Abstract
Background:
There is a little data on Coxiella burnetii (Q fever agent) in Iran. Ticks may play a significant role in the transmission of C. burnetii among animals. The aim of this study was to use polymerase chain reaction for the detection of C. burnetii in ticks collected in Southeast Iran.
Methods:
One hundred and sixty ticks were collected from domestic animals in three localities of Kerman Province, Southeast Iran from November to June 2009. The collected ticks were divided into 35 pools and examined by Trans-PCR for C. burnetii.
Results:
Three pools, each consisting of five female of Hyalomma anatolicum anatolicum and one pool (6 ticks) of Rhipicephalus sanguineus ticks collected from goats and sheep were found to be positive by Trans-PCR.
Conclusion:
This paper documents the first molecular detection of C. burnetii in ticks, which shows their role as putative vectors and reservoirs for this pathogenic agent.
Keywords: Coxiella burnetii, Ticks, Trans-PCR, Iran
Introduction
Ticks are well known for their impact on the health of both human and animals during infestation (Noda et al. 1997, Vilcins et al. 2005). Ticks can transmit the widest range of pathogens, including protozoa, bacteria, rickettsiae, spirochetes and viruses (Kim et al. 2006, Psaroulaki et al. 2006). The etiological agent of Q fever, Coxiella burnetii, has been identified in over 40 tick species (Kazar 2005, Psaroulaki et al. 2006). Coxiella burnetii, an obligate intracellular parasite with a worldwide distribution, is the causative agent of acute and chronic Q fever in humans. Q fever has been described worldwide except in New Zealand. From 1999 to 2004, there were 18 reported outbreaks of Q fever from 12 different countries involving two to 289 people. Six outbreaks involved sheep; three involved goats; one resulted from exposure to goat manure; one from exposure to ovine manure; one involved exposure to wild animals; one involved exposure to cats and dogs; and in two outbreaks the source was unknown (Maurin and Raoult 1999).
A few studies were conducted on Q fever in Iran some 50 years ago. Q fever cases have been reported from some countries neighboring Iran, such as Turkey and Oman (Scrimgeour et al. 2003, Kennerman et al. 2008). Results of a serosurvey undertaken on 42 sheep flocks in Turkey showed that 20% of sheep were seropositive (Kennerman et al. 2008). Recently, an outbreak of Q fever occurred with high morbidity in U.S. marines located in Iraq (Faix et al. 2008).
The reservoirs are extensive but only partially known and include mammals, birds, and arthropods, mainly ticks. Farm animals, mainly cattle, sheep, and goats, are the primary reservoirs of infection. High numbers of C. burnetii are present in the placenta of infected parturient animals and are shed in the environment following labor or abortion. Humans acquire the infection mainly via inhalation of contaminated aerosolized particles or ingestion of unpasteurized dairy products (Maurin and Raoult 1999).
Clinical Q fever in human can present as two basic forms: acute or chronic. Acute Q fever normally manifests as a self-limiting flu-like illness characterized by high-grade fever, peri-orbital headache, and myalgia. However, in some cases pneumonia occurrence requires hospitalization. Coxiella burnetii can establish a persistent, latent infection that may reactivate months or years after initial exposure to the organism to cause chronic disease. Chronic Q fever is typically associated with patients who are immunocompromised and/or who have pre-existing heart valve defects and most commonly presents as endocarditis (Maurin and Raoult 1999)
It is likely that factors such as the route of infection and the inoculum size, affect the expression of C. burnetii infection. Indeed the respiratory route is associated with pneumonia and the intra peritoneal route with hepatitis (Marrie et al. 1996). Coxiella burnetii may induce reproductive disorders such as abortion, stillbirth, and delivery of weak and nonviable neonates in ruminants (Lang et al. 1994).
