Abstract
Background:
Plant extracts and oils may act as alternatives to conventional pesticides for malaria vector control. The aim of this study was to evaluate the larvicidal activity of essential oils of three plants of Apiaceae family against Anopheles stephensi, the main malaria vector in Iran.
Methods:
Essential oils from Heracleum persicum, Foeniculum vulgare and Coriandrum sativum seeds were hydro distillated, then their larvicidal activity were evaluated against laboratory-reared larvae of An. stephensi according to standard method of WHO. After susceptibility test, results were analysis using Probit program.
Results:
Essential oils were separated from H. persicum, F. vulgare and C. sativum plants and their larvicidal activities were tested. Result of this study showed that F. vulgare oil was the most effective against An. stephensi with LC50 and LC90 values of 20.10 and 44.51 ppm, respectively.
Conclusion:
All three plants essential oil can serve as a natural larvicide against An. stephensi. F. vulgare oil exhibited more larvicidal properties.
Keywords: Malaria, Apiaceae, Vector, Anopheles stephensi
Introduction
Mosquitoes play an important role in transmission of some human diseases such as malaria, dengue fever, yellow fever and filariasis, which consider them among the greatest health problems across the glob (James 1992). Anopheles species are considered as vectors of human malaria, filariasis and certain arboviruses (Sedaghat and Harbach 2005). Malaria is considered as one of the most important health problem in Iran especially in southern parts. In the south parts of Iran there are six anopheline vectors including Anopheles culicifacies, An. stephensi, An. dthali, An. fluviatilis, An. superpictus, and An. pulcherrimus, (Naddaf et al. 2003, Vatandoost and Moinvaziri 2004, Vatandoost et al. 2004, 2005a,b, Hanafi-Bojd et al. 2006, Soltani et al. 2008, Hanafi-Bojd et al. 2010, Vatandoost et al. 2006, 2007, 2009, 2011). Anopheles sacharovi and An. maculipennis can transmit human malaria in northern part of the country (Sedaghat et al. 2003a, 2003b, Oshaghi et al. 2003, Doosti et al. 2007).
Anopheles stephensi, an oriental malaria vector, is distributed in Indo-Persian area from India, Pakistan and Iran, to countries around the Persian Gulf (Nagpal and Sharma 1995). In Iran, it occurs in the southern areas of the country in Khuzestan, Fars, Kerman, Hormozgan, Sistan and Baluchistan and southern Kermanshah Provinces (Manouchehri et al. 1976, Sedaghat and Harbach 2005).
Although there are several methods for control of Anopheles mosquitoes however environmental effect and resistance is a main human concern. Synthetic pyrethroids which considered as the most effective insecticides against anophelines, are still expensive and beyond the financial resources of some countries.
Using botanical insecticides is a technique which can apply as an alternative to synthetic chemical formulations. Most botanicals are rapid acting and breakdown quickly in the environment. The extract of whole leaf and essential oil of some certain plants have been investigated against some public health pests (Saxena and Sumithra 1985, Kumar and Dutta 1987, Chariandy et al. 1999, Hadjiakhoondi et al. 2000 a,b, Markouk et al. 2000, Hadjiakhoondi et al. 2003, Tare et al. 2004, Vatandoost et al. 2004, 2008, Hadjiakhoondi et al. 2005–2006, Oshaghi et al. 2008 a).
Apiaceae (Umbelliferae) is one of the best known families of flowering plants, which comprise 300–450 genus and 3000–3700 species (Constance 1971, Pimenov and Leonov 1993). They are aromatic plant and have a distinctive flavor which diverse volatile compounds from the fruits and leaves. This family also encompasses toxic plants that some had used in ancient Athens to execute those sentenced to death (Constance 1971, Pimenov and Leonov 1993). Heracleum persicum Desf., known as Persian Hogweed or “Golpar”, is an annual native plant native to Iran with a wide distribution across the country. It is used in traditional medicine because of its antioxidant and anticonvulsant activity (Parsa 1948, Souri et al. 2000, Sayyah et al. 2005). Foeniculum vulgare Mill., known as Fennel or “Razianeh” in Iran and neighboring countries, has been used by humans since antiquity. It is generally considered indigenous to the shores of the Mediterranean, but now can be found in many parts of the world. (Muckensturm et al. 1997). The bioactivities of this plant have been investigated for its antioxidant activity (Oktaya et al. 2003) fumigant activity (Kim et al. 2003), antimicrobial activity (Ruberto et al. 2000), larvicidal activity (Chantraine et al. 1998, Pitasawat et al. 2007), insecticidal activity (Laurent et al. 1997), and acaricidal activity (Lee 2004).
