Abstract
OBJECTIVE
Elevated serum free fatty acid (FFA) levels are associated with an increased risk for cardiovascular disease and type 2 diabetes. Macrophages are recruited to atherosclerotic plaques and metabolic tissues during obesity and accumulate lipids, including FFAs. We investigated the molecular consequences of intracellular saturated FFA accumulation in macrophages.
METHODS AND RESULTS
Previously, we demonstrated that co-treatment of mouse peritoneal macrophages (MPMs) with stearic acid and triacsin C (TC, an inhibitor of long-chain acyl-CoA synthetases) results in intracellular FFA accumulation and apoptosis. Here, we utilized Western blot analysis, real-time RT-PCR, and TUNEL staining to assess ER stress, inflammation, and apoptosis in MPMs. Intracellular stearic acid accumulation induces toll-like receptor 4/2-independent inflammation that results in ER stress-mediated apoptosis of MPMs. Polarization of MPMs to a pro-inflammatory M1 phenotype increases their susceptibility to inflammation and ER stress, but not apoptosis, in response to co-treatment with stearic acid and TC.
CONCLUSIONS
Intracellular accumulation of stearic acid in MPMs activates inflammatory signaling, leading to ER stress-mediated apoptosis. M1 macrophages are more prone to stearic acid-induced inflammation and ER stress. These same pathways may be activated in macrophages residing in atherosclerotic plaques and metabolic tissues during conditions of obesity and hyperlipidemia.
Keywords: stearic acid, macrophage, ER stress, inflammation, apoptosis
Elevated serum free fatty acid (FFA) levels are independently associated with an increased risk for both cardiovascular disease (CVD) and type 2 diabetes (T2D).1, 2 Importantly, this systemic FFA toxicity may be mediated, in part, by activation of inflammatory pathways in macrophages residing in atherosclerotic plaques3, 4 or metabolic tissues, including adipose tissue5–8 and liver.9 The majority of the studies regarding macrophage lipotoxicity have sought to identify extracellular receptors that are responsible for mediating the inflammatory affects of saturated FFAs. Several groups have suggested that saturated FFAs may act through toll-like receptor 4 (TLR4) to generate macrophage inflammation and lipotoxicity;10 however, others dispute this claim.11 An additional study has demonstrated that saturated FFAs activate a TLR2-dependent apoptotic pathway in macrophages undergoing endoplasmic reticulum (ER) stress.12
Recent studies suggest that intracellular accumulation of FFAs within macrophages may play a pathogenic role in the progression of atherosclerosis. Multiple FFA species are present in macrophage-rich regions of human atherosclerotic lesions, and increased FFA concentrations are observed in inflammatory plaques from diabetic individuals.13 In addition, treatment of human macrophages with dyslipidemic serum increases intracellular FFA concentrations.14, 15 Therefore, determining the consequences of intracellular FFA accumulation within macrophages is of critical importance. Our lab has previously shown that accumulation of the saturated FFA, stearic acid, in macrophages results in apoptotic cell death;16 however, the mechanism of lipotoxicity remains unknown.
The ER is not simply a site of protein synthesis and maturation, but also functions as a nutrient sensor to regulate the energy needs of the cell. Therefore, changes in nutrient availability or inflammatory status can cause ER stress and activation of the unfolded protein response (UPR). In fact, increased levels of free cholesterol in macrophages (induced by inhibition of ACAT activity)17 or exposure of pancreatic β-cells,18 hepatocytes,19 or adipocytes20 to saturated FFAs results in UPR activation. The UPR is controlled by three ER-localized receptors: inositol-requiring enzyme-1α (IRE-1α), double-stranded RNA-dependent protein kinase-like ER kinase (PERK), and activating transcription factor 6 (ATF-6). ER stress initiates a signaling cascade that decreases the protein load entering the ER and increases the folding capacity of the organelle by up-regulating genes including binding immunoglobulin protein (BiP).21 ER stress can also activate inflammatory signaling through c-jun N-terminal kinase (JNK)22 and nuclear factor- κB (NF-κB)23 and initiate apoptosis.24 Interestingly, ER stress is increased in atherosclerotic plaques, whole adipose tissue, and liver of obese, dyslipidemic mice and humans.25–27 Decreasing ER stress through treatment with the chemical chaperone, 4-phenyl butyric acid (PBA), reduces systemic inflammation and insulin resistance in response to a high-fat diet28 and decreases macrophage apoptosis in atherosclerotic plaques during conditions of hyperlipidemia.29
The above data suggest that the systemic elevation of FFAs observed in obese individuals may result in ER stress activation, inflammation, and apoptosis in macrophages residing in metabolic tissues. In addition, recent data indicate that macrophages in atherosclerotic plaques accumulate FFAs;13–15 however, the molecular consequences of FFA accumulation in these immune cells remain ill defined. We have previously demonstrated that triacsin C (TC)-mediated inhibition of long-chain acyl-CoA synthetases (ACSL) during stearic acid treatment of mouse peritoneal macrophages (MPMs) increases intracellular stearic acid concentrations and results in apoptotic cell death.16 Using this model, we now show that stearic acid accumulation in macrophages results in TLR4/2-independent induction of inflammation, leading to ER stress-mediated apoptosis. Polarization of macrophages to a pro-inflammatory M1 phenotype increases the susceptibility of these cells to inflammation and ER stress, but not apoptosis. These findings advance our understanding of the molecular changes that occur in macrophages upon FFA accumulation, and demonstrate that intracellular FFAs induce macrophage ER stress, inflammation, and apoptosis. Activation of these signaling pathways, specifically in macrophages, may play a key role in the progression of atherosclerosis and insulin resistance.
