Abstract
Background:
Culex pipiens complex shows variations in morphological and biological characters including different biological forms and has medical and veterinary importance. Because of having morphological variations, sometimes it is not easy to separate this species from Cx. quinquefasciatus and Cx. torrentium. The aim of this study was to identify the Culex pipiens complex species in order to use in control programs in the future.
Methods:
This study was carried out in two randomly selected rural villages in Yazd County, eastern Iran using dipping technique from April to October 2009. The data were analyzed using SPSS software version 16.
Results:
Average of siphon index in fourth-instrar larvae was 3.86±0.03, the minimum and maximum were calculated 2.43 and 5.14, respectively. Siphon/Saddle index was measured as average, minimum and maximum 3.2±0.2, 2.78, and 4.42 respectively. In our study, only 4 specimens had single seta 1 on segments III and VI (2.5%) and the remaining beard double seta (97.5%). The maximum 3–6 branches seta 1a-S and 1b-S (95%) were observed on siphon.
Conclusion:
More populations of Culex pipiens from different areas of Iran need to be studied to gain complete information about the taxonomy and ecology of the species in the country.
Keywords: Culex pipiens complex, larvae, taxonomy, Iran
Introduction
Culex pipiens Linnaeus shows a wide morphological and biological variations and well known as a broad research topic by many researchers (Knight and Malek (1951). This complex includes several members of the same morphological form and has different variations in physiology and behavior. The species group includes more than 75 binomial names due to the complexity of synonyms (Knight 1978). Barr (1982) believes that the concept of classical complex for the geographical populations. He mentioned Cx. pipiens and Cx. quinquefasciatus as a simple geographical population, which has a great distribution. Ishii (1991) expressed that there are variation among the characteristics of this complex and should not considered being independent species. There are some degree of intra species such as subspecies, variety and form among the members of this complex (Vinogradova 2000). Culex pipiens, Cx. quinquefasciatus and Cx. pipiens form molestus are the most common members of this complex and are widespread in the world (Cornel et al. 2003).
Culex pipiens has wide distribution from Europe, next to tropical regions of Asia and Africa till central part of North America, and one third of South Australia and South America. Culex quinquifasciatus has been distributed in tropical areas and has overlap extensively with Cx. pipiens (Vinogradova 2000). Hybrid forms of this species have been distributed in Australia, Africa, the Middle, and Far East of Asia, North and South America. The habitat of Cx. pipiens form molestus is temperate climates while Cx. quinquefasciatus considered cosmotropical species (Bourguet et al. 1998). Culex pipiens has more adaptation to larval habitats such as the pit containing high degree of waste water up to cavity include dishes of fresh water and presented as the most common species in the subgenus Culex in southwestern Asia (Harbach 1988). In Iran, Cx. pipiens and its form molestus and Cx. quinquefasciatus are recorded (Azari-Hamidian 2007). Culex pipiens (including form molestus) has a wide distribution in most areas of Iran and Cx. quinquefasciatus is recorded in seven southern provinces of the country (Zaim 1987).
Culex pipiens has the major role in human nuisance due to biting. Wuchereria bancrofti and Brugia malayi transmitted by Cx. pipiens and distributed in tropical and subtropical areas of Africa, Asia, Australia, and Pacific Islands. Some arbovirus infections have been transmitted by Cx. pipiens such as West Nile fever, Sindbis, Japanese Encephalitis, St Louise Encephalitis, Western Equine Encephalitis, Rift Valley fever, Tahyna, and Oropouche. Plasmodium gallinaceum and P. relictum, causes of avian malaria, are transmitted by this species (Vinogradova 2000).
Culex pipiens form molestus is separated from Cx. pipiens mainly by ecological and physiological characters. Larvae of this species have been found in underground habitat including the storage of water, septic tanks and sanitary space. Culex pipiens larval habitat is open space accumulated with artificial water.