Coriandrum sativum L, known as Coriander, is native to Iran, however it is widely distributed around the world. The seeds contain an essential oil (up to 1%) and the seeds are used in traditional medicine for indigestion, against worms, rheumatism and pain in the joints (Wangensteen 2004). It has also the nematicidal activity (Kim et al. 2008), antibacterial activity (Cantore et al. 2004) and larvicidal activity (Harve and Kamath 2004).
In recent years, much effort has been focused on the exploration of bioactive chemical compounds from indigenous plants for mosquito control in Iran. So, the purpose of this study was to determine bioactivity of three essential oils obtained from the seeds of plants against 4th instar larvae of An. stephensi.
Materials and Methods
Mosquito rearing
The fourth-instars larvae of An. stephensi used for bioassays. The laboratory-reared An. stephensi Bandar-Abbas strain were reared in the Department of Medical Entomology, Tehran University of Medical Sciences, and maintained at 27° C with a photoperiod of 12 hours light and 12 hours e dark in 80±10% relative humidity. A 10% percentage yeast suspension was used as food source.
Plant materials
In this study, the three plants from Apiaceae (Umbellifera) family were collected from three provinces of Iran in 2009 (Table 1). Examined plants were authenticated and identified by the Department of Pharmacognosy, Faculty of Pharmacy, Tehran University of Medical Sciences. The seeds were air-dried at room temperature and kept in an air-tight light-protected container.
Table 1.
Collection locations, physical properties and yields of volatile oils derived from seeds of H. persicum, F. vulgare and C. sativum
| Plant species | Location | Harvest date (2009) | Color | Appearance | %Yield (w/w) |
|---|---|---|---|---|---|
| H. persicum | Mazandaran | August | Pale Yellow | Liquid | 1.6 |
| C. sativum | Chaharmahal and Bakhtiary | September | Pale Yellow | Liquid | 0.9 |
| F. vulgare | East Azarbaijan | September | Pale yellow | Liquid | 0.8 |
Dried seeds of H. persicum, F. vulgare and C. sativum were subjected to hydro distillation using a modified Clevenger-type apparatus for 3 hours, dried over anhydrous sodium sulphate, and stored in amber-colored vials at 5°C until required for further work.
Bioassays and larval mortality
Fourth instar larvae of An. stephensi Bandar-Abbas strain was exposed to test concentrations of 5, 10, 20, 40, 80,160 and 320 ppm of essential oil for 24 hours according to standard method described by WHO (1981). As the oils do not dissolve in water, they were first dissolved in ethanol. The test 400 ml glass beaker were used by adding 1 ml of appropriate dilution of essential oil in ethanol and mixed with 249 ml of water to make up 250 ml of test solution (Dharmagadda et al. 2005). In control beakers, ethanol was applied into the water (1%). A minimum of 20 larvae per each concentrations were used for all the experiments. The dead larvae were counted after 24 hour recovery period, and percentage of mortality was reported from the average for the five replicates. The larvae considered dead were those that did not move when touched with a needle.
Statistical analysis
The lethal concentrations (LC50 and LC90) were calculated using Probit analysis (Finney 1971). For all bioassays, the percentages of mortality were adjusted for the mortality in controls by using Abbott's correction (Abbot 1925). Differences between means were considered significant at P≤ 0.05 (SAS Institute 2001).
