Summary
This study analysed the initial effects of the combination of oestrogen deficiency with a calcium-deficient diet on alveolar bone repair. Sixty-three 3-month-old female rats were either ovariectomized (OVX, n = 42) or sham-operated (SHAM, n = 21). Among the 42 ovariectomized rats, 21 received standard commercial food (OVX) and 21 received food with low calcium content (ESP). The mandibular first molars were extracted bilaterally 15 days after ovariectomy or sham surgery. The rats were weighed and killed at 7, 21 and 45 days after tooth extraction. The results were evaluated by descriptive microscopic analysis, histomorphometry of the trabecular and osteoid volume and mast cell counts. Analysis of the results indicated that trabecular volume and mast cell counts increased significantly over time, while osteoid volume decreased over time. Comparisons between the SHAM and OVX groups demonstrated no statistical differences, while comparison between the OVX and ESP groups indicated differences in trabecular volume and the number of mast cells. The data suggest that hormonal deficiency does not delay alveolar bone repair in OVX rats; however, oestrogen deficiency associated with calcium deficiency can lead to bone resorption through the activation of mast cells.
Keywords: bone turnover, low calcium intake, ovariectomy, wound healing
Osteoporosis is a syndrome characterized by the reduction in and deterioration of bone microarchitecture, leading to bone fragility and increased risk of fracture (Moreira 1998). Even though osteoporosis may be secondary to several conditions, the most common types occur owing to ageing and menopause (Rosenber 2005). Oestrogen deficiency caused by menopause is the most important aetiological factor for its development (Compston 2010).
The association between osteoporosis in women and bone loss in the jaws, periodontal disease, tooth loss and other oral tissue changes remains controversial (Dervis 2005). Kribbs (1990) showed that osteoporotic postmenopausal women presented lower mandibular bone mass and density than non-osteoporotic women. Furthermore, oestrogen seems to be involved in reduced rates of cutaneous wound healing during the healthy ageing of women (Ashcroft et al. 1997).
Considering that bone remodelling and bone repair share many molecular and cellular events and the first is completely changed in osteoporosis (Moreira 1998), investigating whether this metabolic disease influences alveolar bone remodelling and healing is of interest to dental health professionals.
Ovariectomized rats were used for this purpose, causing loss of bone mass secondary to the hormonal deficiency (Arjmandi et al. 2002). However, the jaws of female rats are seldom affected by the hormonal deficiency owing to ovariectomy (Ishihara et al. 1999; Teófilo et al. 2003). According to Ishihara et al. (1999), in postmenopausal osteoporosis, bone loss predominantly occurs in those areas formed by endochondral ossification, such as distal femurs, rather than areas of intramembranous ossification, such as maxillae.
Besides the type of ossification, there is evidence of other factors that determine a different behaviour in jaw bones compared to long bones. The maintenance of bone mass occurs owing to anatomically diverse bone matrix characteristics generated by heterogeneous osteoblast populations, by diverse osteocyte densities, by different osteoblast proliferation rates and in response to oestrogen. All are associated with distinct mRNA expression. Thus, there are inherent genetic differences between bone of cranial origin and bone of non-cranial origin (Rawlinson et al. 2009).
The peculiar masticatory habits of rodents place large loads on oral bones (Zaffe et al. 1999). By analogy, this mastication is comparable to physical activity to prevent bone loss in osteoporotic postmenopausal woman. Observation verified that ovariectomized rats fed standard grind commercial diet presented significantly lower means of maxillary trabecular volume compared to those fed standard commercial diet or low-calcium diet (Costa et al. 2011).
Considering all the information above, ovariectomy alone does not appear to be a suitable experimental model for studying oral bone loss related to skeletal osteoporosis. Thus, to worsen oral bone loss, combining ovariectomy with mechanical unloading is required (Zaffe et al. 1999).
With improvement in animal models used to study the effects of postmenopausal osteoporosis in the jaws, it may be possible to elucidate how it interferes in the bone repair process and even propose ways to accelerate and improve repair in cases where there is systemic involvement.
An increase in the number of bone marrow mast cells, mostly the mucosal subtype, is also observed following ovariectomy (Lesclous & Saffar 1999). The concept that mast cells play a potentially pathogenic role in osteoporosis bone loss is novel but advances consistently. Augmentation in mast cell numbers accelerates bone turnover, while their deficiency causes delay in osteoclastic recruitment and the osteoblastic formation phase (Chiappetta & Gruber 2006).
Therefore, this study was conducted to investigate the effects of ovariectomy in association with a calcium-deficient diet on the alveolar bone repair process. Our hypothesis was that osteoporosis and low calcium intake may impair new bone formation in postextraction sockets, delaying alveolar bone repair.
Material and methods
Sixty-three 3-month-old female rats (Rattus norvegicus, albinus, Wistar), weighing approximately 300 g, were kept in cages at room temperature supplied by the Animal Laboratory of São José dos Campos School of Dentistry of the State University of Sao Paulo (UNESP). The animals received distilled water ad libitum and were fed standard commercial food (Nutrilabor Guabi®, Mogiana Alimentos, Campinas, SP, Brazil) with a calcium content of up to 1.2% and minimum phosphate content of 0.8% up to day one of study, the day of the ovariectomy.
This study followed the ethical principles for animal studies (COBEA) and was approved by the Research Ethics Committee of São José dos Campos School of Dentistry, UNESP, under protocol n. 027/2005/PA-CEP.
General anaesthesia was performed using a solution of 14.2 mg/kg of 2-(2,6-xylidine)-5,6-dihydro-4H-1,3-thiazine chlorhydrate (Anasedan®; Bayer do Brasil, São Paulo, SP, Brasil) and 38.5 mg/kg of base ketamine (Dopalen®; Agribands do Brasil Ltda., Paulínia, SP, Brasil) by intramuscular injection.
The rats were divided randomly into two groups: OVX, composed of 42 rats submitted to ovariectomy; and SHAM, composed of 21 rats, submitted to the surgical stress.