The main and reliable characters for diagnosis in larval stage are siphon index, siphon/saddle index, the branch number of seta 1a-S and 1b-S and seta 1 of the abdominal segments of III and IV, and the shape of siphon (Harbach 1988, Azari-Hamidian and Harbach 2009).
The Siphon Index has been used widely for separating the members of this complex. The index is defined as the ratio of siphon length to its basal width. Many researchers have been reported considerable variations in Cu. pipiens and Cx. pipiens form molestus, also Harbach (1988) has rejected this character as diagnostic feature for these forms. The siphon index of Cx. pipiens larvae changes based on geographical and ecological variations (Vinogradova 2000). In addition, considerable variations have been cited, for example siphon index of species are grown in contaminated water are shorter than the species reared in the fresh water. Nevertheless, there are considerable ranges due to length of siphon inside the complex species (Harbach 1988). Changes in habitats, locations, sex composition is reflected in larval siphon and can be used for diagnosis analysis among the complex species (Vinogradova 2003). The average of siphon index is correlated to the certain types of larval habitats in ground or underground places. The average index siphon not only correlated with larval habitats, but also has relation to geographical distribution. This index for species in above ground habitats is stronger than other species based on comparing larval habitats. Culex torrentium shows close relationship to the members of Cx. pipiens complex based on morphological characters, even some researchers proposed it as a member of Cx. pipiens complex (Vinogradova 2000, Smith and Fonseca 2004). Harbach (1988) classified Cx. torrentium with Cx. vagans in the Trifilatus Subgroup of the Pipiens Group. This species with Cx. pipiens complex is sympatric in Europe and some areas of Asia including Iran (Azari-Hamidian et al. 2009).
According to Harbach (1988), the most important diagnostic characters of Cx. torrentium, Cx. quinquefasciatus and Cx. pipiens larvae are the branch of seta 1 of abdominal segment of III–V. Cx. quinquefasciatus and Cx. pipiens are sympatric in some parts of the world that their distributions have overlap situation (Jupp 1978).
Since the morphological variations and biological diversity of Culex pipiens can be found in different populations, each study possibly obtains more accurate data of the variability of Cx. pipiens complex to provide a more reasonable discussion about the applied taxonomic issue. It is evident that the final decision about the taxonomic status needs more complete information which should be obtained from different ways among the complex populations.
The aim of this study was to identify the Culex pipiens complex species in order to use in control programs in the future.
Materials and Methods
Study area
A study was carried out from April to October 2009 in Yazd Province, Iran. This study was performed in Zarch and Shahediyeh, two randomly selected rural villages in Yazd County (54°04′N–31°59′E). The province is bounded by Isfahan Province in the west, South Khorasan Province in the east, Kerman and Fars Provinces in the south, Razavi Khorasan and Seman Provinces in the north (Fig. 1). In 2009 the maximum and minimum mean monthly temperatures were 41° C in July and −4.4 °C in January, respectively. The total annual rainfall was 62 mm, the minimum 0.3 mm in May and maximum 18 mm in March. The mean annual relative humidity was 37%. The main occupations are agriculture and husbandry of cow and goat. Based on the available epidemiological data from Yazd Health Center, there are no villages under the entomological survey. Based on this information, two rural areas with 889 houses and 24358 populations were selected.
Fig. 1.
Map of Iran indicating the location of Yazd County situated in the center of Yazd Province
Mosquito sampling and morphological studies
In the present study, two cities of Shahediyeh and Zarch were selected and sampling was carried out in selected larval habitats. The larvae of each habitat collected by dipping technique, were separately transported to the laboratory of Medical Entomology, School of Public Health, Tehran University of Medical Science, Iran and identified using the keys of Zaim and Cranston (1986) and Azari-Hamidian and Harbach (2009).
In order to morphometric studies, the samples of Culex pipiens larvae were separated and sent to the Entomology Laboratory, Department of Entomology and Parasitology, School of Medical Sciences, Tarbiat Modarres University. The fourth-instar larvae were preserved in lactophenol and the microscope slides were prepared using d’Faure’s medium. The diagnostic characters of larvae were measured carefully using a microscope equipped with a glass lens dial.