Results
Hydro distillation of H. persicum, C. sativum and F. vulgare yielded (w/w, dry weight) of volatile oils (Table 1). The highest yield of volatile oil was obtained from H. persicum, whereas that of F. vulgare was the lowest.
The larvicidal activities of the essential oils against An. stephensi larvae under laboratory conditions are given in Table 2. All plants oil exerted significant larvicidal potential against An. stephensi after exposure for 24 h (Table 2). The lethal dosage of 50% (LC50) ranged between 20.10 and 120.95 ppm.
Table 2.
Parameters of probit regression line Anopheles stephensi to essential oil derived from H. persicum, F. vulgare and C. sativum seeds at different interval concentrations
| Specimens | A | B±SE | LC50, 95% C.I. | LC90, 95% C.I. | X2 (df) | P value |
|---|---|---|---|---|---|---|
| C. sativum | −5.25 | 2.52±0652 | 47.68 | 181.62 | 43.82 (3) | <0.05 |
| 120.95 | 389.90 | |||||
| 479.9 | 181172.4 | |||||
|
| ||||||
| H. persicum | −11.73 | 5.81±1.725 | 26.30 | 114.40 | 41.85 (3) | <0.05 |
| 104.80 | 174.22 | |||||
| 336.90 | 1073788.4 | |||||
|
| ||||||
| F. vulgare | −4.83 | 3.71±1.023 | 6.05 | 24.30 | 53.6 (3) | <0.05 |
| 20.10 | 44.51 | |||||
| 67.62 | 15839.5 | |||||
Foeniculum vulgare oil was the most effective against An. stephensi with LC50 and LC90 values of 20.10 and 44.51 ppm, respectively, while C. sativum had the least mortality with LC50 and LC90 values of 120.95 and 389.90 ppm, respectively. This was also 5 times lower than H. persicum, which showed an LC50 of 104.80 ppm.
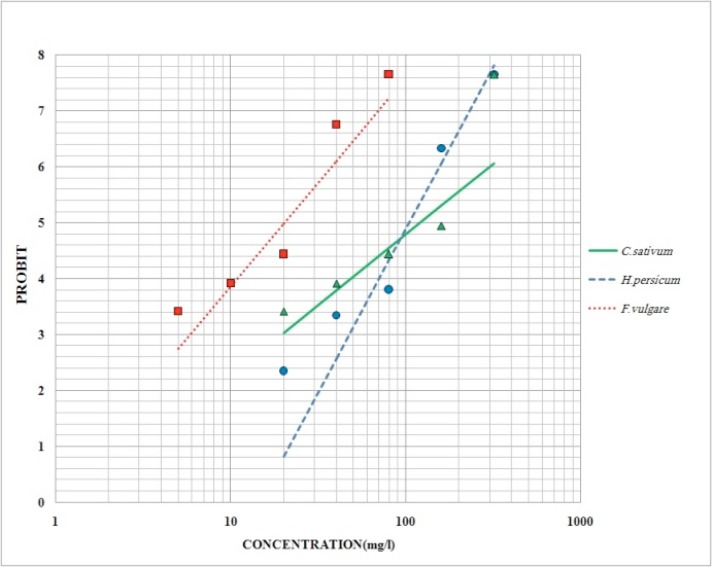
In regression line a positive correlation were observed between the essential oil concentrations and the percent mortality (Fig. 1).
Fig. 1.
