | B. sp | ||||
| Infected | 96 | 16 (16.6) | 0.0 (0) | 95 | 0.0 (0) | 0.0 (0) | |
| Clean | 4 | 0.0 (0) | 0.0 (0) | 130 | 0.0 (0) | 3(2.3) | |
| Total | 100 | 16 (16) | 0.0 (0) | 225 | 0.0 (0) | 3(1.3) | |
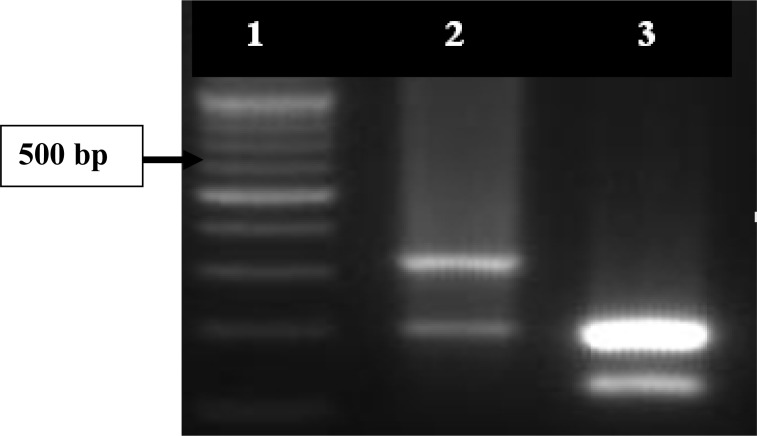
TaqI RFLP and sequence analysis of the amplified products from O. tholozani ticks specimens resulted in unique profile. On the basis of TaqI RFLP patterns of positive controls of B. persica the profiles obtained in this study consist of restriction pattern for B. persica species (Fig. 1).
Fig. 1.
PCR-RFLP profile of 523bp of 16SrDNA gene of Borrelia persica (no. 2) and B. microtti (no. 3) digested by TaqI restriction enzyme (Cinnagen). No. 1: Molecular size marker (100 bp Cinnagen), B. persica PCR was broken down into two fragments of 324 bp and 199 bp and B. microtti into three fragments of 205, 199, and 119 bp.
Discussion
In this study we confirmed the presence of B. persica in 16.7% of the soft tick O. tholozani in the previously reported TBRF areas of Takistan district. This infection rate is higher than the prevalence of 3.7% in the same tick species that were previously collected in Qazvin, neighbor district of Takistan (Aghighi et al. 2007). This is mainly attributable to sensitivity of PCR methods in comparison with microscopic following xenodiagnosis (Rafinejad et al. 2010). In addition, in this study we could use the PCR technique for either dead or alive, and 4 different development stage of ticks. However, this prevalence is comparable to the overall prevalence average in Iran and other countries. This rate was 3.7% in Hamadan (Ghaderi 2001), 8.8% in Qazvin (Aghighi et al. 2007), 20% in different areas of Iran (Rafiei and Rak 1986), 36.6% in Semnan (Nekoui et al. 1999), and 2–40% in other parts of the world (Assous and Wilamowoski 2009). These differences in infection rates among localities could be attributable to geographical and seasonal variations of infected ticks or to different sampling approaches and examination methods. Among these factors, the examination method is more critical since the classical method, which is mainly based on xenodiagnosis, is extremely laborious, slow, expensive, and requires preparation of the samples. Also we should consider the fact that the density of B. persicai in field ticks is very low and hard to be detected by the classical methods.
In Takistan, people are involved with poultry and animal husbandry and there is close relationship between people and animals. Also there is an abundant small wild animals such rodent and canine fauna, thus it can be suggested that B. persica or other Borrelia spp. are maintained particularly in enzootic cycles involving the wild and domestic mammals in the region.
The two soft ticks of O. lahorensis and A. persicus, which are mainly distributed in Takistan, lack any B. persica spirochetes. In spite of high prevalence (41.15%) of O. lahorensis in the area, it was not infected with B. persica. Instead, it was found infected with an unknown Borrelia species. Similarly, other investigators have already revealed infection of O. lahorensis with unknown Borrelia in Iran (Arshi et al. 2002) and Nablos in Lebanon. The results of our research and previous studies on infection of O. lahorensis to Borrelia sp needs further investigations to identify the spirochetes presence in this particular soft tick.