The data were recorded in specific forms according to sampling method, location, and date of collection and were analyzed using SPSS software ver. 16.
Results
The siphon index was measured in 176 fourth-instar larvae. The average siphon index was 3.86±0.03, the minimum were calculated 2.43 and the maximum 5.14. This index had the most frequency in 159 specimens (90%) in the range of 3.3–4.8. Siphon/Saddle index of 176 larvae was measured as average, minimum and maximum 3.2±0.2, 2.78, and 4.42 respectively. This index showed the most prevalence in 174 larvae (99%) with the range of 2.7–3.95.
In addition, the larval abdominal seta 1 on segments III and IV and its branches were studied. This character was analyzed in 159 larvae, only 4 had single seta (2.5%) and the rest bifid seta (97.5%) indicating the presence of Culex pipiens in the area. The number of the branches of seta 1a-S and 1b-S was 3–8 and the most prevalence branches were 3–6 (95%) on siphon. The most number of seta on the siphon was 8–9. In our research, the siphon was gradually narrows in 96% of the samples from base toward the apex. These features of Cx. pipiens larvae are presented in Table 1.
Table 1.
The variations of some morphological characters of siphon in Culex pipiens larvae, Yazd Province, Central Iran, 2009–2010
| Character | n | Distinctiveness | Prevalence | % |
|---|---|---|---|---|
| Seta 1 abdominal segment III and IV | 159 | Single | 4 | 2.5 |
| Double | 155 | 97.5 | ||
| Siphon Seta 1a-S and 1b-S | 158 | 3 | 22 | 14 |
| 4 | 52 | 33 | ||
| 5 | 52 | 33 | ||
| 6 | 24 | 15 | ||
| 7 | 6 | 4 | ||
| 8 | 2 | 1 | ||
| Siphon seta number | 127 | 6 | 12 | 9.5 |
| 8 | 85 | 67 | ||
| 9 | 18 | 14 | ||
| 10 | 12 | 9.5 | ||
| Siphon shape | 181 | Gradually narrowing | 173 | 96 |
| Wide in the middle | 8 | 4 |
Discussion
Culex pipiens complex is cosmopolitan species. The distribution of the member of this complex in Iran is not completely known using reliable characters for identification and a few studies have been performed in this field (Zaim 1987, Azari-Hamidian and Harbach 2009, Azari-Hamidian et al. 2009, Azari-Hamidian et al. 2010).
The larval chaetotaxy and siphon characters are very important in the taxonomy of the genus Culex. In our research three related characters including: siphon index, siphon/saddle ratio, and chaetotaxy were studied.
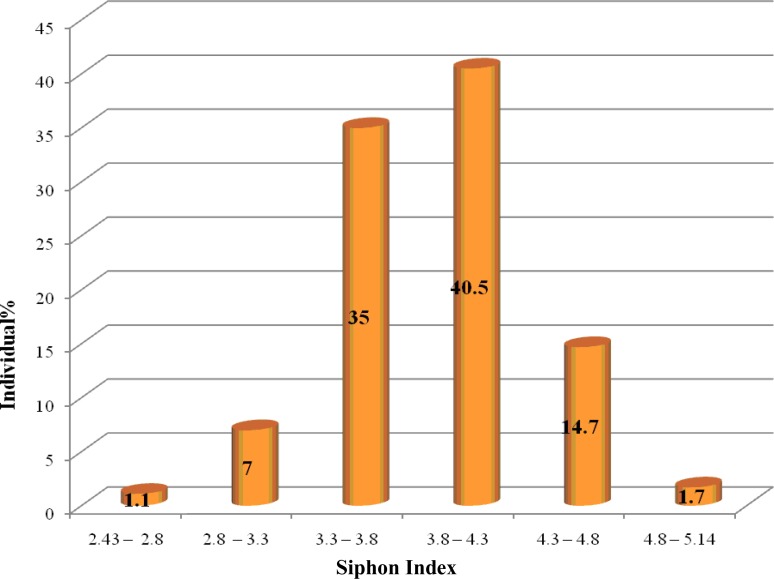