Probit regression line of An. stephensi exposed to different interval concentrations of H. persicum, F. vulgare and C. sativum seed essential oils
Discussion
The use of plant essential oils in vector control is an alternative method for minimizing the side effects of chemical pesticides on the environment (Fatope et al. 1993). In recent surveys, it has been found that some of secondary plant metabolites act as botanical insecticides (Watanabe et al. 1993, Vatandoost et al. 2004, Nathan 2007). According to the biological results of present study, all of three plant oils exerted significant larvicidal potential against An. stephensi and the essential oil of F. vulgare had an appropriate effect. A study in Bolivia reported that essential oil of F. vulgare, was one of the most toxic oils on Ae. aegypti larvae. Also its LC50 and LC90 were 24.3 and 30.8 ppm, respectively (Chantraine et al. 1998). In the other study in Lebanon for assay of repellency and toxicity of aromatic plant extracts against the mosquito Culex pipiens form molestus, results showed that F. vulgare was one of the most significant repellency against this species (52.0 min of protection with concentration of 3%) also the LC50 and LC90 from essential oil of F. vulgare was 24.5 and 34 mg/l respectively (Traboulsi et al. 2005). In several study on chemical compositions of plants by using of gas chromatography and mass spectrometry (GC/MS), results showed that different compounds were present in different parts of the plants. For example, in the study on seeds essential oil of H. persicum it is found that it contains hexyl butyrate (35.5%), octyl acetate (27%) and hexyl isobutyrate (3.2%) (Sefidkon et al. 2004). In the other study on the leaves of C. sativum, Matasyoh et al (2009) reported four main compounds, including: 2E-decenal (15.9%), decanal (14.3%), 2E-decen-1-ol (14.2%) and n-decanol (13.6%). In the two same study on fruit and flower of F. vulgare, the transanethole (85.63%), estragole (5.27%) and D-limonene (3.8%) were present in the fruit (Dadalioglu and Evrendilek 2004), While other report showed limonene (63.6%), anethole (25.5%), fenchyl acetate (2.6%), α-Pinene (0.97%), myrcene (0.98%) and estragole (1.1%) from flower (Traboulsi et al. 2005). It seems that the larviciding effect of these essential oils of plant is attributed to these main compounds. We propose test different components of the plants against mosquitoes.
Results of the current study showed that the essential oil of these plants exhibited the biological effect on the larvae of An. stephensi. The use of botanical pesticide may help in reducing the environmental side effects by the synthetic insecticides. The results obtained suggest that the essential oils of H. persicus, F. vulgare and C. sativum are promising as larvicide against An. stephensi. Moreover, these results could be useful in the search for newer, more selective, and biodegradable larvicidal natural compounds. Further investigations are currently underway to isolate these compounds.
Acknowledgments
The authors would like to appreciate very much for kind collaboration of the Department of Medical Entomology and Vector Control, School of Public Health, Tehran University of Medical Sciences (TUMS) for providing the test mosquito, An. stephensi. This work was supported by grants from the Centre of Environmental Research, TUMS, Project No. 88-01-46-8661. The authors declare that there is no conflict of interests.
References