MATERIALS AND METHODS
Thioglycollate-elicited MPMs were collected from wild-type (WT), TLR4-KO, or TLR2-KO mice on a C57BL/6 background and treated with 90 μM stearic acid and 2.5 μM TC to induce intracellular stearic acid accumulation.30 To determine the role of stearic acid-induced ER stress and inflammation, PBA (6 mM) and sodium salicylate (SS, 1 and 5 mM) co-treatments were performed. Lipopolysacchride (LPS, 10 ng/mL) and IL-13 (4 ng/mL) were utilized to investigate the effect of macrophage polarization on susceptibility to ER stress, inflammation, and apoptosis during stearic acid accumulation. See supplemental material for further description of methods.
RESULTS
Intracellular accumulation of stearic acid induces ER stress in MPMs
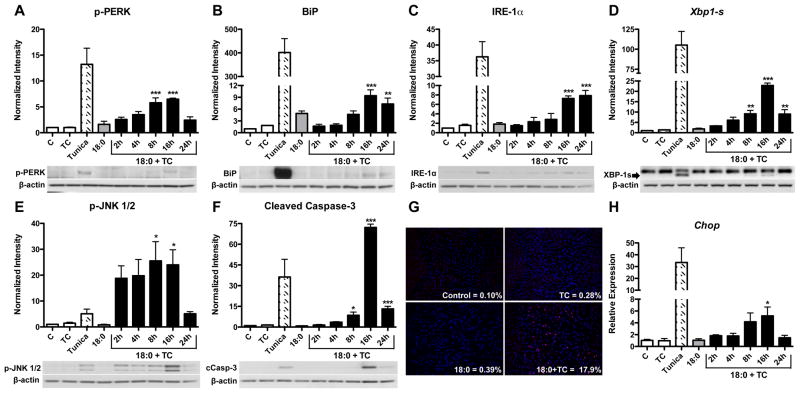
To determine if intracellular stearic acid accumulation within macrophages induces ER stress, MPMs were co-treated with 90 μM stearic acid (identified as 18:0 in figures) and 2.5 μM TC for 2 to 24 h. In addition, cells were treated with either TC or stearic acid alone for 24 h. Tunicamycin (1 μM for 24 h) was used as a positive control for ER stress induction. Co-treatment with stearic acid and TC significantly increased the phosphorylation of PERK after 8 (p<0.001) and 16 h (p<0.001) compared to vehicle-treated control cells (Figure 1A). Treatment with stearic acid or TC alone did not increase PERK phosphorylation, indicating that ER stress activation is due to intracellular stearic acid accumulation as opposed to fatty acid exposure without accumulation (Figure 1A). During the time-course of stearic acid accumulation, BiP and IRE-1α protein levels were increased at 16 (p<0.001) and 24 h (p<0.01) of co-treatment (Figure 1B–C). PCR amplification of the IRE-1α target gene, Xbp1, revealed an increase in the spliced form of Xbp1 after 8 (p<0.01), 16 (p<0.001), and 24 h (p<0.01, Figure 1D).
Figure 1. Intracellular stearic acid accumulation induces ER stress, inflammation, and apoptosis.
MPMs were co-treated with stearic acid (18:0, 90 μM) and TC (2.5 μM) for 2 to 24 h in DMEM containing 5% FBS. All controls [vehicle-treated (C), TC, tunicamycin (1 μM), and 18:0 alone] were treated for 24 h. A–C) Western blot analysis for markers of ER stress: A) phospho-PERK, B) BiP, C) IRE-1α. D) RT-PCR analysis of Xbp1 mRNA splicing. E–F) Western blot analysis of: E) phospho-JNK1/2 and F) cleaved caspase-3. G) Detection of apoptotic cells by TUNEL (red) and DAPI (blue) staining of MPMs treated for 16 h. Magnification: 20X. H) Gene expression analysis of Chop mRNA by real-time RT-PCR. Data are presented as mean ± SD, n = 3/group. Abbreviations: C, vehicle-treated control; TC, triacsin C; Tunica, tunicamycin; 18:0, stearic acid. * p<0.05, ** p<0.01, *** p<0.001 compared to control. Tunicamycin treatment was used as a positive control for Western blotting and is not included in statistical analysis.
Intracellular accumulation of stearic acid increases inflammation in MPMs
ER stress signaling can result in the activation of pro-inflammatory kinases, including JNK and NF-κB.22 A significant increase in the phosphorylation of JNK1/2 was observed after 8 (p<0.05) and 16 h (p<0.05) of stearic acid and TC co-treatment (Figure 1E). Additionally, intracellular stearic acid accumulation increased the expression of the NF-κB target genes, TNF-α (Tnf), IL-6 (Il6), and IL-1β (Il1b, p<0.05, Supplemental Figure 1A–C). These data indicate that intracellular accumulation of stearic acid induces both JNK- and NF-κB-mediated inflammatory pathways. In addition, gene expression of the chemokine, MCP-1 (Ccl2), was also increased by stearic acid and TC co-treatment (p<0.05, Supplemental Figure 1D). MIP-1α (Ccl3) expression was not modulated (Supplemental Figure 1E).
Intracellular accumulation of stearic acid induces apoptosis in MPMs
Cleavage of caspase-3, a key executioner caspase, was significantly increased after 8 (p<0.05) and 24 h (p<0.001) of treatment, with maximal cleavage observed at 16 h (p<0.001, Figure 1F). TUNEL staining was performed on macrophages at the peak of apoptosis (16 h). Approximately 17.9 ± 4.1% of cells co-treated with stearic acid and TC were TUNEL positive, while only 0.10 ± 0.10% of control cells stained positive for TUNEL (p<0.01, Figure 1G). Gene expression of CEBP-homologous protein (Chop), the primary mediator of apoptosis following ER stress, was increased after 16 h (p<0.05) of co-treatment with stearic acid and TC (Figure 1H). Additionally, higher concentrations of stearic acid (250 and 500 μM) in the absence of TC resulted in ER stress, inflammation, and apoptosis (Supplementary Figure 2), indicating that inhibition of ACSL activity accelerates, but is not required for, the lipotoxic effects of stearic acid.