Siphon index was studied in 176 samples and was obtained in average 3.86 and the range of 2.43–5.14. Siphon index was calculated as 2.8–4.8 in 97% of the samples and shown with a scattering in Fig. 2. In similar study in Egypt, this index was reported as 3.7 in average and the range 2.7–4.7 for Cx. pipiens (Knight and Malek 1951). Harbach (1988) reported the average 4.58 and the range 3–5.8 for Cx. pipiens. Ishii (1991) stated the overall ratios of less than 4 as indicator for mosquito larvae developed in pollutant water, whereas high ratio greater than 4.4 for the specimens caught in larval habitats with fresh water. On the other hand, there are some reports about relation between the average of siphon index and underground or above ground larval habitats. Culex pipiens found in above ground habitats, whereas Cx. pipiens form molestus chooses underground habitats. The range of siphon index was reported 4.8–6.2 for 80% of Cx. pipiens populations and was found 3.3–4.4 for 90% of the Cx. pipiens form molestus populations (Vinogradova 2000).
Fig. 2.
The range of siphon index in Culex pipiens, Yazd Province, Iran, 2009–2010
Considering the samples collected in Yazd Province from above ground in all larval habitats, siphon index was compared based on the average amount of water pollution with organic matter. The results of the finding showed that the average of the index was 3.65 in 63 samples found in sewage water near the households. The average index was 4 in 113 specimens collected from the cistern water pool near homes and livestock shelters. The diversity of this index shows relation to larval habitats with fresh water to contaminated water with high levels of organic matter.
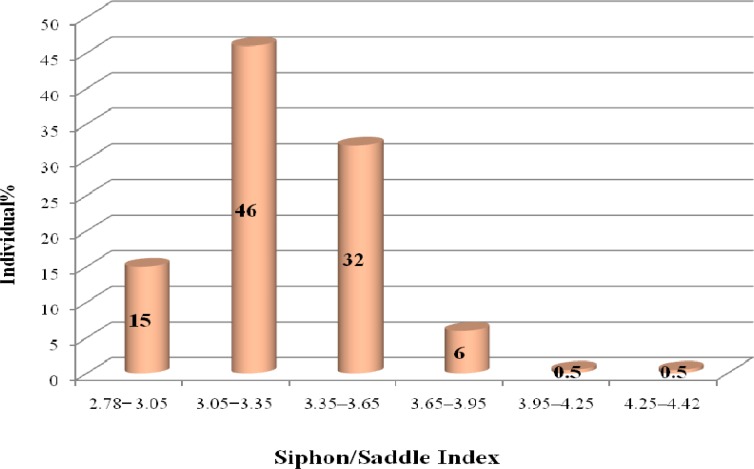
The siphon/saddle index and seta 1 of abdominal segment of III–IV are the main characters for the reliable differentiation of Cu. pipiens from Cx. quinquefasciatus (Harbach 1988, Azari-Hamidian and Harbach 2009). Moosa-Kazemi et al. (2010) identified Cx. pipiens using these characters in Kurdistan Province. In our study, the average rate of the siphon/saddle index was 3.2 with the maximum 4.42, and the minimum 2.78. The diversity of this index was low and in 99% of the samples was calculated as a range between 2.7–3.95 (Fig. 3). The average of this index was reported 4.08 and the range 3.48–4.63 for Cx. pipiens and was mentioned the range 2.77–3.41 for Cx. quinquefasciatus by Harbach (1988). The ratio of this index was reported more than 3.45 for Cx. pipiens and less than 3.45 for Cx. quinquefasciatus by Azari-Hamidian and Harbach (2009).
Fig. 3.