- Abbott WS. A method of computing the effectiveness of an insecticide. J Econ Entomol. 1925;18:265–267. [PubMed] [Google Scholar]
- Cantore PL, Iacobellis NS, Marco AD, Capasso F, Senatore F. Antibacterial activity of Coriandrum sativum L. and Foeniculum vulgare Miller var. vulgare (Miller) essential oils. J Agric Food Chem. 2004;52(26):7862–7866. doi: 10.1021/jf0493122. [DOI] [PubMed] [Google Scholar]
- Chariandy CM, Seaforth CE, Phelps RH, Pollard GV, Khambay BP. Screening of medicinal plants from Trinidad and Tobago for antimicrobial and insecticidal properties. J Ethnopharmacol. 1999;64:265–270. doi: 10.1016/s0378-8741(98)00130-5. [DOI] [PubMed] [Google Scholar]
- Chantraine JM, Laurent D, Ballivian C, Saavedra G, Ibañez R, Vilaseca LA. Insecticidal activity of essential oils on Aedes Aegypti larvae. Phytotherapy Res. 1998;12:350–354. [Google Scholar]
- Constance L. History of the classification of Umbelliferae (Apiaceae) In: Heywood VH, editor. The biology and chemistry of the Umbelliferae, 1–8. Academic Press; London, UK: 1971. p. 438. [Google Scholar]
- Dadalioglu I, Evrendilek GA. Chemichal compositions and antibacterial effects of essential oils of Turkish oregano (Origanum minutiflorum), bay laurel (Laurus nobilis), Spanish lavender (Lavandula stoechas L), and fennel (Foeniculum vulgare) on common foodborne pathogenesis. J Agric Food Chem. 2004;52:8255–8260. doi: 10.1021/jf049033e. [DOI] [PubMed] [Google Scholar]
- Dharmagadda VSS, Naik SN, Mittal PK, Vasudevan P. Larvicidal activity of Tagetes patula essential oil against three mosquito species. Biores Technol. 2005;96:1235–1240. doi: 10.1016/j.biortech.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Doosti S, Vatandoost H, Oshaghi MA, Hosseini M, Sedaghat MM. Applying morphometric variation of seta 2 (Antepalmate Hair) among the larvae of the members of the Maculipennis Subgroup (Diptera: Culicidae) in Iran. Iran J Arthropod-Borne Dis. 2007;1:28–37. [Google Scholar]
- Fatope MO, Ibrahim H, Takeda Y. Screening of higher plants reputed as pesticides using the Brine Shrimp Lethality Assay. Int J Pharmacog. 1993;31:250–254. [Google Scholar]
- Finney DJ. Probit Analysis. 3rd ed. London, UK: Cambridge University Press; 1971. [Google Scholar]
- Gilles HM, Warrell DA. Bruce-Chwatt’s Essential Malariology. third ed. Edward Arnold; London: 1993. [Google Scholar]
- Hadjiakhoondi A, Aghel N, Zamanizadeh N, Vatandoost H. Chemical and Biological study of Mentha spicata L. essential oil from Iran. Daru. 2000a;8:19–21. [Google Scholar]
- Hadjiakhoondi A, Vatandoost H, Abousaber M, Khanavi M, Abdi L. Chemical composition of the essential oil of Tagetes minuta L. and its effects on Anopheles stephensi larvae in Iran. J Med Plants. 2000b;7:33–100. [Google Scholar]
- Hadjiakhoondi A, Vatandoost H, Jamshidi A, Bagherj Amiri E. Chemical constituents and efficacy of Cymbopogon olivieri (Boiss) bar essential oil against malaria vector, Anopheles stephensi. Daru. 2003;11:125–128. [Google Scholar]
- Hadjiakhoondi A, Vatandoost H, Khanavi M, Abai MR. Biochemical investigation of different extracts and larvicidal activity of Tagetes minuta L on Anopheles stephensi larvae. Iran J Pharm Sci. 2005;1:81–84. [Google Scholar]
- Hadjiakhoondi A, Sadeghipour-Roodsari HR, Vatandoost H, Khanavi M, Abai MR, Vosoughi M, Kazemi M. Fatty acid composition and toxicity of Melia azedarach L. fruits against malaria vector Anopheles stephensi. Iran J Pharm Sci. 2006;2:97–102. [Google Scholar]