Inhibition of ER stress during stearic acid loading of MPMs decreases apoptosis
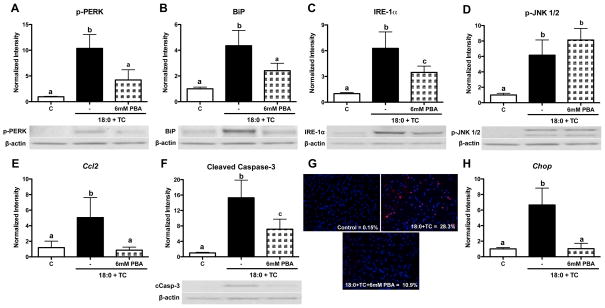
To determine if ER stress induced by intracellular stearic acid accumulation is upstream of inflammation and apoptosis, MPMs were treated with stearic acid and TC in the presence of the chemical chaperone, PBA. As expected, PBA co-treatment during stearic acid accumulation decreased phospho-PERK, BiP, and IRE-1α protein levels by approximately 50% (p<0.05 compared to stearic acid and TC, Figure 2A–C). Activation of ER stress can result in inflammation and secretion of chemokines.22, 23 However, phosphorylation of JNK1/2 was not decreased in the presence of PBA (Figure 2D). Gene expression of Il6 and Il1b was also not modulated by PBA co-treatment (Supplementary Figure 3A–B). In contrast, PBA co-treatment significantly decreased gene expression of the chemokine, Ccl2 (p<0.05 compared to stearic acid and TC, Figure 2E). In regards to apoptosis, PBA co-treatment decreased caspase-3 cleavage, TUNEL staining, and Chop gene expression (p<0.05 compared to stearic acid and TC, Figure 2F–H). Interestingly, PBA treatment in the absence of stearic acid resulted in a significant increase in cleaved caspase-3 protein levels (data not shown, p<0.01). This result suggests that basal ER stress signaling is necessary for these cells to cope with the stressors associated with cell culture.
Figure 2. PBA attenuates ER stress, chemokine secretion, and apoptosis, but not inflammation, induced by stearic acid accumulation.
MPMs were co-treated with stearic acid (18:0, 90 μM) and TC (2.5 μM) in the presence or absence of PBA (6 mM) for 16 h in DMEM containing 5% FBS. A–D) Western blot analysis for: A) phospho-PERK, B) BiP, C) IRE-1α, D) phospho-JNK1/2. E) Real-time RT-PCR analysis of Ccl2 gene expression. F) Western blot analysis for cleaved caspase-3. G) TUNEL (red) and DAPI (blue) staining to detect apoptotic cells. Magnification: 20X. H) Gene expression analysis of Chop mRNA by real-time RT-PCR. Data are presented as mean ± SD, n = 4–5/group. Abbreviations: C, vehicle-treated control; TC, triacsin C; 18:0, stearic acid; PBA, 4-phenyl butyric acid. Groups not connected by the same letter are significantly different, p<0.05.
Inhibition of inflammation during stearic acid loading of MPMs decreases ER stress and apoptosis
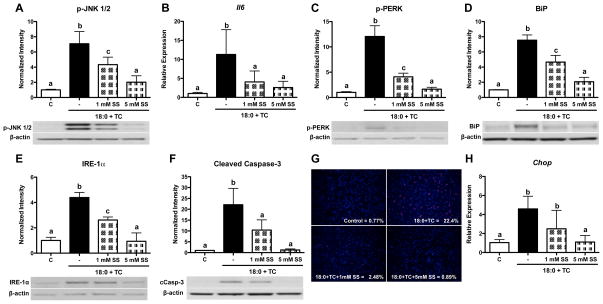
Sodium salicylate (SS) was utilized to further assess the role of inflammation during stearic acid accumulation in MPMs. Cells were co-treated with stearic acid and TC in the presence of 1 or 5 mM SS for 16 h. JNK phosphorylation (Figure 3A) and gene expression of the NF-κB target genes, Il6 (Figure 3B) and Ccl2 (data not shown), were decreased by either 1 or 5 mM SS (p<0.05 compared to stearic acid and TC). Inhibition of inflammation during stearic acid accumulation significantly decreased phospho-PERK, BiP, and IRE-1α protein levels (p<0.05 compared to stearic acid and TC, Figure 3C–E). Additionally, co-treatment with stearic acid, TC, and either 1 or 5 mM SS decreased caspase-3 cleavage, TUNEL staining, and Chop gene expression (p<0.05 compared to stearic acid and TC, Figure 3F–H). These data indicate that inflammation is responsible for the activation of ER stress and apoptosis during stearic acid accumulation. Similar to results obtained using PBA in the absence of stearic acid, treatment with SS alone also increased cleaved caspase-3 levels (data not shown, p<0.01).
Figure 3. Inhibition of inflammation by sodium salicylate decreases MPM ER stress and apoptosis during stearic acid accumulation.
MPMs were co-treated with stearic acid (18:0, 90 μM) and TC (2.5 μM) in the presence or absence of 1 or 5 mM SS for 16 h in DMEM containing 5% FBS. A) Western blot analysis of phospho-JNK1/2, B) Real-time RT-PCR analysis of Il6 gene expression. C–F) Western blot analysis for: C) phospho-PERK, D) BiP, E) IRE-1α, and F) cleaved caspase-3. G) TUNEL (red) and DAPI (blue) staining to detect apoptotic cells. Magnification: 20X. H) Gene expression analysis of Chop mRNA by real-time RT-PCR. Data are presented as mean ± SD, n = 3/group. Abbreviations: C, vehicle-treated control; TC, triacsin C; 18:0, stearic acid; SS, sodium salicylate. Groups not connected by the same letter are significantly different, p<0.05.