All rats were weighed at study onset and before killing. Rats in the ovariectomized group were anaesthetized, submitted to trichotomy and incision of the subcutaneous tissue and musculature. The ovaries were exposed, a ligature was placed for haemorrhage control, and then the ovaries were removed bilaterally. Rats in the SHAM group, submitted to false ovariectomy, were subjected to the same procedures except for ligature and ovary removal.
The muscular layer was sutured with a resorbable suture Catgut 4.0 (Cirumédica-Brazil), and the skin was sutured with silk suture 4.0 (Ethicon-Johnson & Johnson, São José dos Campos, SP, Brasil), followed by final antisepsis with iodine alcohol.
Animals and diet
Administration of the specific foods of each group was initiated on the day of ovariectomy. The quantity of food ingested per day was predetermined as 20 g per rat, assuring the same volume of food for all the groups. Each cage contained seven rats, to which 140 g of diet were supplied daily (Kawamoto & Nagaoka 2000).
The ovariectomized rats were subdivided into two groups: the special food group (ESP) and the OVX group. The first was composed of 21 rats fed a calcium-deficient diet, (AIN-93M®) containing calcium (0.1%) and phosphate (0.5%) (RHOSTER Indústria e Comércio LTDA, Vargem Grande Paulista, SP, Brazil) from day one.
The OVX group was composed of 21 rats that were fed standard commercial food, namely Nutrilabor Guabi®, with a calcium content of up to 1.2% and minimum phosphate content of 0.8%. The 21 rats of the SHAM group were also fed standard food.
Exodontia
The mandibular molar was chosen because its four roots are parallel. All the rats were submitted to extraction of the mandibular first molars, bilaterally, 15 days after ovariectomy or sham surgery, under general anaesthesia. The extraction comprised fiberotomy, luxation, tooth removal and simple stitches with silk suture n. 4.0. All animals received 1 mg/kg of anti-inflammatory (sodium diclofenac 75 mg, injectable Voltaren; Geigy, São Paulo, SP, Brasil) and 0.1 ml of antibiotic solution (Pentabiótico; Fort Dodge Ltda., Campinas, SP, Brasil).
Euthanasia
Seven rats from each group were killed 7 days following extraction, another seven after 21 days, and the remaining seven 45 days following tooth extraction. The rats were anaesthetized and killed in a guillotine. The success of OVX was confirmed at necropsy by observation of marked atrophy of the uterine horns.
Tissue preparation
The mandibles were removed, dissected and fixed in 10% formalin solution. The left mandibles of each period were embedded in methyl methacrylate. The specimens were dehydrated in alcohol, cleared in xylol and immersed in a solution containing 85% methyl methacrylate (Sigma-Aldrich Chemistry St Louis, MO, USA) and 15% dibutyl phthalate (Fluka Sigma-Aldrich Chemistry, St Louis, MO, USA), with increasing doses of benzoyl peroxide catalyst (Vetec Química Fina Ltda, Duque de Caxias, Rio de Janeiro, Brazil) (up to 3 g/100 ml of solution), and finally cured at 37 °C.
The blocks were sectioned along the mandibular long axis and transversally to the roots, using a Labcut 1010 sectioning appliance (Extec, Enfield, CT, USA), up to approximately 50 μm of thickness, then ground and polished in a Labpol 8-12 polishing machine (Extec). After staining with toluidine blue, the sections were photographed for histomorphometric analysis of the trabecular volume, osteoid volume and mast cell counts.
The specimens on the right side of each subgroup were demineralized in (Ethylenen = dinitrilo) tetraacetic acid (EDTA, Titriplex®; Merck DGaA, Darmstadt, Germany) and submitted to routine histological processing and embedding in paraffin. The specimens were sectioned along the mandibular long axis and transversally to the roots, and sections were stained with haematoxylin and eosin (HE) for qualitative histological analysis. The groups were coded for blind analysis.
Weight analysis
The weight of the animals obtained at study onset (IW) and completion (FW) was used to calculate weight gain (WG), according to the following formula: WG = 100 × (FW −IW)/IW.
Qualitative histological analysis
The demineralized sections were submitted to histological analysis under a light microscope for qualitative tissue evaluation of the bone repair process.
Histomorphometric analysis (trabecular volume and osteoid volume)
Five fields of newly formed bone tissue on the apical region of the mesial or distal tooth socket of each rat (n = 7) were photographed using a Zeiss Axiophot 2 microscope (Carl Zeiss, Oberköchen, Germany) with 10× ocular and 20× objective, digitized by a Sony digital camera (model Cybershot) connected to the microscope. The trabecular and osteoid volumes were quantified by points counting planimetry, using the software Image-J. For this purpose, a grid composed of 70 points resulting from intersections between vertical (7) and horizontal lines (10) was positioned on the histological image for counting. This software permitted construction of the grid and positioning on the histological image, to count points that overlapped for the parameter analysed (trabecular or osteoid bone). The following parameters (Parfitt et al. 1987) were observed: trabecular volume, expressed as percentage of volume filled by trabecular bone, mineralized or not, in relation to the volume filled by bone marrow and bone trabeculae; and osteoid volume, expressed as percentage of volume filled by non-mineralized bone, in relation to the volume filled by mineralized and non-mineralized trabecular bone.
In this study, histomorphometric analysis was limited to static parameters. The remodelling of bone surrounding the socket was accessed by the dynamic parameter, mineral apposition rate, using fluorescent labelling with tetracycline in two steps (1 day after extraction and 2 days before euthanasia (data not shown).
Analysis of mast cells
Mast cells were recognized based on their metachromatic reaction to toluidine blue and characteristic morphological aspect. The number of mast cells per rat corresponded to the mean of values obtained in the same five fields analysed in the histomorphometric analysis.
Statistical analysis
Two-way analysis of variance (anova) and the Tukey test for multiple comparisons (5%) were always performed twice for comparison. The first included the variables study period (7, 21 and 45 days) and the factor ovariectomy (presence or absence of ovarian hormones), comparing the SHAM and OVX groups.
