Abstract
Background:
Trypsin Modulating Oostatic Factor (TMOF) terminates trypsin biosynthesis in adult and larval mosquito gut. It will inhibit the growth and development of mosquito larvae feeding on it resulting in death by starvation. The objective of this study is to determine the effective dose of Pichia-TMOF and the combination of Pichia-TMOF with Bacillus thuringiensis (Bt) as larvicide on Ae. aegypti larvae.
Methods:
Aedes aegypti first-instar larvae were exposed to various doses of Pichia-TMOF, Bt and combination of Pichia-TMOF and Bt. The development of the larvae were observed and recorded daily during the bioassay larval test until the adult emergence.
Results:
The results showed that 400 ppm Pichia-TMOF and 300 ppm Pichia-TMOF were able to cause 100% and 67% cumulative mortality on Ae. aegypti larvae on 8th day respectively. At 200 ppm, 100 ppm and 50 ppm concentration Pichia-TMOF showed obvious stunted effect on Ae. aegypti larvae. Moreover, the combination of 400 ppm Pichia-TMOF with 0.1 ppm Bt showed synergistic effect on Ae. aegypti.
Conclusion:
Pichia-TMOF inhibited trypsin biosynthesis is potential to act in larval gut causing stunted growth and larval development and causing mortality. The combination of Pichia-TMOF and Bt increased the effectiveness in causing larval mortality.
Keywords: Pichia-TMOF, Bt combination, Aedes aegypti larvae
Introduction
Mosquitoes are the vectors for the dreadful diseases of mankind. Of all the insects that transmit diseases, mosquitoes represent the greatest menace. WHO has declared the mosquito “public enemy number one” because mosquitoes are responsible for the transmission of various dreadful diseases (WHO 1996). Dengue is the most common and widespread arthropod-borne arboviral infection in the world today. It is estimated that there are at least 100 million cases of dengue fever (DF) annually and 500,000 cases of dengue hemorrhagic fever (DHF) which require hospitalization. In Malaysia, it has become a major public health problem (Hussin 2005).
Chemical insecticides are the most important components of integrated vector control. However, safe and cost-effective insecticides are rapidly disappearing because of the development of resistance, abandonment of many compounds for reasons of environmental safety, and new registration requirements that are more stringent (Rose 2001). When Aedes aegypti (L) and Culex quinquefasciatus Say larvae were fed Trypsin Modulating Oostatic Factor (TMOF) that was adsorbed onto yeast cells the larvae stopped synthesizing trypsin and stopped growing (Borovsky and Meola 2004). These results indicate that shutting off trypsin biosynthesis with TMOF can be used as a new approach to control larval growth and development, possibly leading to new biorational insecticides, which are desperately needed (Zaim and Guillet 2002). Borovsky reported that the mosquito ovary is a rich source of ‘oostatic hormone’. Injections of the hormone into female mosquitoes inhibited yolk deposition and vitellogenin biosynthesis (Borovsky 1985).
Mode of action for TMOF is as follows: After application to water, TMOF is consumed by mosquito larvae who are aquatic particulate filter feeders. As the yeast is broken down by digestive processes, TMOF is released into the insect gut. The TMOF protein (a small decapeptide) then passes through the peritropic membrane and midgut wall into the hemolymph. TMOF then binds to specific receptors which, once activated, trigger a halt in trypsin-like enzyme translation (enzyme biosynthesis) and ultimately transcription (mRNA synthesis). The biochemical process of receptor activation is not well understood, though affected insects are unable to produce digestive proteases needed to provide essential amino acids, resulting in starvation (EPA 2003). TMOF can be degrades by vertebrate digestive protease and technical TMOF is not toxic to the non-target organisms examined (Deborah 2004).
According to Sallehudin (1990), Bacillus thuringiensis (Bt) is not toxic to human and others mammals and it is effective in Ae. aegypti control when put them in water container. However, the high cost of Bt, need for frequent application and possibility of resistance due to widespread application have severaly compromised its use. So the objective of this study is to evaluate the efficacy of TMOF and combination of TMOF with Bacillus thuringiensis on Ae. aegypti larvae in the laboratory.
Materials and Methods
The samples used were Pichia-Trypsin Modulating Oostatic Factor (TMOF) (P7) and Bacillus thuringiensis (Bt) were provided by EntoGenex Industries Sdn Bhd Malaysia and originally developed in the laboratory of Professor D Borovsky at the University of Florida. Aedes aegypti larvae was used in the experiment. Aedes aegypti was a laboratory strain orginated from the Institute for Medical Research (IMR).