Intracellular stearic acid accumulation in MPMs increases inflammation, ER stress, and apoptosis independent of TLR4 and TLR2
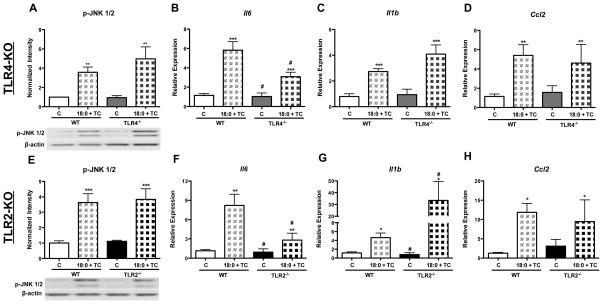
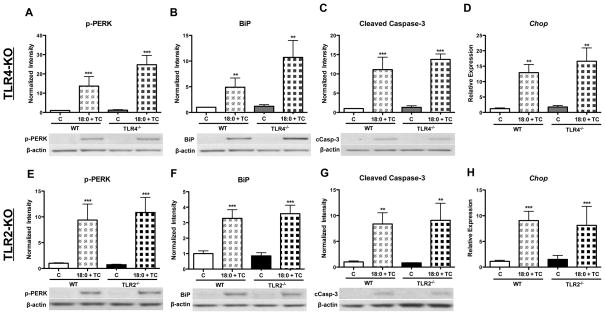
To determine if the inflammation, ER stress, and apoptosis generated by intracellular stearic acid accumulation in MPMs is dependent upon TLR4 or TLR2 signaling, WT, TLR4 knockout (TLR4-KO), and TLR2-KO macrophages were treated with stearic acid and TC for 16 h (Figure 4). Intracellular accumulation of stearic acid increased the phosphorylation of JNK 1/2 to the same extent in WT, TLR4-KO, and TLR2-KO macrophages (genotype effect = ns, Figure 4A and E). Deficiency of either TLR4 or TLR2 resulted in a significant decrease in Il6 gene expression during intracellular FFA accumulation (genotype effect = p<0.05, Figure 4B and F). However, gene expression of Il1b and Ccl2 was not decreased by TLR4 or TLR2 deficiency (Figure 4C–D, G–H). In fact, Il1b gene expression was higher in TLR2-KO macrophages co-treated with stearic acid and TC (genotype effect = p<0.05, Figure 4G). Stearic acid accumulation increased phospho-PERK and BiP protein levels to a similar extent in WT, TLR4-KO, and TLR2-KO macrophages (genotype effect = ns, Figure 5A–B, E–F). Stearic acid accumulation resulted in a significant increase in the cleaved form of caspase-3 in WT, TLR4-KO, and TLR2-KO macrophages (genotype effect = ns, Figure 5C and G). Additionally, gene expression of Chop was also increased upon stearic acid accumulation, regardless of genotype (genotype effect = ns, Figure 4D and H). Together, these data indicate that TLR4 and TLR2 signaling are not necessary for the induction of macrophage inflammation, ER stress, or apoptosis in response to intracellular accumulation of stearic acid.
Figure 4. Absence of TLR4 or TLR2 does not protect MPMs from inflammation induced by stearic acid accumulation.
MPMs were isolated from WT, TLR4-KO, and TLR2-KO mice and co-treated with stearic acid (18:0, 90 μM) and TC (2.5 μM) for 16 h in DMEM containing 5% FBS. A–D) Analysis of inflammatory markers in WT and TLR4-KO MPMs: A) Western blot of phospho-JNK 1/2, B–D) Real-time RT-PCR analysis of: B) Il6, C) Il1b, D) Ccl2. E–F) Analysis of inflammatory markers in WT and TLR2-KO MPMs: E) Western blot of phospho-JNK 1/2, F–H) Real-time RT-PCR analysis of: F) Il6, G) Il1b, H) Ccl2. Abbreviations: C, vehicle-treated control; TC, triacsin C; 18:0, stearic acid; WT, wild-type; TLR4−/−, toll-like receptor 4 knockout, TLR2−/−, toll-like receptor 2 knockout. * Treatment effect, p <0.05; ** Treatment effect, p<0.01; *** Treatment effect, p<0.001; # Genotype effect, p<0.05.
Figure 5. Absence of TLR4 or TLR2 does not protect MPMs from ER stress or apoptosis induced by stearic acid accumulation.
MPMs were isolated from WT, TLR4-KO, and TLR2-KO mice and co-treated with stearic acid (18:0, 90 μM) and TC (2.5 μM) for 16 h in DMEM containing 5% FBS. A–D) Analysis of ER stress and apoptotic markers in WT and TLR4-KO MPMs: A–C) Western blot analysis of: A) phospho-PERK, B) BiP, C) Cleaved caspase-3. D) Real-time RT-PCR analysis of Chop gene expression. E–F) Analysis of ER stress and apoptotic markers in WT and TLR2-KO MPMs: EG) Western blot analysis of: E) phospho-PERK, F) BiP, G) Cleaved caspase-3. H) Real-time RT-PCR analysis of Chop gene expression. Abbreviations: C, vehicle-treated control; TC, triacsin C; 18:0, stearic acid; WT, wild-type; TLR4−/−, toll-like receptor 4 knockout, TLR2−/−, toll-like receptor 2 knockout. ** Treatment effect, p<0.01; *** Treatment effect, p<0.001.