- Hanafi-Bojd AA, Vatandoost H, Jafari R. Susceptibility status of Anopheles dthali and An. fluviatilis to commonly used larvicides in an endemic focus of malaria, southern Iran. J Vect Borne Dis. 2006;43:34–38. [PubMed] [Google Scholar]
- Hanafi-Bojd AA, Vatandoost H, Philip E, Stepanova E, AI Abdi A, Safari R, Mohseni GH, Bruhi MI, Peter A, Abdulrazag SH, Mangal G. Malaria situation analysis and stratification in Bandar Abbas County, Southern Iran, 2004–2008. Iran J Arthropod-Borne Dis. 2010;4:31–41. [PMC free article] [PubMed] [Google Scholar]
- Harve G, Kamath V. Larvicidal activity of plant extracts used alone and in combination with known synthetic larvicidal agents against Aedes aegypti. Indian J Exp Biol. 2004;42:1216–1219. [PubMed] [Google Scholar]
- James AA. Mosquito molecular genetics: the hands that feed bite back. Science. 1992;257:37–38. doi: 10.1126/science.1352413. [DOI] [PubMed] [Google Scholar]
- Kim D, Ahn Y. Contact and fumigant activities of constituents of Foeniculum vulgare fruit against three coleopteran stored-product insects. Pest Manag Sci. 2001;57:301–306. doi: 10.1002/ps.274. [DOI] [PubMed] [Google Scholar]
- Kim SI, Park C, Ohh MH, Cho HC, Ahn YJ. Contact and fumigant activities of aromatic plants extracts and essential oils against Lasioderma serricorne (Coleoptera: Anobiidae) J Stored Prod Res. 2003;39:11–19. [Google Scholar]
- Kim J, Seo SM, Lee SG, Shin SC, Park IK. Nematicidal activity of plant essential oils and components from coriander (Coriandrum sativum), oriental sweetgum (Liquidambar orientalis), and valerian (Valeriana wallichii) essential oils against pine wood nematode (Bursaphelenchus xylophilus) J Agric Food Chem. 2008;56:7316–7320. doi: 10.1021/jf800780f. [DOI] [PubMed] [Google Scholar]
- Kumar A, Dutta GP. Indigenous plant oils as larvicidal agent against Anopheles stephensi mosquitoes. Curr Sci. 1987;56:959–960. [Google Scholar]
- Laurent D, Vilaseca LA, Chantraine JM, Ballivian C, Saavedra G, Ibañez R. Insecticidal activity of essential oils on Triatoma infestans. Phytother Res. 1997;11:285–290. [Google Scholar]
- Lee HS. Acaricidal Activity of constituents identified in Foeniculum vulgare fruit oil against Dermatophagoides spp. (Acari: Pyroglyphidae) J Agric Food Chem. 2004;52:2887–2889. doi: 10.1021/jf049631t. [DOI] [PubMed] [Google Scholar]
- Manouchehri AV, Javadian E, Eshighy N, Motabar M. Ecology of Anopheles stephensi Liston in southern Iran. Trop Geogr Med. 1976;28:228–232. [PubMed] [Google Scholar]
- Matasyoh JC, Maiyo ZC, Ngure RM, Chepkorir R. Chemical composition and antimicrobial activity of the essential oil of Coriandrum sativum. Food Chem. 2009;113:526–529. [Google Scholar]
- Markouk M, Bekkouche K, Larhsini M, Bousaid M, Lazrek HB, Jana M. Evaluation of some Moroccan medicinal plant extracts for larvicidal activity. J Ethnopharmacol. 2000;73:293–297. doi: 10.1016/s0378-8741(00)00257-9. [DOI] [PubMed] [Google Scholar]
- Muckensturm B, Foechterlen D, Reduron JP, Danton P, Hildenbrand M. Pythochemical and chemotaxonomic studies of Foeniculum vulgare. Biochem Syst Ecol. 1997;25:353–358. [Google Scholar]
- Naddaf SR, Oshaghi MA, Vatandoost H, Asmar M. Molecular characterization of the Anopheles fluviatilis species complex in Iran. Eastern Med Health J. 2003;9(3):257–265. [PubMed] [Google Scholar]
- Nagpal BN, Sharma VP. Indian Anophelines. Oxford and IBH Pub. Co Pvt Ltd.; 1995. [Google Scholar]