The second included the variables study period (7, 21 and 45 days) and type of food (standard or calcium-deficient), comparing the OVX and ESP groups.
Results
Fluorescent labelling with tetracycline in two steps could not be used. This analysis was used as a dynamic parameter to calculate mineral deposition rate. However, osteoid bone formation in the socket at day one presented no tetracycline labelling for the first step, impairing this analysis regarding alveolar bone healing.
Weight gain
The percentage weight gain values of the groups following application of the formula showed that the mean weight gain increased with time (Table 1).
Table 1.
Effects of ovariectomy and low-calcium diet on body weight (n = 7)
| Time (days) | |||
|---|---|---|---|
| 7 | 21 | 45 | |
| SHAM | 7.23 ± 15.26c | 7.67 ± 6.07c | 17.44 ± 10.32bc |
| OVX | 12.87 ± 3.92bc | 17.61 ± 10.92bc | 24.39 ± 10.87ab |
| ESP | 11.74 ± 5.51bc | 22.15 ± 5.51b | 39.25 ± 11.43a |
Values are means ± standard deviation (SD).
Values that do not share the same superscript letters are significantly different from each other. (P < 0.05).
Considering the factor absence of ovaries, comparison between the SHAM and OVX groups demonstrated that the OVX groups presented greater weight gain (P = 0.023). Addition of the interactions between time of euthanasia and ovariectomy, the Tukey test comparisons revealed similar homogeneous groups: OVX7 and SHAM7; OVX21 and SHAM21; and OVX45 and SHAM45 (Table 1).
When the OVX and ESP groups were compared, considering only the type of food, significantly greater weight gain was verified in the ESP group (P = 0.028). However, with the interactions of multiple comparisons, similar homogeneous groups were revealed: OVX7 and ESP7; OVX21 and ESP21; and OVX45 and ESP45 (Table 1).
Qualitative histological analysis
Overall, the histological pattern observed was similar in the OVX and SHAM groups, with marked differences according to the study period and some differences in the ESP group. The characteristics were described according to the periods of 7, 21 and 45 days. The analysis also considered the apical, middle and coronal root thirds. The differences observed were highlighted according to the group.
Day 7 postextraction
The mesial and distal sockets of the first molar were analysed in their greatest dimensions. In the apical region, both presented variable quantities of newly formed bone composed of thin and delicate trabeculae irradiating from the alveolar wall towards the centre. The central region of the socket usually exhibited areas of granulation tissue, of variable maturation, without bone tissue (Figure 1). Osteoid tissue was often observed on the surface of thin trabeculae originating from the alveolar bone and occasionally on the surface of mature trabeculae, usually covered by columnar osteoblasts. The osteocytes were large and observed in wide and irregular osteocyte lacunae.
Figure 1.
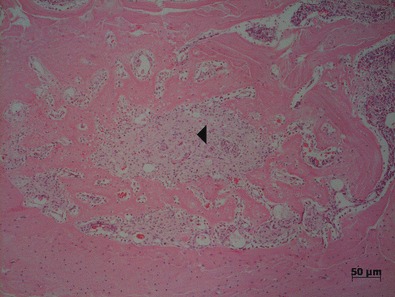
Mesial tooth socket in the SHAM group, after 7 days of bone healing, demonstrating newly formed bone trabeculae from the cortical bone and centrally positioned granulation tissue (◂). At day 7, significantly less bone was observed than after longer periods (HE).
The granulation tissue presented a high rate of cellularity, exhibiting large fibroblasts and dispersed areas of haemorrhage. Foci of chronic inflammatory cells were observed. Most blood vessels were congested.
Greater quantity of bone tissue was often observed on the middle part of the socket, filling the central area, while the apical region was filled with granulation tissue. In the middle third, in addition to the well-defined and distinguishable mesial and distal sockets, it was possible to observe the sockets corresponding to the mesiobuccal and mesiolingual roots of the first molar.
There were areas of bone remodelling characterized by basophilic reverse lines and bone trabeculae presenting irregular surfaces, with resorption lacunae containing or not containing multinucleated giant cells suggestive of osteoclasts.
In the coronal third, a large quantity of inflammatory infiltrate composed of polymorphonuclear and mononuclear cells was evident. Periosteal new bone formation was also identified on the external surface of the lingual and buccal cortical bone.
Extensive bone resorption occurred around the socket, prevailing over the new bone formation inside it. There was often partial coverage of the alveolar process by stratified squamous epithelium. Fracture of the cortical bone secondary to tooth extraction was frequently observed in the region, concomitant with delayed repair in these areas. The SHAM group presented the same characteristics yet exhibited more evident and compact alveolar cortical bone, delimiting the mesial socket, different from the OVX and ESP groups.
Day 21 postextraction
At day 21, the trabeculae were denser in the apical region, delineating small spaces filled by richly vascularized tissue (Figure 2), while smaller osteocytes and osteoclasts appeared more frequently inside the socket. Flat or columnar osteoblasts arranged in rows were observed around the trabeculae. Non-mineralized bone tissue (osteoid) was observed around the newly formed bone trabeculae. Sections stained by toluidine blue showed the presence of mast cells dispersed throughout the tissue.
Figure 2.
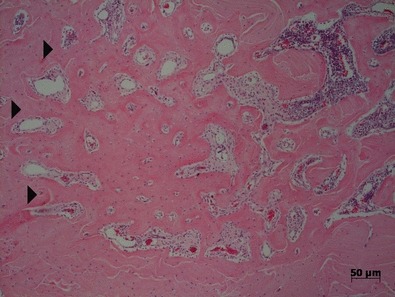
Mesial tooth socket in the SHAM group after 21 days of bone healing showing more mature trabecular bone irradiating from the periphery towards the centre ( ). The presence of vascularized connective tissue between trabeculae and on the right, haematopoietic bone marrow can be observed (HE).
). The presence of vascularized connective tissue between trabeculae and on the right, haematopoietic bone marrow can be observed (HE).
The central area was filled by a smaller amount of fibrous connective tissue compared to the previous period.
The middle third was filled by bone tissue with small medullary spaces and basophilic reverse lines.