Polarization of MPMs to an M1 phenotype increases susceptibility to inflammation and ER stress, but not apoptosis, in response to intracellular stearic acid accumulation
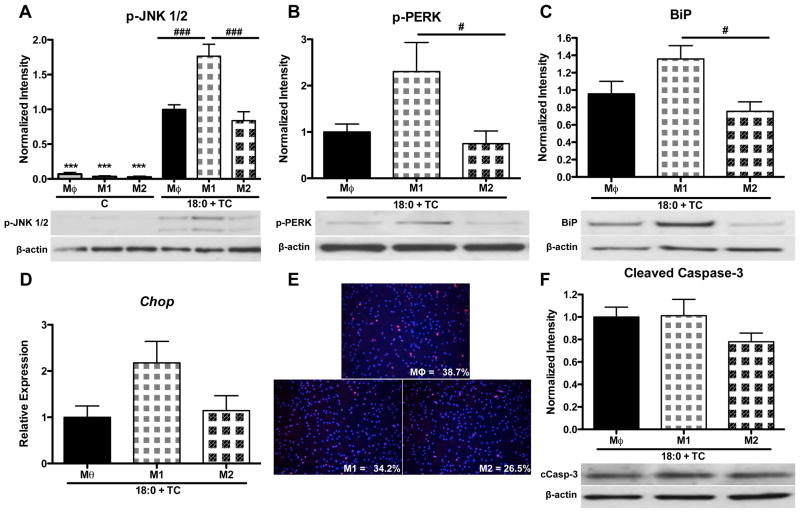
To determine if macrophage polarization modulates the susceptibility of these cells to stearic acid-induced inflammation, ER-stress, and apoptosis, MPMs were polarized for 24 h with vehicle (Mθ, un-polarized macrophages), LPS (M1 macrophages), or IL-13 (M2 macrophages). Polarization was confirmed by real-time RT-PCR analysis of M1 and M2 gene expression (Supplemental Figure 4). After polarization, each group was co-treated with stearic acid (90 μM) and TC (2.5 μM) in the presence of either vehicle (Mθ), LPS (M1), or IL-13 (M2) for an additional 16 h (40 h total treatment). To control for the effects of LPS and IL-13, a subset of macrophages was incubated with the respective polarizing agent in the absence of stearic acid and TC for the duration of the experiment. These control cells exhibited low JNK1/2 phosphorylation that was not modulated by macrophage phenotype (Figure 6A). Polarization in the absence of stearic acid and TC did not modulate the expression of ER stress or apoptotic markers (data not shown). However, when polarized macrophages were exposed to stearic acid and TC, M1 macrophages exhibited increased JNK phosphorylation when compared to either Mθ or M2 macrophages (Figure 6A, p<0.001), suggesting that M1 polarized macrophages are more susceptible to stearic acid-induced inflammation.
Figure 6. Polarization of MPMs to an M1 phenotype increases susceptibility to inflammation and ER stress in response to intracellular stearic acid accumulation.
MPMs were polarized for 24 h with vehicle, LPS (10 ng/ml), or IL-13 (4 ng/ml) to induce a Mθ, M1, or M2 phenotype, respectively. Cells were then co-treated with stearic acid (18:0, 90 μM) and TC (2.5 μM) for 16 h in the presence of polarization agents. Controls were treated for 40 h with polarizing agents in the absence of stearic acid and TC. A–C) Western blot analysis for: A) phospho-JNK 1/2, B) phospho-PERK, C) BiP. D) Gene expression analysis of Chop mRNA by real-time RT-PCR. E) Detection of apoptotic cells by TUNEL (red) and DAPI (blue) staining. Magnification: 20X. F) Western blot analysis of cleaved caspase-3. Data are presented as mean ± SD. n = 5–9/group. Abbreviations: C, control; TC, triacsin C; 18:0, stearic acid; Mθ, unpolarized macrophage; M1, LPS polarized macrophage; M2, IL-13 polarized macrophage. *** p<0.001 compared to Mθ of 18:0 + TC group, # p<0.05, ### p<0.001 compared to M1 of 18:0 + TC group.
Intracellular accumulation of stearic acid in M1 macrophages resulted in a 3-fold increase in PERK phosphorylation and a 45% increase in BiP protein levels (p<0.05 compared to M2, Figure 6B–C). There was a non-significant trend towards an increase in Chop gene expression in M1 macrophages (Figure 6D); however, there was no difference in TUNEL staining (Figure 6E) or caspase-3 cleavage (Figure 6F) between groups. Collectively, these results suggest that polarization of macrophages to an M1 phenotype increases susceptibility to stearic acid-induced inflammation and ER stress, but not apoptosis.
DISCUSSION
Our previous studies have demonstrated that co-treatment of MPMs with low-level stearic acid and TC blocks ACSL activity, thus inducing intracellular FFA accumulation and lipotoxicity.30 Using this model, we now show that stearic acid accumulation in macrophages activates ER stress, inflammation, and apoptosis independent of TLR4/2 signaling. While exposure of macrophages to high concentrations of stearic acid induced ER stress, inflammation, and apoptosis (Supplemental Figure 2), exposure to low-level stearic acid in the absence of TC had no effect on these parameters (Figure 1), suggesting that intracellular accumulation of stearic acid accelerates lipotoxicity. It is possible that the ability of a macrophage to metabolize or store stearic acid is overwhelmed by higher concentrations of stearic acid, thus leading to intracellular FFA accumulation and lipotoxicity. Together, the PBA and SS studies demonstrate that stearic acid accumulation in MPMs initiates inflammatory signaling that culminates in the activation of ER stress-mediated apoptosis. Polarization of macrophages to a pro-inflammatory M1 phenotype increases susceptibility to stearic acid-induced inflammation and ER stress, but not apoptosis (See Supplemental Figure 5 for diagram).