Bioassay
The method was slightly modified from protocol for testing Pichia-TMOF with mosquito larvae by Professor D Borovsky (2006). Mosquito eggs were hatched a day or few hours before the test in the presence of Brewer’s yeast. The first instar larvae that just hatched were used for the test. Paper cup was filled with distilled water till 200 ml. 20 Aedes aegypti larvae were added to each paper cup.
Stock solutions of 10,000 ppm of Pichia-TMOF and 500 ppm Bt were prepared. 20 larvae were added to each container with different amount of Pichia-TMOF and Bt respectively. The doses used to test Aedes aegypti larvae were 400 ppm, 300 pm, 200 ppm, 100 ppm and 50 ppm Pichia-TMOF; 1 ppm, 0.1 ppm and 0.01 ppm Bt and combination of every concentration of Pichia-TMOF with each concentration of Bt. Each assay was run in duplicate and repeated 3 times for each assay. 400 ppm Pichia cells without TMOF or baker yeast was used as negative control while Bt with baker yeast and without baker yeast were used as positive control.
The test was run until all the larvae died or became adults. The larvae were considered dead if they did not wriggle or move after touching them gently with a micropipette tip. The dead larvae were removed from containers daily and no additional yeast was given as food after starting the test. Futhermore, the test was run in a laboratory at 27° C and 80% humidity. All the containers were covered with netting. The data were analysed statistically using ANOVA, independant T-test, Kruskal Wallis and Mann-Whitney.
Results
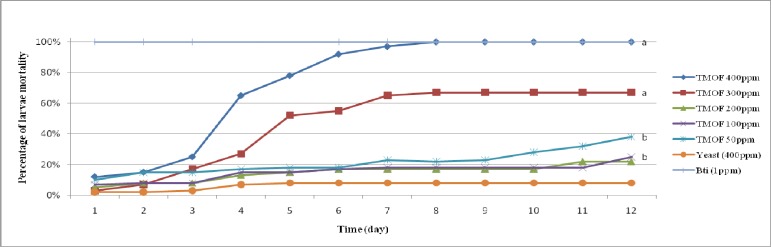
Figure 1 shows the effectiveness of Pichia-TMOF on cumulative mortality of Aedes aegypti larvae in 12 days. The graph shows that 400 ppm Pichia-TMOF increased exponentially on the 4th day and achieved 100% cumulative mortality on 8th day. This shows that 400 ppm Pichia-TMOF cause starvation of larvae to death. However from statistical analysis using Kruskal-wallis,there was significant different between the groups on 12th day χ2(5, N=18)= 12.552, P< 0.05. Analysis of Mann-Whitney was done and showed that there was a significant different between 400 ppm Pichia-TMOF and 300 ppm Pichia-TMOF with 400 ppm baker yeast (P< 0.05). However there was no significant different between 200 ppm, 100 ppm and 50 ppm Pichia-TMOf with 400 ppm baker yeast (P> 0.05). One ppm Bt is used as positive control.
Fig. 1.
Line graph shows effectiveness of Pichia-TMOF on Aedes aegypti by percentage of cumulative mortality (a: P< 0.05 compared to control, b: P>0.05 compared to control).
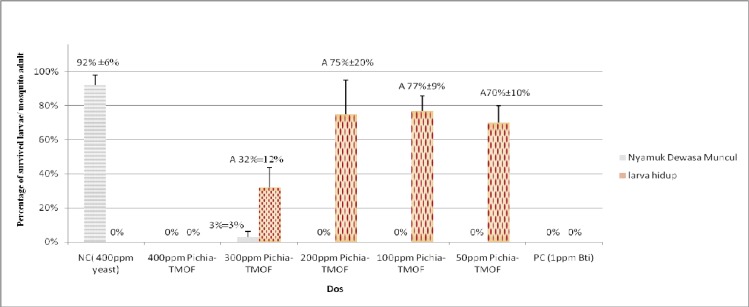
Figure 2 shows the percentages of development and survival of Ae. aegypti larvae treated with Pichia-TMOF. From the graph 300 ppm Pichia-TMOF, 200 ppm Pichia-TMOF, 100 ppm Pichia-TMOF and 50 ppm Pichia-TMOF show 32%, 75%, 77% and 70% of larvae still survived in larval stage on 12th day. According to the analysis of one way ANOVA, there is significant different between 300 ppm Pichia-TMOF, 200 ppm Pichia-TMOF, 100 ppm Pichia-TMOF and 50 ppm Pichia-TMOF with 400 ppm baker yeast (P< 0.05). This proved that the Pichia-TMOF is effective in stunted growth of Ae. aegypti larvae. Although 1ppm Bt used as positive control and show 100% mortality after few hours, in real life there is a lot of other factors in the environment that will affect the effectiveness of Bt such as natural food and sunlight.