Argas persicus, with 43% prevalence, which are mainly distributed in Takistan (43%), was not found infected with any kind of Borrelia. These diversities in vectorial capacity of different tick species is attributable to the highly specific Borrelia-tick interactions as each species of Borrelia is only transmitted by one or a few closely related species of ticks. For example, O. tholozani is the main vector of B. persica, and O. erraticus is vector of B. microtti (Assmar et al. 2002, Masoumi Asl et al. 2009). In Azerbaijan, O. verrucosus is a vector of B. caucasica, which causes a very severe TBRF. In Iran and the countries of Central Asia of the former USSR, O. tartakowskyi is the vector of B. latyshewii, which causes a very mild RF without apparent clinical relapses (Parola and Raoult 2001). Ornithodouros sonrai is recognized as the only vector of B. crocidurae causing human relapsing fever in West Africa (Vial et al. 2006). The mechanisms responsible for this strict species specificity in transmission of one species of spirochete by only one species of tick are not known (Schwan and Piesman 2002).
In conclusion, pathogenic B. persica infection in O. tholozani ticks poses a potential health threat both to local residents and to tourists and animals in this area. It will be necessary to alert public health officials and clinicians about the existence of B. persica-positive in ticks in previously infected areas of Takistan district to continue vector control measures.
Acknowledgments
This work was supported by a grant from Tehran University of Medical Sciences (TUMS) (Grant no. 86-03-27-6268) to M. A. Oshaghi. We thank from manager of Health Office of Qazvin Province and Takistan Health Center. We also thank Eng, Taghi Satvat, Mrs S. Charedar from TUMS for technical assistance. The authors declare that they have no conflicts of interest.
References
- Aghighi Z, Assmar M, Piazak N, Javadian E, Seyedi Rashti MA, Kia EB, Rassi Y, Vatandoost H. Distribution of Soft Ticks and Their Natural Infection with Borrelia in a Focus of Relapsing Fever in Iran. Iran J Arthropod-Borne Dis. 2007;1:14–18. [Google Scholar]
- Arshi S, Majidpoor A, Sadeghi H, Asmar M, Emdadi D, Derakhshan MH. Relapsing fever in Ardabil, a northwestern province of Iran. Arch Iranian Med. 2002;5:141–145. [Google Scholar]
- Assmar M, Soleimani M, Oreizi F, Piazak N, Hossini SM, Saghiri R, Zamani Z. Purification of Periplasmic Flagellar Antigen from Borrelia microtti. Scand J Infect Dis. 2002;34:267–272. doi: 10.1080/00365540110080575. [DOI] [PubMed] [Google Scholar]
- Assous MV, Wilamowoski A. Review of relapsing fever borreliosis in Eurasia-forgotten, but certainly not gone. Clin Microbiol Infec. 2009;15(5):407–414. doi: 10.1111/j.1469-0691.2009.02767.x. [DOI] [PubMed] [Google Scholar]
- Barbour AG, Hayes SF. Biology of Borrelia species. Microbiol Rev. 1986;50:381–400. doi: 10.1128/mr.50.4.381-400.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao WC, Gao YM, Zhang PH, Zhang XT, Dai QH, Dumler JS, Fang LQ, Yang H. Identification of Ehrlichia chaffeensis by nested PCR in ticks from southern China. J Clin Microbiol. 2000;38:2778–2780. doi: 10.1128/jcm.38.7.2778-2780.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerar T, Ogrinc K, Cimperman J, Lotri-Furlan S, Strle F, Ruzić-Sabljić E. Validation of Cultivation and PCR Methods for Diagnosis of Lyme Neuroborreliosis. J Clin Microbiol. 2008;46(10):3375–3379. doi: 10.1128/JCM.00410-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada-Peñ AA, Bouattour A, Camicas JL, Walker AR. A Guide to Identification of Species. University of Zaragoza Press; Zaragoza, Spain: 2004. Ticks of Domestic Animals in Mediterranean Region. [Google Scholar]
- Ghaderi A. Study on distribution of soft ticks in indoor and outdoor and potential infection of O.tholozani to B. persica in Hamadan. School of Public Health, Tehran University of Medical Sciences; Iran: 2001. [MSc Thesis]. [Google Scholar]
- Horka H, Cerna-Kyckova K, Fiserova L, Kopecky G. Efficiency of experimental infection of Ixodes ricinus ticks with Borrelia burgdorferi spirochetes. Int J Med Microbiol. 2008;298:177–179. [Google Scholar]
- Karimi Y. 1980. Recurrent fever and its epidemiology Tehran, Iran: Pasteur Institute of Iran Publications 55–58.