Research conducted in the past several decades has established a role for saturated FFAs in the development of CVD and T2D.1, 2 Additionally, recent studies indicate that macrophages of atherosclerotic plaques accumulate FFAs;13–15 however, the molecular consequences of intracellular FFAs remain relatively unknown. Increased partitioning of FFA to TG (via DGAT-1 overexpression) protects macrophages from FFA-induced inflammation,31 suggesting that accumulation of FFAs, rather than TG, in these immune cells may be responsible for inflammatory signaling. Our results support these findings and indicate that intracellular FFAs may be especially pathogenic, as blocking the incorporation of stearic acid into TG (through inhibition of ACSL activity) greatly increases the intracellular accumulation and lipotoxic potential of this FFA. Interestingly, studies have shown that polymorphisms in ACS genes, including ACSL-1 and medium-chain acyl-CoA synthetase 2, confer an increased risk for metabolic syndrome in human subjects.32, 33 Although the direct functional consequences of polymorphisms in these ACS genes are not known, it is believed that disruption of ACS activity is responsible for the increased risk for metabolic syndrome. Several studies have shown that insulin sensitizing thiazolidinediones increase the transcription of ACSL-1 (the primary ACS isoform in macrophages) in the adipose tissue and liver.34, 35 Together with our current data, these studies demonstrate that FFA accumulation occurs in plaque macrophages and may exacerbate inflammation during obesity/hyperlipidemia.
Previous studies have shown that ER stress signaling can culminate in apoptotic cell death.24 Our results demonstrate that reducing ER stress signaling (via PBA) also abrogates apoptosis, indicating that stearic acid accumulation in MPMs induces ER stress-mediated apoptosis. Surprisingly, treatment with 6 mM PBA during stearic acid accumulation only decreased ER stress activation by 50%. This finding is in contrast to previously published studies showing that 3 mM PBA is sufficient to eliminate ER stress caused by extracellular exposure to high levels of palmitic acid.29 These results imply that intracellular and extracellular saturated FFAs may activate ER stress pathways by different mechanisms. Because correction of the protein folding defect only partially reduces UPR signaling, it is interesting to speculate that intracellular stearic acid accumulation may alter the composition of the ER membrane to activate ER stress signaling independently of an increase in unfolded proteins. In support of this hypothesis, a recent paper has shown that alterations in the lipid composition of the ER membrane during obesity initiate ER stress signaling.36
Activation of ER stress pathways has been shown to initiate inflammation through JNK and NF-κB.22, 23 However, in the current study, reducing ER stress during FFA accumulation in macrophages had no effect on inflammatory cytokine expression (Figure 2). Interestingly, PBA co-treatment was sufficient to decrease expression of the chemokine, Ccl2. These findings indicate that intracellular stearic acid activates inflammatory signaling independent of the ER stress response, whereas Ccl2 expression is dependent upon ER stress signaling. Inhibition of inflammation by co-treatment with SS demonstrated that the inflammation generated by stearic acid accumulation initiates ER stress-mediated apoptosis, suggesting that inflammation is the main driving force for the lipotoxic effects of intracellular FFA accumulation.
Our data point to the importance of FFA-induced inflammation in activating macrophage ER stress and apoptosis. Some have suggested that saturated FFAs directly activate TLR4 or TLR2.10,12 However, our data support recent reports indicating that FFAs may not act through these pattern recognition receptors.11 We have shown that intracellular stearic acid accumulation potently activates inflammation, ER stress, and apoptosis in WT, TLR4-KO, and TLR2-KO macrophages. Additionally, low-level stearic acid in the absence of TC does not result in either FFA accumulation or inflammation. If cell surface TLR signaling were involved, extracellular exposure to stearic acid (as opposed to intracellular accumulation) should be a more potent activator of inflammation.
Interestingly, a recent study reported that saturated FFAs initiate inflammation by modifying the composition of the cell membrane rather than acting through extracellular receptors.37 Holzer and colleagues show that saturated FFAs alter the localization and activity of c-Src, a membrane-anchored tyrosine kinase involved in the activation of inflammation.37 Exposure of fibroblasts to palmitic or stearic acid partitions c-Src into intracellular lipid rafts where the active kinase phosphorylates JNK, leading to inflammation.37 It is possible that c-Src also plays a role in saturated FFA-mediated inflammation in macrophages. In fact, c-Src is activated in macrophages of human atherosclerotic lesions and may contribute to plaque inflammation and instability.38
Pro-inflammatory M1 polarized macrophages aberrantly accumulate in atherosclerotic lesions,3, 4 adipose tissue,5–8 and liver,9 and during obesity. We have shown that polarization to a M1 phenotype increases the susceptibility of macrophages to stearic acid-induced inflammation and ER stress when compared to anti-inflammatory M2 macrophages. Our data demonstrates that FFA-induced inflammation is responsible for the initiation of macrophage ER stress (Figure 3). Therefore, the increased susceptibility of M1 macrophages to stearic acid-induced ER stress is likely due to increased inflammatory signaling in the presence of LPS. Although M1 polarization augmented inflammation and ER stress activation during stearic acid accumulation, there was no significant difference in the expression of apoptotic markers between M1 and M2 macrophages. Recent studies have demonstrated that engagement of TLRs reduces apoptotic cell death in response to ER stress by promoting the up-regulation of the guanine nucleotide exchange factor, eIF2B.39, 40 It is possible that this process also occurs when macrophages are polarized to an M1 phenotype. Thus, M1 macrophages would be protected from accelerated apoptosis even in the presence of elevated ER stress signaling during FFA accumulation. These findings could suggest that M1 macrophages are particularly pathogenic because they resist apoptotic cell death and continue to secrete inflammatory cytokines into their local environment.
ER stress activation and inflammation are observed in atherosclerotic lesions, adipose tissue, and liver of obese/hyperlipidemic mice and humans.25–27 M1 macrophages are recruited these tissues during obesity and contribute to the development of CVD and T2D.3–9 Our data suggest that FFA accumulation in macrophages residing in atherosclerotic plaques and metabolic tissues may play an essential role in the pathogenesis of obesity-related disorders.
Supplementary Material
Acknowledgments
We would like to thank the members of our laboratory for their careful reading of our manuscript.
