Summary
Transforming growth factor-β (TGF-β) is known to act as a tumour suppressor early in carcinogenesis, but then switches to a pro-metastatic factor in some late stage cancers. However, the actions of TGF-β are context dependent, and it is currently unclear how TGF-β influences the progression of human squamous cell carcinoma (SCC). This study examined the effect of overexpression of TGF-β1 or TGF-β2 in Ras-transfected human malignant epidermal keratinocytes that represent the early stages of human SCC. In vitro, the proliferation of cells overexpressing TGF-β1 or TGF-β2 was inhibited by exogenous TGF-β1; cells overexpressing TGF-β1 also grew more slowly than controls, but the growth rate of TGF-β2 overexpressing cells was unaltered. However, cells that overexpressed either TGF-β1 or TGF-β2 were markedly more invasive than controls in an organotypic model of SCC. The proliferation of the invading TGF-β1 overexpressing cells in the organotypic assays was higher than controls. Similarly, tumours formed by the TGF-β1 overexpressing cells following transplantation to athymic mice were larger than tumours formed by control cells and proliferated at a higher rate. Our results demonstrate that elevated expression of either TGF-β1 or TGF-β2 in cells that represent the early stages in the development of human SCC results in a more aggressive phenotype.
Keywords: invasion, isoform, keratinocytes, SCC, TGF-β
Transforming growth factor-β (TGF-β) is a ubiquitously expressed growth factor that signals through canonical (Smad) and non-canonical transduction pathways to regulate a wide variety of cellular processes, including proliferation, differentiation and apoptosis (Heldin et al. 2009). The role of TGF-β in cancer has been the subject of much attention over the past 20 years, and it is now known that TGF-β can function both as a tumour suppressor or tumour promoter, depending upon the cellular context and/or the stage of carcinogenesis (Massague 2008; Meulmeester & ten Dijke 2011). In mammals, there are three highly conserved TGF-β isoforms (TGF-β1, TGF-β2, TGF-β3) that show 70–80% sequence identity at the amino acid level. It has been known for some time that although the TGF-β isoforms bind to the same cell surface receptors and elicit similar responses in vitro, they are differentially expressed in vivo and display non-overlapping functions in development (Pelton et al. 1991; Letterio & Bottinger 1998). It is perhaps surprising, therefore, that the majority of studies only examined the role of TGF-β1 in tumorigenesis, even though isoform-specific differences have been identified (Laverty et al. 2009).
Squamous cell carcinoma (SCC) is the most common epithelial malignancy in man, and SCC of the skin is a significant world health problem. Early studies using the mouse model of skin carcinogenesis were pioneering in our understanding of the role of TGF-β1 in cancer and demonstrated that TGF-β1 promoted metastasis in late stage SCC (Cui et al. 1996; Portella et al. 1998). However, a number of studies, including our own, have failed to demonstrate a tumour promoting role for TGF-β1 in the development and progression of SCC in the skin of humans. For example, Gold et al. (2000) showed in tumours derived from cells in the HaCaT model of human epidermal tumour progression that TGF-β1 was associated with a more differentiated state, and we demonstrated that expression of a dominant-negative TβRII in cells representing the later stages of tumour progression in the HaCaT model inhibited metastasis (Ganapathy et al. 2010). Whether the discrepancies in the apparent role of TGF-β1 between mouse and human models are because of fundamental differences between murine and human cells or simply represent differences in experimental approach is uncertain. Interestingly, Gold et al. (2000) also demonstrated that large, invasive SCCs expressed high levels of TGF-β2, particularly at the invasive front, suggesting that TGF-β2 might be more likely to promote tumour invasion than TGF-β1.
The Ha-ras transformed clonal cell line, I-7, formed slowly growing and encapsulated keratinizing cysts when transplanted subcutaneously into athymic mice (Fusenig & Boukamp 1998). In surface transplants, I-7 cells gave rise to slightly dysplastic but keratinizing epithelia in which TGF-β1 expression was restricted to the upper differentiated layers, and TGF-β2 showed weak cytoplasmic expression in the upper epithelial layers (Gold et al. 2000). In the present study, we examined the effect of overexpressing either TGF-β1 or TGF-β2 on the behaviour of I-7 cells in organotypic invasion assays, and we demonstrate that overexpression of both isoforms resulted in a more invasive phenotype in vitro. In addition, transplantation of TGF-β1 overexpressing I-7 cells to the floor of the mouth in athymic mice resulted in faster growing, larger, weakly metastatic tumours, as compared to small, non-metastatic tumours formed after the transplantation of control cells. Our data show that both TGF-β1 and TGF-β2 can function as tumour promoters in early human SCC.
Materials and methods
Cell lines and culture
The human keratinocyte cell clone, I-7 (a kind gift from Prof. Norbert Fusenig, Heidelberg, Germany), was created following the transfection of the immortalized from normal adult skin cell line, HaCaT, with mutant c-Ha-ras and subsequent selection with G418. I-7 also contains mutant p53, a common initiating event in human skin cancer and is representative of early stage skin SCC in the HaCaT model of tumour progression. The series of cell lines derived from HaCaT show increasing tumorigenic/metastatic potential following subcutaneous/tail vein transplantation to athymic mice and are an excellent model of the multistep processes of epidermal transformation (Fusenig & Boukamp 1998).