Ticks can serve as indicators of infection in nature. For example, 10 C. burnetii strains were isolated from Ixodes ricinus, Dermacentor reticulatus, D. marginatus, Haemaphysalis concinna, and H. inermis ticks species collected in different habitats in Slovakia (Rehacek et al. 1991). The organism multiply in the gut cells of ticks and large numbers of C. burnetii are shed in tick feces. Infected ticks are probably the most important agents in maintaining the whole cycle of C. burnetii. Ticks may play a significant role in the transmission of C. burnetii among the wild vertebrates, especially in rodents, lagomorphs, and wild birds. Also, experimental transmission of C. burnetii from infected to uninfected guinea pigs via tick bite has been performed with Ixodes holocyclus, Haemaphysalis bispinosa and Rhipicephalus sanguineus (Angelakis and Raoult 2010).
DNA-based methods have been successfully used for detection of C. burnetii in ticks, fresh tissues, paraffin-embedded clinical samples, frozen samples, formalin-fixed tissues, serum samples, and milk (Maurin and Raoult 1999). The isolation of the pathogen is a reliable diagnostic method, but it remains time-consuming and hazardous and requires biosafety level BL3 practices. Therefore, the diagnosis of C. burnetii infection is usually done by PCR or serological examination. The PCR assay with primers targeting IS1111, the repetitive, transposon-like element (Trans-PCR), has been found to be very specific and sensitive for the detection of C. burnetii in different clinical samples (Vaidya et al. 2008).
The aim of our study was to use polymerase chain reaction for the detection of C. burnetii in ticks collected in Southeast Iran.
Materials and Methods
Standard strain of C. burnetii
Phenol-killed, purified, and lyophilized cells of the C. burnetii Nine Mile, phase I, strain (RSA 493) were used for this study.
Tick collection
During November and June 2009, one hundred and sixty ticks were collected from domestic animals (goats, sheep) in Kerman and Bardsir cities, Kerman Province (southeast of Iran) and put them in 35 pools (each pool formed from 5–7 ticks). The collected ticks were properly noted and placed in properly-labeled bottles containing denatured alcohol (95% ethanol+4% methanol+1% pyridine). The ticks were transferred to the parasitology laboratory of School of Veterinary Medicine, Shahid Bahonar University of Kerman and adults were identified using a stereomicroscope, according to general identification keys (Kaiser and Hoogstraal 1963, Mazlum 1968, Walker et al. 2003).
DNA extraction
Prior to DNA extraction, ticks were repeatedly washed with 70% ethanol and allowed to air dry for 10 min on sterile paper. Ticks were divided into pools of 3–10, according to species, gender and locality, and were homogenized in minimum essential medium (Biochrom AG, Germany) supplemented with 4% fetal bovine serum. DNA was extracted from 300 μl of the homogenized suspension using the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany), according to the manufacturer’s instructions. The extracted DNA stored at −20 °C until used.
Trans-PCR
A PCR assay targeting IS1111 fragment, a transposon-like repetitive region, was used to detect C. burnetii in clinical samples. In this study, trans-1 and trans-2 primers were used from the published data sequence of a transposon-like repetitive region of the C. burnetii genome (Hoover et al. 1992). The primers trans-1 (5′-TAT GTA TCC ACC GTA GCC AGT C-3′) and trans-2 (5′-CCC AAC AAC ACC TCC TTA TTC-3′) were synthesized by Copenhagen (Denmark). The trans-1 and trans-2 primers were designed to amplify a 687-bp fragment of the transposon-like repetitive element. The trans-PCR assay was performed as described previously (Vaidya et al. 2008). The PCR mixture (25 μl) included 2.5 μl of 10× PCR buffer (100 mM Tris-HCl buffer, pH 8.3, 500 mM KCl, 15 mM MgCl2, and 0.01% gelatin), 200 μM deoxynucleoside triphosphate mix, 2 μM of each primers, 0.3 U of Taq DNA polymerase, 3 μl of template DNA, and sterilized water to make up the reaction mixture volume. The DNA amplification reaction was performed in a MG thermocycler (Eppendorf, Germany). The cycling conditions for PCR included an initial denaturation of DNA at 95° C for 2 min, followed by five cycles at 94° C for 30 s, 66 to 61° C (the temperature was decreased by 1° C between consecutive steps) for 1 min, and 72° C for 1 min. These cycles were followed by 35 cycles consisting of 94° C for 30 s, 61° C for 30 s, and 72° C for 1 min and then a final extension step of 10 min at 72° C. Amplicons were visualized by agarose gel electrophoresis, stained with ethidium bromide at a final concentration of 0.5 mg/mL, and photo documented.