Sources of Funding: This project was funded by NIH grant HL089466. A.H.H. is also supported by an American Diabetes Association Career Development Award (1-07-CD-10). E.K.A was supported by the Cellular, Biochemical, and Molecular Sciences Training Program (NIH T32GM008554). A.A.H is supported by the Cardiovascular Research Training Program (T32 HL007411-30). Imaging experiments were performed in the Vanderbilt Cell Imaging Shared Resources Core (NIH grants CA68485, DK20593, DK58404, HD15052, DK59637 and EY08126).
Footnotes
Disclosures: The authors have no conflict of interest.
References
- 1.Mathew M, Tay E, Cusi K. Elevated plasma free fatty acids increase cardiovascular risk by inducing plasma biomarkers of endothelial activation, myeloperoxidase and pai-1 in healthy subjects. Cardiovasc Diabetol. 2010;9:1–9. doi: 10.1186/1475-2840-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pankow JS, Duncan BB, Schmidt MI, Ballantyne CM, Couper DJ, Hoogeveen RC, Golden SH. Fasting plasma free fatty acids and risk of type 2 diabetes: The atherosclerosis risk in communities study. Diabetes Care. 2004;27:77–82. doi: 10.2337/diacare.27.1.77. [DOI] [PubMed] [Google Scholar]
- 3.Ball RY, Stowers EC, Burton JH, Cary NR, Skepper JN, Mitchinson MJ. Evidence that the death of macrophage foam cells contributes to the lipid core of atheroma. Atherosclerosis. 1995;114:45–54. doi: 10.1016/0021-9150(94)05463-s. [DOI] [PubMed] [Google Scholar]
- 4.Smith JD, Trogan E, Ginsberg M, Grigaux C, Tian J, Miyata M. Decreased atherosclerosis in mice deficient in both macrophage colony-stimulating factor (op) and apolipoprotein e. Proc Natl Acad Sci U S A. 1995;92:8264–8268. doi: 10.1073/pnas.92.18.8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. The Journal of clinical investigation. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008;57:3239–3246. doi: 10.2337/db08-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kiki I, Altunkaynak BZ, Altunkaynak ME, Vuraler O, Unal D, Kaplan S. Effect of high fat diet on the volume of liver and quantitative feature of kupffer cells in the female rat: A stereological and ultrastructural study. Obes Surg. 2007;17:1381–1388. doi: 10.1007/s11695-007-9219-7. [DOI] [PubMed] [Google Scholar]
- 10.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. Tlr4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erridge C, Samani NJ. Saturated fatty acids do not directly stimulate toll-like receptor signaling. Arterioscler Thromb Vasc Biol. 2009;29:1944–1949. doi: 10.1161/ATVBAHA.109.194050. [DOI] [PubMed] [Google Scholar]
- 12.Seimon TA, Nadolski MJ, Liao X, Magallon J, Nguyen M, Feric NT, Koschinsky ML, Harkewicz R, Witztum JL, Tsimikas S, Golenbock D, Moore KJ, Tabas I. Atherogenic lipids and lipoproteins trigger cd36-tlr2-dependent apoptosis in macrophages undergoing endoplasmic reticulum stress. Cell Metab. 2010;12:467–482. doi: 10.1016/j.cmet.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mas S, Martinez-Pinna R, Martin-Ventura JL, Perez R, Gomez-Garre D, Ortiz A, Fernandez-Cruz A, Vivanco F, Egido J. Local non-esterified fatty acids correlate with inflammation in atheroma plaques of patients with type 2 diabetes. Diabetes. 2010;59:1292–1301. doi: 10.2337/db09-0848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong BX, Kyle RA, Myhill PC, Croft KD, Quinn CM, Jessup W, Yeap BB. Dyslipidemic diabetic serum increases lipid accumulation and expression of stearoyl-coa desaturase in human macrophages. Lipids. 2011;46:931–941. doi: 10.1007/s11745-011-3578-5. [DOI] [PubMed] [Google Scholar]
- 15.Wong BX, Kyle RA, Croft KD, Quinn CM, Jessup W, Yeap BB. Modulation of macrophage fatty acid content and composition by exposure to dyslipidemic serum in vitro. Lipids. 2011;46:371–380. doi: 10.1007/s11745-011-3528-2. [DOI] [PubMed] [Google Scholar]
- 16.Saraswathi V, Hasty AH. Inhibition of long-chain acyl coenzyme a synthetases during fatty acid loading induces lipotoxicity in macrophages. Arterioscler Thromb Vasc Biol. 2009;29:1937–1943. doi: 10.1161/ATVBAHA.109.195362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng B, Yao PM, Li Y, Devlin CM, Zhang D, Harding HP, Sweeney M, Rong JX, Kuriakose G, Fisher EA, Marks AR, Ron D, Tabas I. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat Cell Biol. 2003;5:781–792. doi: 10.1038/ncb1035. [DOI] [PubMed] [Google Scholar]
- 18.Karaskov E, Scott C, Zhang L, Teodoro T, Ravazzola M, Volchuk A. Chronic palmitate but not oleate exposure induces endoplasmic reticulum stress, which may contribute to ins-1 pancreatic beta-cell apoptosis. Endocrinology. 2006;147:3398–3407. doi: 10.1210/en.2005-1494. [DOI] [PubMed] [Google Scholar]
- 19.Pfaffenbach KT, Gentile CL, Nivala AM, Wang D, Wei Y, Pagliassotti MJ. Linking endoplasmic reticulum stress to cell death in hepatocytes: Roles of c/ebp homologous protein and chemical chaperones in palmitate-mediated cell death. Am J Physiol Endocrinol Metab. 2010;298:E1027–1035. doi: 10.1152/ajpendo.00642.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alhusaini S, McGee K, Schisano B, Harte A, McTernan P, Kumar S, Tripathi G. Lipopolysaccharide, high glucose and saturated fatty acids induce endoplasmic reticulum stress in cultured primary human adipocytes: Salicylate alleviates this stress. Biochem Biophys Res Commun. 2010;397:472–478. doi: 10.1016/j.bbrc.2010.05.138. [DOI] [PubMed] [Google Scholar]