I-7 cells were cultured in standard medium (SM) consisting of Dulbecco’s modified Eagle’s medium (DMEM):Ham’s F12 (1:1; BioWhittaker) contains 10% (v/v) foetal bovine serum (FBS), 0.5 μg/ml hydrocortisone and 400 μg/ml geneticin sulphate (G418; PAA laboratories, Yeovil, UK). The amphotrophic packaging cell line AM-12 and fibroblast cell line ICRF 23 (both kind gifts from Dr John Marshall, UCL) were cultured in DMEM containing 10% (v/v) FBS, supplemented with 0.6 mg/ml l-glutamine or DMEM, containing 20% (v/v) foetal clone III (Hyclone) and l-glutamine 0.3 μg/ml, respectively.
Overexpression of TGF-β
TGF-β1 cDNA was subcloned in a sense orientation into pIRESpuro2 (Takahara Bio Europe/Clontech Saint-Germain-en-Laye, France) from pcDNA3-TGF-β1, the preparation of which has been described previously (Davies et al. 1997). I-7 cells at 30–50% confluent were transfected with 10 μg pIRES-puro or pIRES-TGF-β1 DNA using Fugene-6 reagent (Roche, Burgess Hill, UK) according to the manufacturer’s instructions. Stable transfectants were selected in medium containing 0.5 μg/ml puromycin and cloned to isolate colonies overexpressing high quantities of TGF-β1.
The plasmid pRK5-β2 (encoding the full length coding sequence for the human TGF-β2 precursor; Graycar et al. 1989) was used as a template for gene amplification by PCR. The primers 5′TTCTGTTGGGCATTGAC-3′ (left primer) and 5′-GCATCATCGTTGTCGTC-3′, deduced from the published sequence, were used to amplify the full coding sequence of TGF-β2. The 1.6 Kb PCR product was TA-cloned into pCR4-TOPO (Invitrogen, Paisley, UK), excised with EcoR1 and then subcloned in a sense orientation into the mammalian expression vector pBABE-puro (a kind gift from Dr John Marshall, UCL). The amphotrophic packaging cell line AM-12 was transfected with pBABE-puro-TGF-β2 or empty vector using Fugene-6 as described previously and stable transfectants selected in medium containing 3.5 μg/ml puromycin. I-7 cells were transduced with conditioned media (CM) collected from stably transfected AM-12 cells as described previously (Davies et al. 2005). Stably transduced cells were selected in medium containing 0.5 μg/ml puromycin, expanded and cloned to isolate colonies overexpressing high quantities of TGF-β2.
ELISA
Clones were grown to 50% confluence, incubated in serum-free medium consisting of DMEM:Ham’s F12 (1:1) containing 0.5 μg/ml hydrocortisone and 200 μg/ml BSA (Sigma; Sigma-Aldrich, Dorset, UK SFM + BSA) for 24 h during which the media were replaced three times and then incubated for a further 24 h in a reduced volume of SFM + BSA. CM was collected into siliconized tubes and cell numbers were determined by counting. The total autocrine production of TGF-β1 or TGF-β2 in CM following acid activation was determined using the relevant Quantikine immunoassay kit (R&D Systems, Abingdon, UK). The quantity of total TGF-β1/2 in each sample was expressed as pg TGF-β/24 h/106 cells. Clones expressing the highest levels of TGF-β1 and TGF-β2 were selected for further study and are listed in Table 1.
Table 1.
Clones of I-7 stably transfected or transduced with TGF-β used in this study
| Construct | Clone name |
|---|---|
| pIRES-puro | ppC3 |
| pIRES-TGF-β1 | I-7β1C3 |
| I-7β1C6 | |
| pBABEpuro | VC1 |
| VC3 | |
| pBABEpuro-TGF-β2 | I-7β2C2 |
| I-7β2C5 |
Proliferation rates and response to TGF-β
In experiments to determine growth rates, cells were seeded in 60 mm dishes at 3 × 104 cells per dish in a standard medium supplemented with G418 and puromycin. Medium was replenished every 3 days and triplicate dishes were counted at days 4, 7 and 10.
In experiments to examine growth inhibitory response to TGF-β, cells were grown to 20–40% confluence in a standard medium containing antibiotics as appropriate. Cells were treated daily for a total period of 72 h (three treatments) with TGF-β1 (R&D systems; 1 ng/ml) or TGF-β vehicle control (4 mM HCl, 0.1% (w/v) BSA) in DMEM:Ham’s F12 (1:1) containing 1% (v/v) FBS and 0.5 μg/ml hydrocortisone and the cell number determined by counting after 72 h.