The range of siphon/saddle index in Culex pipiens, Yazd Province, Iran, 2009–2010
In our study, we found the present of double seta 1 of abdominal segment of III–IV in 96.9% of the samples. In parallel to Harbach (1988) and Azari-Hamidian and Harbach (2009), this character attributed to Culex pipiens.
Siphonal seta 1-S of Culex pipiens complex, especially 1a-S and 1b-S, was used for identification, particularly Cx. pipiens and Cx. quinquefasciatus. In our study, siphonal seta 1a-S and 1b-S were observed in 158 samples, and was found range 3–8 branches, whereas 95% of the samples have 3–6 branches. Harbach (1988) cited the variation of the number of branches and mentioned the more branches of seta in Cx. quinquefasciatus than Cx. pipiens. In agree with the present research, Knight and Malek (1951) reported an average 4 and a range 2–9 branches in Cx. pipiens. In our study, seta 1-S was counted in 127 larval samples and 8–9 setae were observed in 103 samples (81%). The most number of tuft setae (10 setae) were counted in 12 samples (Table 1). Harbach (1988) mentioned four pairs of seta 1-S in Cx. pipiens, sometimes fifth tuft was observed on one or both sides of siphon.
Shape of siphon was studied in relation to siphon width in the middle and its base in 181 larvae. This character in the middle was less than in the base in 173 larvae samples, and was wide in the middle of others (Table 1). In general, siphon of Cx. pipiens is longer than in Cx. quinquefasciatus (Harbach 1988, Azari-Hamidian and Harbach 2009). This feature can be used for the differentiation of Cx. pipiens and Cx. quinquefasciatus.
In conclusion, our study indicated that siphon/saddle index is specific diagnostic character, in contrast, siphon index has relative diagnostic value due to more scattering range (Fig. 2 and 3). According to our observations, branch of seta 1 of abdominal segment of III–IV and shape of siphon in larval samples are valuable characters which can easily separate Culex pipiens and Cx. quinquefasciatus. According to the results reported previously, some characters were overlap in Cx. pipiens and Cx. quinquefasciatus, therefore to determine the species it is recommended studying all characters together and then making a decision about such a complex species. Based on our study, Cx. pipiens larvae were detect in Yazd area and in order to determination of the distribution of the complex species more study should be carried out in other areas of Iran.
Acknowledgments
The authors are grateful to Dr Akhond, PhD student in Biostatistics, School of Medical Sciences, Tarbiat-Modares University. The authors appreciate Mrs Akbari, Noruzi, and Ghasemi for their collaborations in the laboratory. Also the authors thank Mr Solaimani, Yazd Health Training and Research Center, Mr Kalantari, Head of Zarch City Council, Mr Rezaei, Mr Eslami, Environmental Health Director of Zarch and Shahediyeh Health Center, for supporting the investigation. Dr Azari-Hamidian, School of Health, Guilan University of Medical Sciences, is appreciated for reviewing the manuscript. We also would like to express our appreciation to the people of Abrand-Abad, Nosrat-Abad, Gerd-e-Faramarz, Elah Abad, and Sarcheshmeh Villages in Yazd County for their kind cooperation during the study. Many thanks also dedicated to the efforts of the field staff of the Yazd Health Training and Research Center. This study was financially supported by grant of Tarbiat Modares University of Medical Sciences and partly by Tehran University of Medical Sciences. The authors declare that they have no conflicts of interest.