In the coronal third, in cases without fractures of the bone plate, re-epithelization was complete without loss of bone crest height. The ESP group presented a different aspect in the apical third, within thin trabeculae, delineating spaces filled by haematopoietic bone marrow containing many mast cells, lymphoid cells and megakaryocytes.
Resorption lacunae were observed with or without osteoclasts and many basophilic reverse lines. The presence of fractures delayed the repair process, leading to persistence of mixed inflammatory infiltrate, as well as contamination by food fragments and microbial colonies.
Day 45 postextraction
At day 45, the bone was compact in the apical region, with small osteocytes and bone surface covered by flat osteoblasts. The quantity of osteoid tissue was considerably reduced compared to the previous periods. In all groups, the sockets were poorly defined, without differentiation of the alveolar cortical bone and neoformed bone owing to intensive remodelling, in the area.
In the OVX and SHAM groups, medullary spaces were filled by haematopoietic bone marrow (Figure 3); however, comparison with the ESP group demonstrated a marked difference, with greater quantity and width of these spaces in the latter (Figure 4).
Figure 3.
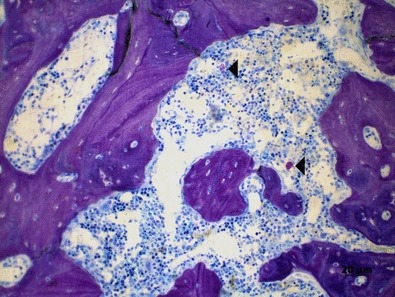
Mesial tooth socket in the OVX group after 45 days of bone healing showing bone tissue interspersed with spaces containing haematopoietic marrow and some mast cells (◂) (toluidine blue).
Figure 4.
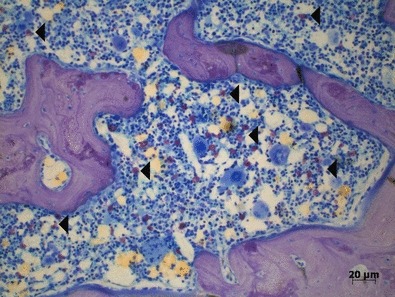
Mesial tooth socket in the ESP group after 45 days of bone healing showing extensive medullar spaces with several metachromatic mast cells (◂). In the ESP group, less bone can be observed than in OVX group for the same period (toluidine blue).
The OVX and ESP groups presented smaller mandibular width in the region analysed, revealing greater bone resorption in the area, as well as larger medullary spaces, altering the bone microarchitecture compared to the SHAM group. The ESP group exhibited smaller quantities of bone trabeculae and wider medullary spaces compared to the OVX group. Bone remodelling was demonstrated by the presence of osteoclasts in some areas, while others exhibited columnar osteoblasts on bone surfaces.
Mast cells were present in all groups, especially in the ESP group (Figure 4).
Analysis of trabecular volume
The data in Table 2 show that the trabecular volume increased over time (P = 0.000) in all groups, independent of ovariectomy or type of food.
Table 2.
Effects of ovariectomy and low-calcium diet on trabecular volume on apical socket bone repair (n = 7)
| Time (days) | |||
|---|---|---|---|
| 7 | 21 | 45 | |
| SHAM | 23.08 ± 6.72a | 39.85 ± 8.56b | 52.32 ± 8.33c |
| OVX | 28.98 ± 4.23a | 41.44 ± 4.56b | 51.55 ± 6.78c |
| ESP | 14.41 ± 4.20d | 38.01 ± 5.00b | 36.92 ± 7.48ab |
Values are means ± SD.
Values that do not share the same superscript letters are significantly different from each other. (P < 0.05).
Considering only the effect of ovariectomy, data analysis of the OVX and SHAM groups showed that oestrogen deficiency did not modify the trabecular volume.
Type of food (P = 0.000) presented a significant effect when comparing OVX and ESP. The OVX group presented a higher mean compared to the ESP group, showing a statistically significant difference, except for day 21 following extraction (Table 2).
Osteoid volume
Osteoid volume diminished over time. Descriptive statistics of these data is presented in Table 3 below. Greater osteoid volume was verified for the 7-day period for all groups (P = 0.000).
Table 3.
Effects of ovariectomy and low-calcium diet on osteoid volume on apical socket bone repair (n = 7)
| Time (days) | |||
|---|---|---|---|
| 7 | 21 | 45 | |
| SHAM | 9.98 ± 3.45a | 2.97 ± 0.90b | 0.88 ± 0.57b |
| OVX | 8.70 ± 4.29a | 3.55 ± 2.59b | 2.04 ± 2.13b |
| ESP | 8.86 ± 2.95a | 2.74 ± 1.94b | 1.47 ± 0.54b |
Values are means ± SD.
Values that do not share the same superscript letters are significantly different from each other. (P < 0.05).
Number of mast cells
Descriptive statistics of these data is presented in Table 4. Analysis of the means of the groups showed an increase in the number of mast cells over time (P = 0.003), with the exception of the SHAM group, which presented a reduced number at 45 days compared to day 21. The number of mast cells in all groups at day 7 was smaller compared to the other periods.
Table 4.
Effects of ovariectomy and low-calcium diet on mast cell numbers on apical socket bone repair (n = 7)
| Time (days) | |||
|---|---|---|---|
| 7 | 21 | 45 | |
| SHAM | 0.09 ± 0.12a | 3.43 ± 3.34b | 2.12 ± 2.32b |
| OVX | 0.02 ± 0.06a | 1.58 ± 1.82b | 2.16 ± 0.90b |
| ESP | 0.00 ± 0.00b | 1.50 ± 0.88b | 9.44 ± 8.40c |
Values are means ± SD.
Values that do not share the same superscript letters are significantly different from each other. (P < 0.05).
Type of food was a significant variable (P = 0.036) and the Tukey test demonstrated that the ESP group presented a higher mean number of mast cells at day 45 postextraction, showing a statistically significant difference compared to the other groups.