- Nathan SS. The use of Eucalyptus tereticornis Sm. (Myrtaceae) oil (leaf extract) as a natural larvicidal agent against the malaria vector Anopheles stephensi Liston (Diptera: Culicidae) Bioresource Technol. 2007;98:1856–1860. doi: 10.1016/j.biortech.2006.07.044. [DOI] [PubMed] [Google Scholar]
- Oktaya M, Gu I, I’rfan Ku O. Determination of in vitro antioxidant activity of fennel (Foeniculum vulgare) seed extracts Lebensm.-Wiss. U Technol. 2003;36:263–271. [Google Scholar]
- Oshaghi MA, Ghalandari R, Vatandoost H, Shayeghi M, Kamali-nejad M, Tourabi-Khaledi H, Abolhassani H, Hashemzadeh M. Repellent effect of extracts and essential oil of Citrus limon (Rutaceae) and Melissa officinalis (Labiatae) against main malaria vector, Anopheles stephensi (Diptera: Culicidae) in Iran. Iran J Public Health. 2003a;32(4):47–52. [Google Scholar]
- Oshaghi MA, Sedaghat MM, Vatandoost H. Molecular characterization of the Anopheles maculipennis complex in the Islamic Republic of Iran. East Mediterr Hlth J. 2003b;9(4):659–66. [PubMed] [Google Scholar]
- Parsa A. Flore de l’Iran. Tehran Danesh Press. 1948;2:481–482. [Google Scholar]
- Pimenov M, Leonov V. The Genera of the Umbelliferae. Royal Botanic Gardens; Kew, UK: 1993. [Google Scholar]
- Pitasawat B, Champakaew D, Choochote W, Jitpakdi A, Chaithong U, Kanjanapothi D, Rattanachanpichai E, Tippawangkosol P, Riyong D, Tuetun B, Chaiyasit D. Aromatic plant-derived essential oil: An alternative larvicide for mosquito control. Fitoterapia. 2007;78:205–210. doi: 10.1016/j.fitote.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Ruberto G, Baratta MT, Deans SG, Dorman HJD. antioxidant activity and antimicrobial activities of Foeniculum vulgare and Crithum maritimum essential oils. Planta Med. 2000;66(8):687–693. doi: 10.1055/s-2000-9773. [DOI] [PubMed] [Google Scholar]
- SAS Institute . The SAS System for Windows, release 8.1. Cary, NC: 2001. [Google Scholar]
- Saxena SC, Sumithra L. Laboratory evaluation of leaf extract of new plant to suppress the population of malaria vector Anopheles stephensi Liston (Diptera: Culicidae) Curr Sci. 1985;54:201–202. [Google Scholar]
- Sayyah M, Moaied S, Kamalinejad M. Anticonvulsant activity of Heracleum persicum seed. J Ethnopharmacol. 2005;98:209–211. doi: 10.1016/j.jep.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Soltani A, Vatandoost H, Jabbari H, Mesdaghinia AR, Mahvi AH, Younesian M, Hanafi-Bojd AA, Bozorgzadeh S, Abai MR, Pakari A, Shabkhiz H. Use of expanded polystyrene (EPS) and shredded waste polystyrene (SWAP) beads for control of mosquitoes. Iran J Arthropod-Borne Dis. 2008;2:12–20. [Google Scholar]
- Sedaghat MM, Linton YM, Nicolescu G, Smith L, Koliopoulos G, Zounos AK, Oshaghi MA, Vatandoost H, Harbach RE. Morphological and molecular characterization of Anopheles (Anopheles) sacharovi Favre, a primary vector of malaria in the Middle East. Syst Entomol. 2003a;28:241–256. [Google Scholar]
- Sedaghat MM, Linton YM, Oshaghi MA, Vatandoost H, Harbach RE. The Anopheles maculipennis complex (Diptera: Culicidae) in Iran: molecular characterisation and recognition of a new species. Bull Entomol Res. 2003b;93:527–535. doi: 10.1079/ber2003272. [DOI] [PubMed] [Google Scholar]
- Sedaghat MM, Harbach RE. An annotated checklist of the Anopheles mosquitoes (Diptera: Culicidae) in Iran. J Vect Ecol. 2005;30:272–276. [PubMed] [Google Scholar]
- Sefidkon F, Dabiri M, Mohammad N. Analysis of the Oil of Heracleum persicum L. (Seeds and Stems) J Essent Oil Res. 2004;14(4):295–297. [Google Scholar]