Fig. 2.
Bar graph show percentages of development and survival of Aedes aegypti with treatment and without treatment (A: P< 0.05 compared with control,NC: negative control, PC: positive control).
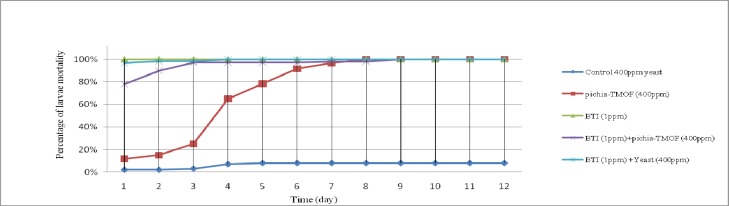
Figure 3 shows the cumulative mortality of combination Pichia-TMOF with 1 ppm Bt on Ae. aegypti larvae. One ppm Bt used as positive control while 400 ppm baker yeast as negative control showing 100% and 8% cumulative mortality respectively. Combination of 1 ppm Bt with 400 ppm baker yeast and 400 ppm Pichia-TMOF show rapid mortality than 400 ppm Pichia-TMOF. Pichia-TMOF at 8th day caused 100% cumulative mortality of Ae. aegypti larvae.Also shown that with the addition of Bt to TMOF will increase the rapidness of mortality of Ae. aegypti larvae.
Fig. 3.
Line graph show effectiveness of combination of Pichia-TMOF and 1 ppm Bt on Aedes aegypti by percentages of cumulative mortality
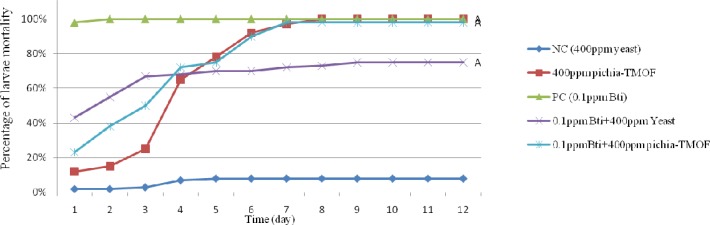
Figure 4 shows the cumulative mortality of combination Pichia-TMOF with 0.1 ppm Bt on Ae. aegypti larvae. 0.1 ppm Bt act as positive control which show 98% mortality after 24 hours and achieved 100% on day 2. From the graph, it is indicated that the combination of 0.1 ppm Bt with 400 ppm baker yeast shows higher percentage of cumulative mortality than combination of 0.1 ppm Bt with Pichia-TMOF in the first 3 days. However starting day 4 the percentage cumulative mortality of combination of 0.1 ppm Bt with Pichia-TMOF were higher than 0.1 ppm Bt with 400 ppm baker yeast and achieved 98% and 75% cumulative mortality on day 12 respectively.
Fig. 4.
Line graph show effectiveness of combination of Pichia-TMOF with 0.1 ppm Bt by percentages of cumulative mortality (A: P> 0.05 compared between group, PC: positive control,NC: negative control).
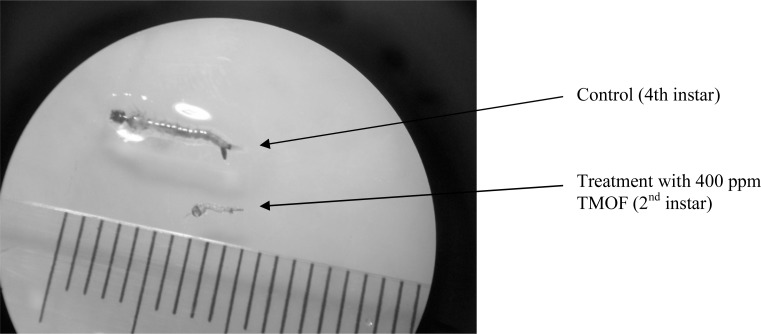
The Photo shows the different between development of larvae with the treatment of 400 ppm Pichia-TMOF and with 400 ppm baker yeast. Larvae with the treatment were dead on second instart while additional of 400 ppm baker yeast shows fourth instar on day 7 and be able to become adult.