Results
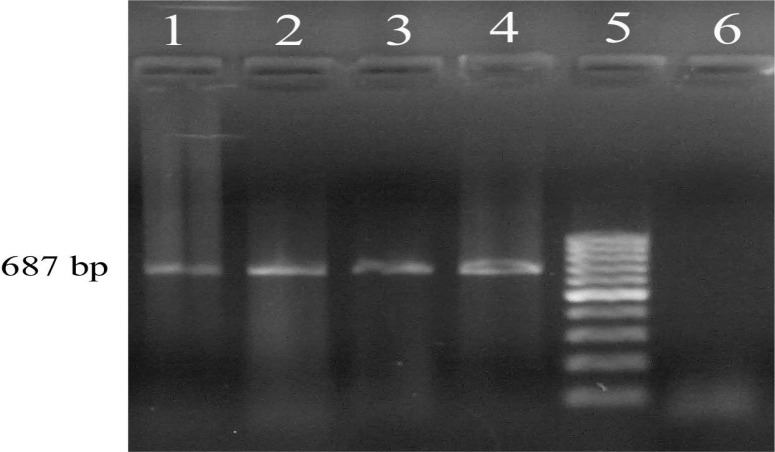
Of a total of one hundred and sixty ticks, three pools consisting of 5 female Hyalomma anatolicum anatolicum and one pool of three Rhipicephalus sanguineus ticks collected in June 2009 on goats and sheep were found positive using Trans-PCR (Fig. 1).
Fig. 1.
Lanes 1–3, an example of positive samples, lane 4, DNA template from the standard C. burnetii Nine Mile, lane 5, 100-bp DNA ladder, and lane 6, Non Template Control (NTC)
Discussion
Serologic evidences indicate people and animals in Iran are exposed to C. burnetii (Khalili and Sakhaee 2009, Khalili et al. 2010, Sakhaee and Khalili 2010), but there is not information available regarding the presence of this agent in specific vectors. This report presents the first detection C. burnetii in ticks in Iran.
It is well known that ticks participate in the transmission of different pathogenic micro-organisms to man and to animals. In nature, C. burnetii is found primarily in cycles involving ticks and vertebrates particularly rodents. C. burnetii is transmitted to domestic animals either by tick bites or through contact with infected excreta. Ticks are considered as natural primary reservoirs of C. burnetii and are responsible for transmission of the infection to wild animals and domestic animals (Norlander 2000). Ticks may play a significant role in the transmission of C. burnetii among the wild vertebrates, especially in rodents, lagomorphs, and wild birds (Angelakis and Raoult 2010). In ticks, C. burnetii can multiply to very high titers, remains viable during their entire life, and can be transmitted transovarially to next generations. In the enzootic cycle, ticks and vertebrates such as rodents are important components (Aitken 1987). Also, experimental transmission of C. burnetii from infected to uninfected guinea pigs via tick bite has been demonstrated by Ixodes holocyclus, Haemaphysalis bispinosa, and Rhipicephalus sanguineus (Maurin and Raoult 1999). Ticks expel heavy loads of C. burnetii with their feces onto the skin of the animal host at the time of feeding. Appropriate tick control strategies and good hygiene practice can decrease environmental contamination (Angelakis and Raoult 2010).
This study examined the occurrence of C. burnetii, the infectious agent of Q fever, in ticks in Iran, as a possible rout of infection in Q-fever outbreaks.
We used pools of ticks for DNA extraction, which offers the possibility of testing a large number of ticks collected in the field. We demonstrated the presence of C. burnetii by Trans-PCR in Hyalomma anatolicum anatolicum and Rhipicephalus sanguineus ticks collected in southeast Iran. Among the various species of hard ticks, Hyalomma anatolicum anatolicum is one of the most frequently found tick throughout Iran (Abbasian 1961). This paper documents the first molecular detection of C. burnetii in ticks. This communication confirms the presence of C. burnetii in Rhipicephalus sanguineus and Hyaloma spp. as previously reported in Cyprus and Italy (Spyridaki et al. 2002, Satta et al. 2010).