- 21.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 22.Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D. Coupling of stress in the er to activation of jnk protein kinases by transmembrane protein kinase ire1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 23.Hu P, Han Z, Couvillon AD, Kaufman RJ, Exton JH. Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through ire1alpha-mediated nf-kappab activation and down-regulation of traf2 expression. Mol Cell Biol. 2006;26:3071–3084. doi: 10.1128/MCB.26.8.3071-3084.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H, Stevens JL, Ron D. Chop is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12:982–995. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 26.Sharma NK, Das SK, Mondal AK, Hackney OG, Chu WS, Kern PA, Rasouli N, Spencer HJ, Yao-Borengasser A, Elbein SC. Endoplasmic reticulum stress markers are associated with obesity in nondiabetic subjects. The Journal of clinical endocrinology and metabolism. 2008;93:4532–4541. doi: 10.1210/jc.2008-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Myoishi M, Hao H, Minamino T, Watanabe K, Nishihira K, Hatakeyama K, Asada Y, Okada K, Ishibashi-Ueda H, Gabbiani G, Bochaton-Piallat ML, Mochizuki N, Kitakaze M. Increased endoplasmic reticulum stress in atherosclerotic plaques associated with acute coronary syndrome. Circulation. 2007;116:1226–1233. doi: 10.1161/CIRCULATIONAHA.106.682054. [DOI] [PubMed] [Google Scholar]
- 28.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, Hotamisligil GS. Chemical chaperones reduce er stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erbay E, Babaev VR, Mayers JR, Makowski L, Charles KN, Snitow ME, Fazio S, Wiest MM, Watkins SM, Linton MF, Hotamisligil GS. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat Med. 2009;15:1383–1391. doi: 10.1038/nm.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saraswathi V, Hasty AH. Inhibition of long-chain acyl coenzyme a synthetases during fatty acid loading induces lipotoxicity in macrophages. Arterioscler Thromb Vasc Biol. 2009;29:1937–1943. doi: 10.1161/ATVBAHA.109.195362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koliwad SK, Streeper RS, Monetti M, Cornelissen I, Chan L, Terayama K, Naylor S, Rao M, Hubbard B, Farese RV., Jr Dgat1-dependent triacylglycerol storage by macrophages protects mice from diet-induced insulin resistance and inflammation. J Clin Invest. 2010;120:756–767. doi: 10.1172/JCI36066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phillips CM, Goumidi L, Bertrais S, Field MR, Cupples LA, Ordovas JM, Defoort C, Lovegrove JA, Drevon CA, Gibney MJ, Blaak EE, Kiec-Wilk B, Karlstrom B, Lopez-Miranda J, McManus R, Hercberg S, Lairon D, Planells R, Roche HM. Gene-nutrient interactions with dietary fat modulate the association between genetic variation of the acsl1 gene and metabolic syndrome. J Lipid Res. 2010;51:1793–1800. doi: 10.1194/jlr.M003046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lindner I, Rubin D, Helwig U, Nitz I, Hampe J, Schreiber S, Schrezenmeir J, Doring F. The l513s polymorphism in medium-chain acyl-coa synthetase 2 (macs2) is associated with risk factors of the metabolic syndrome in a caucasian study population. Mol Nutr Food Res. 2006;50:270–274. doi: 10.1002/mnfr.200500241. [DOI] [PubMed] [Google Scholar]
- 34.Martin G, Schoonjans K, Lefebvre AM, Staels B, Auwerx J. Coordinate regulation of the expression of the fatty acid transport protein and acyl-coa synthetase genes by pparalpha and ppargamma activators. J Biol Chem. 1997;272:28210–28217. doi: 10.1074/jbc.272.45.28210. [DOI] [PubMed] [Google Scholar]
- 35.Clemenz M, Frost N, Schupp M, Caron S, Foryst-Ludwig A, Bohm C, Hartge M, Gust R, Staels B, Unger T, Kintscher U. Liver-specific peroxisome proliferator-activated receptor alpha target gene regulation by the angiotensin type 1 receptor blocker telmisartan. Diabetes. 2008;57:1405–1413. doi: 10.2337/db07-0839. [DOI] [PubMed] [Google Scholar]
- 36.Fu S, Yang L, Li P, Hofmann O, Dicker L, Hide W, Lin X, Watkins SM, Ivanov AR, Hotamisligil GS. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature. 2011;473:528–531. doi: 10.1038/nature09968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holzer RG, Park EJ, Li N, Tran H, Chen M, Choi C, Solinas G, Karin M. Saturated fatty acids induce c-src clustering within membrane subdomains, leading to jnk activation. Cell. 2011;147:173–184. doi: 10.1016/j.cell.2011.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Al-Khalili O, Duke BJ, Zeltwanger S, Eaton DC, Spier B, Stockand JD. Cloning of the proto-oncogene c-src from rat testis. DNA Seq. 2001;12:425–429. doi: 10.3109/10425170109084469. [DOI] [PubMed] [Google Scholar]
- 39.Woo CW, Cui D, Arellano J, Dorweiler B, Harding H, Fitzgerald KA, Ron D, Tabas I. Adaptive suppression of the atf4-chop branch of the unfolded protein response by toll-like receptor signalling. Nat Cell Biol. 2009;11:1473–1480. doi: 10.1038/ncb1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Woo CW, Kutzler L, Kimball SR, Tabas I. Toll-like receptor activation suppresses er stress factor chop and translation inhibition through activation of eif2b. Nat Cell Biol. 2012 doi: 10.1038/ncb2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.