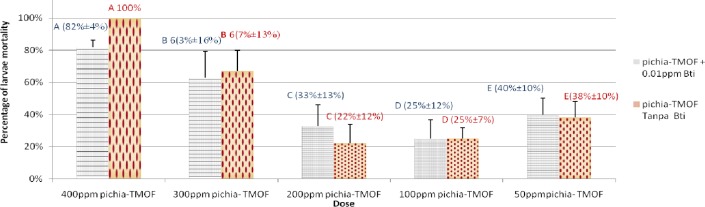
Table 1 shows the development and survival of Ae. aegypti with the treatment of combination of Pichia-TMOF and 0.01 ppm Bt on day 12.At day 12 all the larvae being given yeast as food emerged as adults. It indicated that 65%, 75% and 60% of larvae still survived on day 12 with the dose of combination 0.01ppm Bt with 200 ppm Pichia-TMOF, 100 ppm Pichia-TMOF and 50 ppm Pichia-TMOF respectively. When 0.01 ppm Bt is added to a higher concentration TMOF (400 ppm) larval survival was low at 5% and adult survival 7% respectively.This shows that with the addition of Bt do not affect the effectiveness of Pichia-TMOF to stunt the Ae. aegypti larvae.
Table 1.
Percentage of larval survival/adult survival after treatment with Pichia-TMOF on the 12th day
| Treatment | Larval survival (%) | Adults survived (%) |
|---|---|---|
| 0.01 ppm Bt plus 400 ppm yeast | 0 | 70±6 |
| 0.01 ppm Bt | 2±2 | 0 |
| 0.01 ppm Bt plus 400 ppm TMOF | 5±3 | 7±2 |
| 0.01 ppm Bt plus 300 ppm TMOF | 35±15 | 2±2 |
| 0.01 ppm Bt plus 200 ppm TMOF | 65±14 | 2±2 |
| 0.01 ppm Bt plus 100 ppm TMOF | 75±12 | 0 |
| 0.01 ppm Bt plus 50 ppm TMOF | 60±10 | 0 |
Discussion
In this study Pichia-TMOF was able to stunt the Aedes aegypti larvae. At 400 ppm Pichia-TMOF was able to cause 100% cumulative mortality of Ae. aegypti larvae within 8 days which maintain in second instar. Three hundred ppm Pichia-TMOF caused 67% cumulative mortality of Ae. aegypti in 12 days. The larvicidal effect decreased when the doses of Pichia-TMOF decreased but it still able to give significant stunted effect on Ae. aegypti. This study support the research of Prof. Borovsky which shows that first instar Ae. aegypti when fed with TMOF organic analogues in microtiter plates in the presence of Brewer’s yeast caused larval mortality within 5–6 days of the feeding (Borovsky 2007). According to Borovsky et al. (2010) Cry toxins and TMOF are synergists to Ae. aegypti larvae when jointly fed or expressed in recombinant Pichia pastoris.
From the study, Bt with the addition of baker yeast decreased the effect of Bt alone and it was shown when high concentration of baker yeast was present. Thus, in the present study the combination of Pichia-TMOF with Bt on Aedes aegypti caused the cumulative mortality of larvae decreased at first then achieve better effect compared to combination of baker yeast with Bt. This is probably due to the effect of the Pichia-TMOF which prevent the biosyntesis of trypsin and decreased the rate of digesting the crystal of Bt and caused the effect of Bt became slower. However, after sometimes when crystal is digested and toxin released increased the percentages of cumulative mortality. Combination of 0.1 ppm Bt with 400 ppm Pichia-TMOF gave the desired result on Ae. aegypti larvae because with the lower dose of Bt able to give 100% cumulative mortality on 8th day. In this case it shows synergistic effect because the effect is greater than 400ppm Pichia-TMOF alone and combination of 0.1 ppm Bt with 400 ppm baker yeast. The study shows that the combination of 0.01 ppm Bt with Pichia-TMOF is too low to give any significant effect compared with Pichia-TMOF alone. Borovsky (2008) and Borovsky et al. (2009) studied the posiblle synergism between TMOF and Bt toxins and found that TMOF enhances the effect of Bt toxins making the toxins more effective at low concentratins or in the presence of organic materials that negate their activity.
In conclusion, combination of lower Bt concentration with Pichia-TMOF is able to give better larvacidal effect on Ae. aegypti 1st instar larvae compared to Bt with added food. Combination of Bt with Pichia-TMOF could eliminate the development of resistance in Aedes mosquitoes and strengthen the dengue vector control programme.