- Souri E, Farsam H, Sarkheil P, Ebadi F. Antioxidant Activity of Some Furanocoumarins Isolated from Heracleum persicum. Pharm Biol. 2004;6:396–399. [Google Scholar]
- Tare V, Deshpande S, Sharma RN. Susceptibility of two different strains of Aedes aegypti (Diptera: Culicidae) to plant oils. J Econ Entomol. 2004;97:1734–1736. doi: 10.1603/0022-0493-97.5.1734. [DOI] [PubMed] [Google Scholar]
- Traboulsi AF, El-Haj S, Tueni M, Taoubi K, Abi Nader N, Mrad A. Repellency and toxicity of aromatic plant extracts against the mosquito Culex pipiens molestus (Diptera: Culicidae) Pest Manag Sci. 2005;61:597–604. doi: 10.1002/ps.1017. [DOI] [PubMed] [Google Scholar]
- Vatandoost H, Moinvaziri VM. Larvicidal activity of neem tree extract (Neemarin) against mosquito larvae in the Islamic Republic of Iran. Eastern Med Health J. 2004;10:573–578. [PubMed] [Google Scholar]
- Vatandoost H, Shahi H, Abai MR, Hanafi-Bojd AA, Oshaghi MA, Zamani G. Larval habitats of main malaria vectors in Hormozgan Province and their susceptibility to different larvicides. Southeast Asian J Trop Med Pub Hlth. 2004;35:22–25. [PubMed] [Google Scholar]
- Vatandoost H, Mashayekhi M, Abaie MR, Aflatoonian MR, Hanafi-Bojd AA, Sharifi I. Monitoring of insecticides resistance in main malaria vectors in a malarious area of Kahnooj District, Kerman Province, southeastern Iran. J Vector Borne Dis. 2005a;42:100–108. [PubMed] [Google Scholar]
- Vatandoost H, Hanafi-Bojd AA. Current resistant status of Anopheles stephensi Liston to different larvicides in Hormozgan Province, southeastern Iran. Pakistan J Biol Sci. 2005b;8:1568–1570. [Google Scholar]
- Vatandoost H, Oshaghi MA, Abaie MR, Shahi M, Yaghoobi F, Baghai M, Hanafi-Bojd AA, Zamain G, Townson H. Bionomics of Anopheles stephensi Liston in the malarious area of Hormozgan Province, southern Iran. Acta Trop. 2006;97:196–205. doi: 10.1016/j.actatropica.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Vatandoost H, Shahi M, Hanafi-Bojd AA, Abai MR, Oshaghi MA, Rafii F. Ecology of Anopheles dthali Patton in Bandar Abbas District, Hormozgan Province, Southern Iran. Iran J Arthropod-Borne Dis. 2007;1:21–27. [Google Scholar]
- Vatandoost H, Khazani A, KebriainZadeh A, Rafinejad J, Khoobdel M, Abai MR, Hanafi-Bojd AA, Akhavan AA, Abtahi SM, Rafi F. Comparative efficacy of Neem and dimethyl phthalate (DMP) against malaria vector, Anopheles stephensi (Diptera: Culicidae) Asian Pacific J Trop Med. 2008;1(3):1–6. [Google Scholar]
- Vatandoost H, Ramin E, Rassi Y, Abai MR. Stability and wash resistance of local made mosquito bednets and detergents treated with pyrethroids against Anopheles stephensi. Iran J Arthropod-Borne Dis. 2009;3:19–28. [PMC free article] [PubMed] [Google Scholar]
- Vatandoost H, Emami SN, Oshaghi MA, Abai MR, Raeisi A, Piazzak N, Mahmoodi M, Akbarzadeh K, Sartipi M. Ecology of malaria vector Anopheles culicifacies in a malarious area of Sistan va Baluchestan Province, south-east Islamic Republic of Iran. East Mediterr Health J. 2011;17:439–445. [PubMed] [Google Scholar]
- Wangensteen H, Samuelsen AB, Malterud KE. Antioxidant activity in extracts from coriander. Food Chem. 2004;88:293–297. [Google Scholar]
- Watanabe K, Shono Y, Kakimizu A, Okada A, Matsuo N, Satoh A, Nishimura H. New mosquito repellent from Eucalyptus camaldulensis. J Agri Food Chem. 1993;41:2164–2166. [Google Scholar]
- WHO Instructions for determining susceptibility or resistance of mosquito larvae to insecticides. WHO/ VBC. 1981;81:807. [Google Scholar]