Discussion
New knowledge that can guide the therapeutic approaches of health professionals assisting women with postmenopausal osteoporosis could contribute to enhancing the quality of life of these patients.
In postmenopause osteoporosis bone loss affects trabecular bone more than cortical bone because of the accelerated bone turnover in the former (Riggs & Melton 1986).
Thus, it is expected that lack of oestrogen in experimental osteoporosis will influence the maxilla more than the mandible, owing to the higher percentage of trabecular bone. However, certain studies in the literature report alterations in the mineral status in the mandible evaluated with dual-energy quantitative computed tomography (QCT). In newly diagnosed postmenopausal women, the bone mineral density (BMD) of the mandibular cortex is diminished, and in postmenopausal women that had been previously diagnosed, the mandibular trabecular bone presented density reduction (Taguchi et al. 1996). Some reliable studies using QCT verified that cortical bone loss in humans is related to oestrogen deficiency; however, trabecular bone loss can be oestrogen-independent (Khosla et al. 2011).
The time elapsed after ovariectomy and before tooth extraction was determined following a review of different studies involving bone remodelling, delineating the shortest period of oestrogen deficiency that may cause osteopenia (Wronski et al. 1988; Most et al. 1997; Lane et al. 1998; Tanaka et al. 2002, 2003; Kwan Tat et al. 2004).
The difficultly in achieving osteopenic changes in the jaws of rodents has been widely discussed. Some studies do not demonstrate significant differences between the maxillae and mandibles of rats in the SHAM-operated control group and the OVX group (Moriya et al. 1998; Teófilo et al. 2003; Shoji et al. 2008). The association of ovariectomy with another methodology that causes bone loss was necessary, such that our group chose the administration of a calcium-deficient diet (Moriya et al. 1998; Hara et al. 2001; Jiang et al. 2003; Teófilo et al. 2003).
The amount of food provided to all the groups was controlled to avoid exaggerated weight gain in ovariectomized rats (Zhang et al. 2007). This was avoided because fatty tissue can provide protection against bone loss in ovariectomized rats (Wronski et al. 1987; Hollinger & Wong 1996) as a source of oestrogenic hormones.
When comparing the weight of OVX and sham-operated rats, observation verified greater gain among rats in the groups with hormonal deficiency compared to controls. According to Deyhim et al. (2003), weight gain could be related to reduced metabolism in OVX rats.
In the present study, weight gain in the ESP group was significantly greater compared to the OVX group. However, based on data from the literature (Omi & Ezawa 1995; Kubo et al. 1999), the mechanism of postovariectomy weight gain combined with a calcium-deficient diet has not yet been elucidated. Patients with hyperparathyroidism are usually heavier than their eucalcaemic peers (Bolland et al. 2005). It was expected that a low-calcium diet would induce secondary hyperparathyroidism in the OVX rats and consequently weight gain.
Regarding alveolar bone healing, the data obtained were compared to other studies in which only static parameters were used (Shimizu et al. 1998, 2000; Tanaka et al. 2001; Teófilo et al. 2004; Pereira et al. 2007 and Silveira et al. 2008). Instead of optic microscopic, some studies used other microscopy technologies, such as backscattered electron microscopy and scanning electron microscopy obtaining percentages and volume fractions. Their results were compared to ours below.
Data from the OVX and SHAM groups indicated no effect on trabecular bone volume owing to oestrogen deficiency. These results are supported by Silveira et al. (2008) and Tanaka et al. (2001), who reported no differences in trabecular volume for newly formed bone tissue in the tooth socket. Thus, we can affirm that the initial acute effects of ovariectomy do not impair alveolar bone healing.
Conversely, Shimizu et al. (1998) observed a smaller quantity of bone in ovariectomized rats in later periods of alveolar repair that could be related to the time elapsed after ovariectomy before tooth extraction. Shimizu et al. (1998) allowed 60 days of hormonal deficiency before molar extraction, while Silveira et al. (2008) allowed 15 days and Tanaka et al. (2001) performed extractions only 7 days after ovariectomy.
When 21 days are allowed following ovariectomy before the extraction of mandibular molars, the tooth sockets of OVX rats present lower bone content compared to control group rats (Pereira et al. 2007). This fact can be attributed to the enzymatic activity of metalloproteinases (MMPs) 2 and 9 in the collagen matrix. For instance, researchers have demonstrated that hormone deficiency can alter the activity of MMPs 2 and 9; moreover, it has been reported that ovariectomized rats present diminished formation of collagen I and III (Zecchin et al. 2005; Prado et al. 2010). These data suggest that delay in alveolar bone repair owing to oestrogen deficiency is time-dependent, which supports the finding of this study.
Hara et al. (2001) observed an increase in the osteoid surface rate of OVX and SHAM rats with standard food compared to OVX and SHAM rats fed a calcium-deficient diet. Therefore, an increase in osteoid volume in rats fed a low-calcium diet was expected. However, in this study, the osteoid measured was not affected by oestrogen deficiency nor by calcium deficiency.
Osteoblast synthesis is fairly active, required by many growth factors of coagulum and granulation tissue, parathyroid hormone (PTH) and calcitriol. A low calcium intake could reduce the rate of mineral juxtaposition, consequently permitting an increase in the rate of osteoid volume, that is, non-mineralized matrix, awaiting the deposition of hydroxyapatite (Eklou-Kalonji et al. 1999). This effect was not observed in the present study, because the osteoid volume was very similar between rats receiving the standard and calcium-deficient diets. Secondary hyperparathyroidism releasing PTH could promote calcium mobilization from skeletal bones, thus supplying the necessary calcium for the new matrix mineralization in postextraction sockets.
Concerning the mast cell counts, ovariectomy did not alter the number of mast cells in the OVX groups compared to the SHAM groups. This may be due to the study duration, because at day 45, the specimens exhibited greater presence of these cells. However, despite the limited numerical analysis, the function of mast cells in bone resorption seems to be confirmed.