The preventive measures in animals should incorporate: the quarantine of imported animals, routinely testing of animals for antibodies to C. burnetii, tick control and maintenance of surrounding bushes.
Furthermore, considering the lack of information on the specific tick vector(s) of Q fever in the country, present preliminary data are meaningful. We are presently working on tick species identification, and hope to carry out similar studies covering other sites in the country. Investigations on C. burnetii using Trans-PCR are important method for diagnosis and disease control of Q fever. In conclusion, this paper documents the first detection of C. burnetii in tick species in Iran. To have a better insight into the epidemiology of C. burnetii infections in Iran and its role in domestic animals diseases and human, further studies are needed.
Acknowledgments
We thank Dr. Prof. Rudolf Toman for kindly providing phenol-killed, purified, and lyophilized cells of the C. burnetii Nine Mile, phase I, strain (RSA 493) for this study.
This research was financially supported by the research council of Shahid Bahonar University of Kerman, Iran.
References
- Abbasian L. Records of tick (Acarina: Ixodidae) occurring in Iran and their distributional data. Acarologia. 1961;3:546–559. [Google Scholar]
- Aitken ID, Bogel K, Cracea E, Edlinger E, Houwers D, Krauss H, Rady M, Rehacek J, Schiefer HG, Schmeer N, Tarasevich IV, Tringali G. Q Fever in Europe: current aspects of aetiology, epidemiology, human infection diagnosis and therapy. Infection. 1987;15:323–327. doi: 10.1007/BF01647731. [DOI] [PubMed] [Google Scholar]
- Angelakis E, Raoult D. Q-fever. Vet Microbiol. 2010;140:297–309. doi: 10.1016/j.vetmic.2009.07.016. [DOI] [PubMed] [Google Scholar]
- Hoover TA, Vodkin MH, Williams JC. A Coxiella burnetii repeated DNA element resembling a bacterial insertion sequence. J Bacteriol. 1992;174:5540–5548. doi: 10.1128/jb.174.17.5540-5548.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faix DJ, Harrison DJ, Riddle MS, Vaughn AF, Yingst SL, Earhart K, Thibault G. outbreak of Q fever among US military in Western Iraq, June–July 2005. Clin Infect Dis. 2008;46:65–68. doi: 10.1086/528866. [DOI] [PubMed] [Google Scholar]
- Kazar J. Coxiella burnetii infection. Ann N Y Acad Sci. 2005;1063:105–114. doi: 10.1196/annals.1355.018. [DOI] [PubMed] [Google Scholar]
- Kaiser MN, Hoogstraal H. The Hyalomma ticks (Ixodoidae) of Afghanistan. The Journal of Parasitology. 1963;49:130–139. [PubMed] [Google Scholar]
- Kaplan MM, Bertagna P. The geographical distribution of Q fever. Bull Wld Hlth Org. 1955;13:829–860. [PMC free article] [PubMed] [Google Scholar]
- Kennerman E, Rousset E, Gölcü E, Dufour P. Seroprevalence of Q fever (coxiellosis) in sheep from the Southern Marmara Region, Turkey. Comp Immunol Microbiol Infect Dis. 2010;33(1):37–45. doi: 10.1016/j.cimid.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Khalili M, Shahabi-Nejad N, Golchin M. Q fever serology in febrile patients in Southeast Iran. Trans Roy S Trop Med Hyg. 2010;104:623–624. doi: 10.1016/j.trstmh.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Khalili M, Sakhaee E. An update on a serologic survey of Q fever in domestic animals in Iran. Am J Trop Med Hyg. 2009;80:1031–1032. [PubMed] [Google Scholar]
- Kim C, Yi Y, Yu D. Tick-borne rickettsial pathogens in ticks and small mammals in Korea. Appl Environ Microbiol. 2006;72:5766–5776. doi: 10.1128/AEM.00431-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakhaee E, Khalili M. The first report of Q fever in Kerman sheep flocks. Trop Anim Health Prod. 2010;42:1561–1564. doi: 10.1007/s11250-010-9606-2. [DOI] [PubMed] [Google Scholar]