- Karimi Y, Hovind-Hougen K, Birch-Andersen A, Asmar M. Borrelia persica and B baltazardi sp. nov.: experimental pathogenicity for some animals and comparison of the ultrastructure. Ann Microbiol (Paris) 1979;130B:157–168. [PubMed] [Google Scholar]
- Masoumi Asl H, Goya MM, Vatandoost H, Zahraei SM, Mafi M, Asmar M, Piazak N, Aghighi Z. The epidemiology of tick-borne relapsing fever in Iran during 1997–2006. Travel Med Infect Dis. 2009;7(3):160–164. doi: 10.1016/j.tmaid.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Moemenbellah-Fard MD, Benafshi O, Rafinejad J, Ashraf H. Tick-borne relapsing fever in a new highland endemic focus of western Iran. Ann Trop Med Parasitol. 2009;103(6):529–537. doi: 10.1179/136485909X451852. [DOI] [PubMed] [Google Scholar]
- Nekoui N, Asmar M, Amirkhani A, Piazak N. Distribution of ticks and their association with Borrelia in Semnan province. Iranian J Publ Health. 1999;28:103–109. [Google Scholar]
- Oshaghi MA, Rafinejad J, Choubdar N, Piazak N, Vatandoost H, Telmadarraiy Z, Mohtarami F, Maleki Ravasa N. 2010. Discrimination of Relapsing Fever Borrelia persica and B. microtti by Diagnostic Species-Specific Primers and PCR-Restriction Fragment Length Polymorphism. Vector Borne and Zoonotic Disease, Epub ahead of print (In Press). [DOI] [PubMed]
- Parola P, Raoult D. Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin Infect Dis. 2001;32:897–928. doi: 10.1086/319347. [DOI] [PubMed] [Google Scholar]
- Rafiei A, Rak H. Arthropod Parasitology. Tehran University Publication 1986 [Google Scholar]
- Ras NM, Lascola B, Postic D, Culter SJ, Rodhain F, Baranton G, Raoult D. Phylogenesis of relapsing fever Borrelia spp. Int J Syst Bacteriol. 1996;46(4):859–865. doi: 10.1099/00207713-46-4-859. [DOI] [PubMed] [Google Scholar]
- Rafinejad J, Oshaghi MA, Choubdar N, Piazak N, Savat T, Mohtarami F, Banafshi O, Taghiloo B, Maleki N. Diagnostic value of PCR for detection of Borrelia persica DNA in soft ticks. Iranian J Public Health. 2010. In press. [PMC free article] [PubMed]
- Rebaudet S, Parola P. Epidemiology of relapsing fever borreliosis in Europe. FEMS Immunol Med Microbiol. 2006;48:11–15. doi: 10.1111/j.1574-695X.2006.00104.x. [DOI] [PubMed] [Google Scholar]
- Rauter C, Hartung T. Prevalence of Borrelia burgdorferi sensu lato genospecies in Ixodes ricinus ticks in Europe: a metaanalysis. Appl Environ Microbiol. 2005;71:7203–7216. doi: 10.1128/AEM.71.11.7203-7216.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz I, Varde S, Nadelman RB, Wormser GP, Fish D. Inhibition of efficient polymerase chain reaction amplification of Borrelia burgdorferi DNA in blood-fed ticks. Am J Trop Med Hyg. 1997;56:339–342. doi: 10.4269/ajtmh.1997.56.339. [DOI] [PubMed] [Google Scholar]
- Zaim M, Shayeghi M. 1989. Pictorial Key of Mites and Ticks. Tehran University of Medical Sciences, Faculty of Health Publication. (In Persian).