Mast cell activity appears to be involved in more advanced stages of repair. Mast cell enzymes may facilitate bone degradation. Some studies have reported that the kinase enzyme promotes nearly complete digestion of the non-collagen protein of the bone matrix (Banovac et al.1995). These data may be important in elucidating the function of mast cells observed in this study, in all groups, at days 21 and 45.
Together with other mast cell enzymes, histamine, an active inflammatory mediator in vascular and exudative inflammation phenomena, is important for bone resorption (Fouilloux et al. 2006), even though its mechanisms are not yet fully understood. Antagonists of histamine receptors reduced bone resorption, the number of active osteoclasts and mast cells and caused vascular alterations (Dobigny & Saffar 1997).
A hypothesis has been proposed that these cells may mediate bone disorders, through the release of granule-associated stem cell factor, a ligand of a tyrosine kinase growth factor receptor (KIT). Systemic mastocytosis usually exhibits mutation in KIT, regulating accelerated bone turnover (Tharp & Longley 2001). Although the data regarding mast cells in SHAM and OVX rats were similar, the ovariectomy-associated low-calcium diet improved the presence of these cells in alveolar bone healing. Secondary hyperparathyroidism caused by calcium deficiency could be the mechanism behind these phenomena, because PTH increases the number of mast cells in bone (Turner et al. 2010).
It is known that that oestrogen depletion rapidly initiates the release of histamine, interleukin-6 (IL-6) and tumour necrosis factor-alpha (TNF-α) and other precursors in the osteoclastic pathway. Mast cells enhance cell lineages involved in osteoclast differentiation and also T-cell activation through mast cell–released TNF-α (Lesclous & Saffar 1999). Oestrogen deficiency did not cause diminished trabecular bone in alveolar healing in OVX rats. The group with the largest numbers of mast cells (ESP45) exhibited lower trabecular volume. This difference was statistically significant compared to the OVX45 group.
The action of mast cells does not seem to be limited to the direct activation of osteoclasts, because no effects of histamine were observed on osteoclasts in isolated cell cultures in the study by Hall & Schaueblin (1994). A recent study by Biosse-Duplan et al. (2009) showed that histamine has an important action on osteoclast precursor recruitment and differentiation. Several cell types are involved in osteoclastic resorption, and histamine appears to play a critical role in the interplay between osteoclasts and osteoblasts, modulating the expression of receptor activator of nuclear factor kappa-B ligand (RANKL) mRNA in immature osteoblasts.
In summary, analysis of the results verified an increase in bone quantity (trabecular volume) and the number of mast cells and a decrease in osteoid volume over time. Extraction socket healing was not impaired by acute oestrogen deficiency; however, according to the literature, the delay in alveolar bone repair appears to be time dependent in the presence of oestrogen deficiency.
Differences verified in comparisons between the ovariectomized groups with different diets permit the conclusion that alveolar bone repair is altered by low calcium intake. This information, combined with other reports in the literature, may encourage the delineation of future studies to investigate therapies that can be applied by dental professionals when tooth extractions are indicated in patients with osteoporosis, particularly those showing indications of poor nutritional status.
Commercial softwares are available for bone analysis that comprise a complete list of the histomorphometric parameters proposed by Parfitt et al. in 1987. These softwares can be used to fully comprehend the effects of hormonal and dietary conditions on bone healing. Analyses using one of these softwares will be a future project of the current research group.
Conclusions
Analysing the results obtained under the experimental conditions described in this study with regard to postextraction socket repair, it can be concluded that (i) hormonal deficiency does not delay alveolar bone repair in OVX female rats; (ii) hormonal deficiency associated with a calcium-deficient diet causes diminished filling of the tooth socket during the bone repair process; (iii) a possible mechanism of bone resorption is activated when calcium and oestrogen are deficient, involving the recruitment of mast cells; and (iv) increased mast cell numbers exacerbate bone resorption.
Acknowledgments
The authors would like to thank the National Council of Scientific and Technological Development (CNPq) for their financial support during this research process 476143/2006-3.
References
- Arjmandi BH, Khalil DA, Hollis BW. Soy protein: its effects on intestinal calcium transport, serum vitamin D, and insulin-like growth factor-I in ovariectomized rats. Calcif. Tissue Int. 2002;70:483–487. doi: 10.1007/s00223-001-1100-4. [DOI] [PubMed] [Google Scholar]
- Ashcroft GS, Dodsworth J, van Boxtel E, et al. Estrogen accelerates cutaneous wound healing associated with an increase in TGF-beta1 levels. Nat. Med. 1997;3:1209–1215. doi: 10.1038/nm1197-1209. [DOI] [PubMed] [Google Scholar]
- Banovac K, Renfree K, Makowski AL, Latta LL, Altman RD. Fracture healing and mast cells. J. Orthop. Trauma. 1995;9:482–490. doi: 10.1097/00005131-199509060-00005. [DOI] [PubMed] [Google Scholar]