- Lang GH, Prescott JF, Williams JC. Serological response in sheep vaccinated against Coxiella burnetii (Q fever) Can Vet J. 1994;35(6):373–374. [PMC free article] [PubMed] [Google Scholar]
- Marrie TJ, Stein A, Janigan D, Raoult D. Route of infection determines the clinical manifestations of acute Q fever. J Infect Dis. 1996;173:484–487. doi: 10.1093/infdis/173.2.484. [DOI] [PubMed] [Google Scholar]
- Mazlum Z. Hyalomma asiaticum asiaticum (Schulze and Schlottke) 1929: its distribution, hosts, seasonal activity, life cycle and role in transmission of bovine theileriosis in Iran. Acarologia. 1968;10:437–442. [PubMed] [Google Scholar]
- Maurin M, Raoult D. Q fever. Clin Microbiol Rev. 1999;12:518–553. doi: 10.1128/cmr.12.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda H, Munderloh UG, Kurtti TJ. Endosymbionts of ticks and their relationship to Wolbachia spp. and tick-borne pathogens of humans and animals. Appl Environ Microbiol. 1997;63:3926–3932. doi: 10.1128/aem.63.10.3926-3932.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norlander L. Q-fever epidemiology and pathogenesis. Microb and Infect. 2000;2:417–424. doi: 10.1016/s1286-4579(00)00325-7. [DOI] [PubMed] [Google Scholar]
- Psaroulaki A, Ragiadakou D, Kouris G, Papadopoulos B, Chaniotis B, Tselentis Y. Ticks, tick-borne Rickettsiae, and Coxiella burnetii in the Greek Island of Cephalonia. Ann N Y Acad Sci. 2006;1078:389–399. doi: 10.1196/annals.1374.077. [DOI] [PubMed] [Google Scholar]
- Rehacek J, Urvolgyi J, Kocianova E, Sekeyova Z, Vavrekova M, Kovacova E. Extensive examination of different ticks species for infestation with Coxiella burnetii in Slovakia. Eur J Epidemiol. 1991;7:299–303. doi: 10.1007/BF00145682. [DOI] [PubMed] [Google Scholar]
- Satta G, Chisu V, Cabras P, Fois F, Masala G. Pathogens and symbionts in ticks: a survey on tick species distribution and presence of tick-transmitted micro-organisms in Sardinia, Italy. J Med Microbiol. 2010 doi: 10.1099/jmm.0.021543-0. [DOI] [PubMed] [Google Scholar]
- Scrimgeour EM, Al-Ismaily SI, Rolain JM, Al-Dhahry SH, El-Khatim HS, Raoult D. Q fever in human and livestock populations in Oman. Ann NY Acad Sci. 2003;990:221–225. doi: 10.1111/j.1749-6632.2003.tb07366.x. [DOI] [PubMed] [Google Scholar]
- Spyridaki I, Gikas A, Kofteridis D, Psaroulaki A, Tselentis Y. Isolation of coxiella burnetii by a centrifugation shellvial assay from ticks collected in Cyprus: detection by nested polymerase chain reaction (PCR) and by PCR-restriction fragment length polymorphism analyses. Am J Trop Med Hyg. 2002;66(1):86–90. doi: 10.4269/ajtmh.2002.66.86. [DOI] [PubMed] [Google Scholar]
- Vaidya VM, Malik SVS, Simranpreet K, Kumar S, Barbuddh SB. Comparison of PCR, Immunofluorescence Assay, and Pathogen Isolation for Diagnosis of Q fever in Humans with Spontaneous Abortions. J Clin Microbiol. 2008;46:2038–2044. doi: 10.1128/JCM.01874-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilcins IM, Old J, Deane E. The impact of ticks and tick-borne diseases on native animal species in Australia. Microbiol Aus. 2005;26(2):76–79. [Google Scholar]
- Walker AR, Bouattour A, Camicas JL, Estrada-Pena A, Horak IG, Latif AA, Pegram RG, Preston PM. 2003. Ticks of domestic animals in Africa: a guide to identification of species Bioscience Reports 42 Comiston Drive, Edinburgh EH10 5QR, Scotland, U.K. [DOI] [PubMed]