References
- Azari-Hamidian S. Checklist of Iranian mosquitoes (Diptera: Culicidae) J Vect Ecol. 2007;32:235–242. doi: 10.3376/1081-1710(2007)32[235:coimdc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Azari-Hamidian S, Harbach RE. Keys to the adult females and forth-instar larvae of the mosquitoes of Iran. Zootaxa. 2009;2078:1–33. [Google Scholar]
- Azari-Hamidian S, Yaghoobi-Ershadi MR, Javadian E, Abai MR, Mobedi I, Linton Y-M, Harbach RE. Distribution and ecology of mosquitoes in a focus of dirofilariasis in northwestern Iran, with the first finding of filarial larvae in naturally infected local mosquitoes. Med Vet Entomol. 2009;23:111–121. doi: 10.1111/j.1365-2915.2009.00802.x. [DOI] [PubMed] [Google Scholar]
- Azari-Hamidian S, Linton Y-M, Abai MR, Ladonni H, Oshaghi MA, Hanafi-Bojd AA, Moosa-Kazemi SH, Shabkhiz H, Pakari A, Harbach RE. Mosquito (Diptera: Culicidae) fauna of the Iranian islands in the Persian Gulf. J Nat Hist. 2010;44:913–925. [Google Scholar]
- Barr AR. The Culex pipiens complex. In: Steiner WWM, Tabachnick WJ, Rai KS, Narang S, editors. Recent Developments in the Genetics of Insect Disease Vectors. Vol. 1. Champaign; Illinois, Stipes: 1982. pp. 551–572. [Google Scholar]
- Bourguet D, Fonseca D, Vouch G, Dubois MP, Chandre F, Severini C, Raymond M. The Acetylcholinesterase gene Ace: a diagnostic marker for the pipiens and quinquefasciatus forms of the Culex pipiens complex. J Am Mosq Control Assoc. 1998;14:390–396. [PubMed] [Google Scholar]
- Cornel AJ, McAbee RD, Rasgon J, Stanich MA, Scott TW, Coetzee M. Differences in extent of genetic introgression between sympatric Culex pipiens and Culex quinquefasciatus (Diptera: Culicidae) in California and South Africa. J Med Entomol. 2003;40:36–51. doi: 10.1603/0022-2585-40.1.36. [DOI] [PubMed] [Google Scholar]
- Harbach RE. The mosquitoes of the subgenus Culex in southwestern Asia and Egypt (Diptera: Culicidae) Contrib Amer Ent Inst. 1988;24:1–237. [Google Scholar]
- Ishii T. Integrated study on the Culex pipiens complex. Akaieka newsletter. 1991;14:35–40. [Google Scholar]
- Jupp PG. Culex (Culex) pipiens pipiens Linnaeus and Culex (Culex) pipiens quinquefasciatus Say in South Africa: morphological and reproductive evidence in favor of their status as two species. Mosq Syst. 1978;10:461–473. [Google Scholar]
- Knight KL. Supplement to a Catalog of the Mosquitoes of the World (Diptera: Culicidae) Thomas Say Foundation; College Park, MD: 1978. [Google Scholar]
- Knight KL, Malek AA. A morphological and biological study of Culex pipiens in the Cairo area of Egypt. Sot Fouad Ent Bull. 1951;35:175–185. [Google Scholar]
- Moosa Kazemi SH, Karimian F, Davari B. Culicinae mosquitoes in Sanandaj County, Kurdistan Province, Western Iran. J Vector Borne Dis. 2010;47:103–107. [PubMed] [Google Scholar]
- Smith JL, Fonseca DM. Rapid assays for identification of members of the Culex (culex) pipiens complex, their hybrids, and other sibling species (Diptera: Culicidae) Am J Trop Med Hyg. 2004;70:339–345. [PubMed] [Google Scholar]
- Vinogradova EB. Culex pipiens pipiens Mosquitoes: Taxonomy, Distribution, Eecology, Physiology, Genetics, Applied Importance and Control. Pensoft Publishers; Sofia-Moscow: 2000. [Google Scholar]
- Vinogradova EB. Ecophysiological and morphological variations in mosquitoes of the Culex pipiens complex (Diptera: Culicidae) Acta Soc Zool Bohem. 2003;67:41–50. [Google Scholar]
- Zaim M. The distribution and larval habitat characteristics of Iranian Culicinae. J Am Mosq Control Assoc. 1987;3:568–573. [PubMed] [Google Scholar]
- Zaim M, Cranston PS. Checklist and keys to the Culicinae of Iran (Diptera: Culicidae) Mosq Syst. 1986;18:233–245. [Google Scholar]