- Biosse-Duplan M, Baroukh B, Dy M, de Vernejoul MC, Saffar JL. Histamine promotes osteoclastogenesis through the differential expression of histamine receptors on osteoclasts and osteoblasts. Am. J. Pathol. 2009;174:1426–1434. doi: 10.2353/ajpath.2009.080871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolland MJ, Grey AB, Gamble GD, Reid IR. Association between primary hyperparathyroidism and increased body weight: a meta-analysis. J. Clin. Endocrinol. Metab. 2005;90:1525–1530. doi: 10.1210/jc.2004-1891. [DOI] [PubMed] [Google Scholar]
- Chiappetta N, Gruber B. The role of mast cells in osteoporosis. Semin. Arthritis Rheum. 2006;36:32–36. doi: 10.1016/j.semarthrit.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Compston J. Osteoporosis: social and economic impact. Radiol. Clin. North Am. 2010;48:477–482. doi: 10.1016/j.rcl.2010.02.010. [DOI] [PubMed] [Google Scholar]
- Costa GP, Leite DS, do Prado RF, Silveira VA, Carvalho YR. Effect of low-calcium diet and grind diet on bone turnover of ovariectomized female rats. Med. Oral Patol. Oral Cir. Bucal. 2011;16:e497–e502. doi: 10.4317/medoral.16.e497. [DOI] [PubMed] [Google Scholar]
- Dervis E. Oral implications of osteoporosis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2005;100:349–356. doi: 10.1016/j.tripleo.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Deyhim F, Stoecker BJ, Brusewitz GH, Arjmandi BH. The effects of estrogen depletion and isoflavones on bone metabolism in rats. Nutr. Res. 2003;23:123–130. [Google Scholar]
- Dobigny C, Saffar JL. H1 and H2 histamine receptors modulate osteoclastic resorption by different pathways: evidence obtained by using receptor antagonists in a rat synchronized resorption model. J. Cell. Physiol. 1997;173:10–18. doi: 10.1002/(SICI)1097-4652(199710)173:1<10::AID-JCP2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Eklou-Kalonji E, Zerath E, Colin C, et al. Calcium-regulating hormones, bone mineral content, breaking load and trabecular remodeling are altered in growing pigs fed calcium-deficient diets. J. Nutr. 1999;129:188–193. doi: 10.1093/jn/129.1.188. [DOI] [PubMed] [Google Scholar]
- Fouilloux I, Duplan MB, Baroukh B, Cherruau M, Saffar JL, Lesclous P. Mast cell activation and degranulation occur early during induction of periosteal bone resorption. Bone. 2006;38:59–66. doi: 10.1016/j.bone.2005.07.026. [DOI] [PubMed] [Google Scholar]
- Hall TJ, Schaueblin M. Promethazine inhibits osteoclastic bone resorption in vitro. Calcif. Tissue Int. 1994;55:68–70. doi: 10.1007/BF00310171. [DOI] [PubMed] [Google Scholar]
- Hara T, Sato T, Oka M, Mori S, Shirai H. Effects of ovariectomy and/or dietary calcium deficiency on bone dynamics in the rat hard palate, mandible and proximal tibia. Arch. Oral Biol. 2001;46:443–451. doi: 10.1016/s0003-9969(00)00135-7. [DOI] [PubMed] [Google Scholar]
- Hollinger J, Wong MEK. The integrated processes of hard tissue regeneration with especial emphasis on fracture healing. Oral Surg. Oral Med. Oral Pathol. 1996;82:594–606. doi: 10.1016/s1079-2104(96)80431-8. [DOI] [PubMed] [Google Scholar]
- Ishihara A, Sasaki T, Debari K, et al. Effects of ovariectomy on bone morphology in maxillae of mature rats. J. Electron. Microsc. (Tokyo) 1999;48:465–469. doi: 10.1093/oxfordjournals.jmicro.a023703. [DOI] [PubMed] [Google Scholar]
- Jiang G, Matsumoto H, Fujii A. Mandible bone loss in osteoporosis rats. J. Bone Miner. Metab. 2003;21:388–395. doi: 10.1007/s00774-003-0433-7. [DOI] [PubMed] [Google Scholar]
- Kawamoto S, Nagaoka E. The effect of oestrogen deficiency on the alveolar bone resorption caused by traumatic occlusion. J. Oral Rehabil. 2000;27:587–594. doi: 10.1046/j.1365-2842.2000.00542.x. [DOI] [PubMed] [Google Scholar]
- Khosla S, Melton LJ, III, Riggs BL. The unitary model for estrogen deficiency and the pathogenesis of osteoporosis: is a revision needed? J. Bone Miner. Res. 2011;26:441–451. doi: 10.1002/jbmr.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kribbs PJ. Comparison of mandibular bone in normal and osteoporotic women. J. Prosthet. Dent. 1990;63:218–222. doi: 10.1016/0022-3913(90)90108-o. [DOI] [PubMed] [Google Scholar]
- Kubo T, Shiga T, Hashimoto J, et al. Osteoporosis influences the late period of fracture healing in a rat model prepared by ovariectomy and low calcium diet. J. Steroid Biochem. Mol. Biol. 1999;68:197–202. doi: 10.1016/s0960-0760(99)00032-1. [DOI] [PubMed] [Google Scholar]
- Kwan Tat S, Padrines M, Théoleyre S, Heymann D, Fortun Y. IL-6, RANKL, TNF-alpha/IL-1: interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev. 2004;15:49–60. doi: 10.1016/j.cytogfr.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Lane NE, Thompson JM, Haupt D, Kimmel DB, Modin G, Kinney JH. Acute changes in trabecular bone connectivity and osteoclast activity in the ovariectomized rat in vivo. J. Bone Miner. Res. 1998;13:229–236. doi: 10.1359/jbmr.1998.13.2.229. [DOI] [PubMed] [Google Scholar]
- Lesclous P, Saffar JL. Mast cells accumulate in rat bone marrow after ovariectomy. Cells Tissues Organs. 1999;164:23–29. doi: 10.1159/000016639. [DOI] [PubMed] [Google Scholar]
- Moreira C. Osteoporose. In: Rocha MOC, Pedroso ERP, Fonseca JGM, Silva AO, editors. Terapêutica Clínica. Rio de Janeiro: Guanabara Koogan; 1998. pp. 588–604. [Google Scholar]
- Moriya Y, Ito K, Murai S. Effects of experimental osteoporosis on alveolar bone loss in rats. J. Oral Sci. 1998;40:171–175. doi: 10.2334/josnusd.40.171. [DOI] [PubMed] [Google Scholar]
- Most W, van der Wee-Pals L, Ederveen A, Papapoulos S, Lowik C. Ovariectomy and orchidectomy induce a transient increase in the osteoclastogenic potential of bone marrow cells in the mouse. Bone. 1997;20:27–30. doi: 10.1016/s8756-3282(96)00309-2. [DOI] [PubMed] [Google Scholar]