Fig. 5.
shows the cumulative mortality of combination Pichia-TMOF with 0.01 ppm Bt and Pichia-TMOF on Ae. aegypti larvae on day 12. According to independent T-test, there was no difference between 400 ppm Pichia-TMOF and combination of 400 ppm Pichia-TMOF with 0.01 ppm Bt (P> 0.05), 300 ppm Pichia-TMOF and combination of 300 ppm Pichia-TMOF with 0.01 ppm Bt (P> 0.05), 200 ppm Pichia-TMOF and combination of 200 ppm Pichia-TMOF with 0.01 ppm Bt (P> 0.05), 100 ppm Pichia-TMOF and combination of 100 ppm Pichia-TMOF with 0.01 ppm Bt (P> 0.05) and also between 50 ppm Pichia-TMOF and combination of 50 ppm Pichia-TMOF with 0.01 ppm Bt (P>0.05).
Fig. 6.
Effectiveness of Pichia-TMOF and combination of 0.01 ppm Bt with Pichia TMOF on Aedes aegypti on day-12 (Min ± SEM)
Acknowledgments
We would like to thank EntogeneX Industry Sdn Bhd for financing the study through research grant NN001-2008. Also we wish to thank the Department of Biomedical Science, Faculty of Alied Health Sciences for providing research facilities.
References
- Borovsky D. Isolation and characterization of highly purified mosquito oostatic hormone. Arch Insect Biochem Physiol. 1985;2:333–349. [Google Scholar]
- Borovsky D. Oostatic hormone inhibits biosynthesis of midgut proteolytic enzymes and egg development in mosquitoes. Arch Insect Biochem Physiol. 1988;7:187–210. [Google Scholar]
- Borovsky D. Trypsin-modulating oostatic factor: a potential new larvicide for mosquito control University of Florida-IFAS. Florida Medical Entomology Laboratory; 200, 9th Street. SE Vero Beach, FL 332962, USA: 2003. [DOI] [PubMed] [Google Scholar]
- Borovsky D, Nauen R. Biological and biochemical effects of organo-synthetic analogues of Trypsin Modulating Oostatic Factor (TMOF) on Aedes aegypti, Heliothis virescens and Plutella xylostella. Pestycydy. 2007;3–4:17–26. [Google Scholar]
- Borovsky D, Meola SM. Biochemical and cytoimmunological evidence for the control of Aedes aegypti larval trypsin with Aea-TMOF. Arch Insect Biochem Physiol. 2004;55:124–139. doi: 10.1002/arch.10132. [DOI] [PubMed] [Google Scholar]
- Borovsky D. A new biopesticide against mosquitoes and agricultural pest insects. KualaLumpur Convention Centre Conference and Exhibition; 7–9 October 2008.2008. [Google Scholar]
- Borovsky D, Laeremans A, Theunis C, Bertier L, Khasdan V, Ben-Dov E, Zaritsky A. Does TMOF enhance the effect of BT toxins on larval Aedes aegypti? 2009. VIth ARTHROPODS, 21–26 June 2009, Ochotnica Dolna, Poland.
- Borovsky D, Khasdan V, Nauwelaers S, Theunis C, BertieOr L, Bendov E, Zaritsky A. Synergy between Aedes aegypti trypsin modulating oostatic factor and δ-endotoxins. The Open Toxin J. 2010;3:116–125. [Google Scholar]
- Deborah MT, Hugh PY, Frank WE, Allen WO, Gerald AL, Ernest H, Michel RR. Pest Biochem Physiol. 2004;80:131–142. [Google Scholar]
- EPA . 2004. Biopesticide registration action document Trypsin modulating oostatic factor (TMOF). [Google Scholar]
- Hussin N, Jaafar J, Naing NN, Mat HA, Muhamad AH, Mamat MN. A review of dengue fever in Kota Bharu, Kelantan, Malaysia during the years 1998–2003. Southeast Asian J Trop Med Public Health. 2005;36(5):1179–1186. [PubMed] [Google Scholar]
- Gagliardi J, Kough J. 2003. Review of submitted studies, background material and waiver requests to support registration of the manufacturing use product Skeetercide containing heat-killed Pichia pastoris yeast with integral Trypsin Modulating Oostatic Factor (TMOF), U.S. EPA.
- Rose RI. Pesticides and public health: integrated methods of mosquito management. Emer Infect Dis. 2001;7:17–23. doi: 10.3201/eid0701.010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallehudin S. Entomologi Perubatan. Bangi: Penerbit Universiti Kebangsaan Malaysia; 1990. [Google Scholar]
- WHO 1996. Report of WHO informal consultation on the evaluation and testing insecticides. CTD/WHO PES/IC/96.
- Zaim M, Guillet P. Alternative insecticides: an urgent need. Trends Parasitol. 2002;18:161–163. doi: 10.1016/s1471-4922(01)02220-6. [DOI] [PubMed] [Google Scholar]