- Omi N, Ezawa I. The effect of ovariectomy on bone metabolism in rats. Bone. 1995;17:163–168. doi: 10.1016/8756-3282(95)00329-c. [DOI] [PubMed] [Google Scholar]
- Parfitt AM, Drezner MK, Glorieux FH, et al. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- Pereira MC, Zecchin KG, Campagnoli EB, Jorge J. Ovariectomy delays alveolar wound healing after molar extractions in rats. J. Oral Maxillofac. Surg. 2007;65:2248–2253. doi: 10.1016/j.joms.2006.11.040. [DOI] [PubMed] [Google Scholar]
- Prado RF, Silveira VAS, Carvalho YRC. Análise imunoistoquímica do colágeno III e do TGF-B1 no reparo ósseo alveolar de ratas osteopênicas. Braz. Oral Res. 2010;24:279. [Google Scholar]
- Rawlinson SC, McKay IJ, Ghuman M, et al. Adult rat bones maintain distinct regionalized expression of markers associated with their development. PLoS ONE. 2009;4:e8358. doi: 10.1371/journal.pone.0008358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs BL, Melton LJ., III Involutional osteoporosis. N. Engl. J. Med. 1986;314:1676–1686. doi: 10.1056/NEJM198606263142605. [DOI] [PubMed] [Google Scholar]
- Rosenber AE. Bone, joints and soft tissue tumors. In: Kumar V, Abbas AK, Fausto N, editors. Robbins and Cotran Pathologic Basis of Disease. Philadelphia: Elsevier Saundes; 2005. pp. 1273–1325. [Google Scholar]
- Shimizu M, Sasaki T, Ishihara A, Furuya R, Kawawa T. Bone wound healing after maxillary molar extraction in ovariectomized aged rats. J. Electron. Microsc. (Tokyo) 1998;47:517–526. doi: 10.1093/oxfordjournals.jmicro.a023623. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Furuya R, Kawawa T, Sasaki T. Bone wound healing after maxillary molar extraction in ovariectomized aged rats: quantitative backscattered electron image analysis. Anat. Rec. 2000;259:76–85. doi: 10.1002/(SICI)1097-0185(20000501)259:1<76::AID-AR9>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Shoji K, Basso N, Elsubeihi ES, Heersche JNM. Short-term effect of ovariectomy on osteoprogenitors in the healing rat mandibular incisor extraction socket. Osteoporos. Int. 2008;19:1193–1201. doi: 10.1007/s00198-007-0558-y. [DOI] [PubMed] [Google Scholar]
- Silveira VAS, Prado RF, Carvalho YR. Efeito do tratamento com estrógenos e as isoflavonas da soja, isolados e associados, no reparo ósseo alveolar de ratas. Braz. Oral Res. 2008;22:318. [Google Scholar]
- Taguchi A, Tanimoto K, Suei Y, Ohama K, Wada T. Relationship between the mandibular and lumbar vertebral bone mineral density at different postmenopausal stages. Dentomaxillofac. Radiol. 1996;25:130–135. doi: 10.1259/dmfr.25.3.9084261. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Shimizu M, Debari K, Furuya R, Kawawa T, Sasaki T. Acute effects of ovariectomy on wound healing of alveolar bone after maxillary molar extraction in aged rats. Anat. Rec. 2001;262:203–212. doi: 10.1002/1097-0185(20010201)262:2<203::AID-AR1030>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Ejiri S, Toyooka E, Kohno S, Ozawa H. Effects of ovariectomy on trabecular structures of rat alveolar bone. J. Periodontal Res. 2002;37:161–165. doi: 10.1034/j.1600-0765.2002.01601.x. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Toyooka E, Kohno S, Ozawa H, Ejiri S. Long-term changes in trabecular structure of aged rat alveolar bone after ovariectomy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2003;95:495–502. doi: 10.1067/moe.2003.135. [DOI] [PubMed] [Google Scholar]
- Teófilo JM, Azevedo AC, Petenusci SO, Mazaro R, Lamano-Carvalho TL. Comparison between two experimental protocols to promote osteoporosis in the maxilla and proximal tibia of female rats. Pesqui. Odontol. Bras. 2003;17:302–306. doi: 10.1590/s1517-74912003000400002. [DOI] [PubMed] [Google Scholar]
- Teófilo JM, Brentegani LG, Lamano-Carvalho TL. Bone healing in osteoporotic female rats following intra-alveolar grafting of bioactive glass. Arch. Oral Biol. 2004;49:755–762. doi: 10.1016/j.archoralbio.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Tharp MD, Longley B. Mastocytosis. Dermatol. Clin. 2001;19:679–696. doi: 10.1016/s0733-8635(05)70308-9. [DOI] [PubMed] [Google Scholar]
- Turner RT, Iwaniec UT, Marley K, Sibonga JD. The role of mast cells in parathyroid bone disease. J. Bone Miner. Res. 2010;25:1637–1649. doi: 10.1002/jbmr.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wronski TJ, Schenck PA, Cintrón M, Walsh CC. Effect of body weight on osteopenia in ovariectomized rats. Calcif. Tissue Int. 1987;40:155–159. doi: 10.1007/BF02555700. [DOI] [PubMed] [Google Scholar]
- Wronski TJ, Cintrón M, Dann LM. Temporal relationship between bone loss and increased bone turnover in ovariectomized rats. Calcif. Tissue Int. 1988;43:179–183. doi: 10.1007/BF02571317. [DOI] [PubMed] [Google Scholar]
- Zaffe D, Paganelli C, Cocchi D. Induction and pharmacological treatment of oral osteopenia in rats. Minerva Stomatol. 1999;48:45–62. [PubMed] [Google Scholar]
- Zecchin KG, Pereira MC, Coletta RD, Graner E, Jorge J. Ovariectomy reduces the gelatinolytic activity and expression of matrix metalloproteinases and collagen in rat molar extraction wounds. Calcif. Tissue Int. 2005;76:136–145. doi: 10.1007/s00223-004-0013-4. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Lai WP, Wu CF, Favus MJ, Leung PC, Wong MS. Ovariectomy worsens secondary hyperparathyroidism in mature rats during low-Ca diet. Am. J. Physiol. Endocrinol. Metab. 2007;292:e723–e731. doi: 10.1152/ajpendo.00445.2006. [DOI] [PubMed] [Google Scholar]