Organotypic invasion assays
Organotypic cultures with an air–tissue interface were prepared, as described previously (Nystrom et al. 2005). Briefly, gels comprising a 50:50 mixture of Matrigel (Becton-Dickinson, Oxford, UK) and type I collagen (Upstate) containing 1% (v/v) FBS, 1% (v/v) Hank’s Balanced Salt Solution (HBSS) and 1 × 106/ml ICRF 23 fibroblasts were prepared in cell culture inserts (pore size 3 μm) placed in six-well biocoat plates (Becton Dickinson). After 1 h, sterile glass rings were placed on the gels, and 45 min later DMEM, containing 20% (v/v) foetal clone III (Hyclone), l-glutamine 0.3 μg/ml (FCM) was added to the wells to the level of the insert bases and inside the glass rings. After a further 20 h, the FCM in the glass rings was replaced by 1 ml FAD medium [DMEM: Hams F12 (3:1), 5% FBS(v/v), 5 μg/ml insulin, 10 ng/ml hEGF, 24 ng/ml adenine, 0.4 μg/ml hydrocortisone, 100 U/ml penicillin, 10 μg/ml streptomycin, 250 ng/ml amphotericin B] containing 1 × 106 I-7 cells. After a further 24 h, the rings and the media were removed and the media in the wells replaced with rFAD [DMEM:Hams F12 (3:1), 10% FBS, 50 μg/ml l-ascorbic acid, 100 U/ml penicillin, 10 μg/ml streptomycin, 250 ng/ml amphotericin B]. After 7–14 days, the gels were fixed in formal-saline, embedded in a solution containing 1% (v/v) agarose, 10% (v/v) formol saline and processed to paraffin. Sections (2 μm) were stained with H&E. Cells were assessed to have invaded only if they had penetrated deep in the Matrigel.
Transplantation studies
5 × 106 cells in 10 μl serum-free medium were injected into the floor of the mouth of 4–6-week-old athymic mice (nu/nu), using techniques described previously (Davies et al. 1997). Mice were examined daily and sacrificed by cervical dislocation after 50 days. Tissues were processed routinely and sections stained with haematoxylin and eosin (H&E). Local spread of the primary tumours to the bone, blood and lymphatic system, and metastatic dissemination to the lungs and lymph nodes were determined by a specialist oral and maxillofacial pathologist (JE). Large tumours occupied more than 50% of the tongue, medium 25–50% and small <25%. These experiments were approved by the UK Home Office and carried out by Prof S Prime (personal licence PCD 3002302, project licence PL 3002084).
Immunohistochemistry
Dewaxed, rehydrated paraffin embedded tissue sections (2 μm) were treated with 3% (v/v) hydrogen peroxide (15 min) to block endogenous peroxide activity. Immunohistochemical staining was carried out using standard techniques and reagents. Details of the antibody concentrations and antigen retrieval methods are given in Table 2. Staining controls included omission of the primary antibody and substitution of the primary layer with a monoclonal antibody of irrelevant specificity but similar immunoglobulin isotype and were consistently negative. The percentage of cells positive for the protein of interest was counted in a minimum of five random fields in a minimum of five tumours formed in the transplantation studies, or three random fields in a minimum of three organotypic cultures.
Table 2.
Primary antibody concentrations used for immunohistochemical staining
| Retrieval | Dilution (incubated 1 h RT unless stated) | Company | |
|---|---|---|---|
| Primary antibody | |||
| Polyclonal rabbit anti human CC3 (Asp 175) | Microwave* | 1:80 | Cell signalling (New England Bioloabs, Hitchin, UK) |
| Monoclonal mouse anti human Ki67, clone MIB1 | Microwave* | 1:150 | Dako (Ely, UK) |
| Rabbit anti human pSmad2, (ser465/467) | Microwave* | 1:1500 | Millipore (Watford, UK) |
| Secondary antibody | |||
| Multi-link (anti rat, rabbit mouse) | N/A | 1:50 | Biogenex (Freemont, CA, USA) |
| Tertiary antibody | |||
| Multi-label (strep HRP) | N/A | 1:50 | Biogenex |
Microwaving was carried out in 0.01 M citrate buffer for 25 min at 800 Watts.
Statistical analysis
Statistical analyses were performed using the Student’s t-test, one-way anova with Tukey’s multiple comparison or χ2 test as appropriate, with P < 0.05 taken to be statistically significant.
Results
Expression of TGF-β1/β2 and TGF-β responses in I-7 cells and transfectants
Total levels of TGF-β produced by I-7 parental cells, vector, TGF-β1 and TGF-β2 expressing clones were determined by isoform-specific ELISA. Vector-only controls secreted similar amounts of TGF-β1 and TGF-β2 as parent I-7 cells. Clones transfected with TGF-β1 expressed significantly (P < 0.05) more ligand than parental or vector control cells, and clones transduced with TGF-β2 expressed significantly (I-7β2C2; P < 0.01) or markedly (I-7 β2C5; P = 0.0618) more ligand than parent or vector controls (Figure 1a,b).
Figure 1.
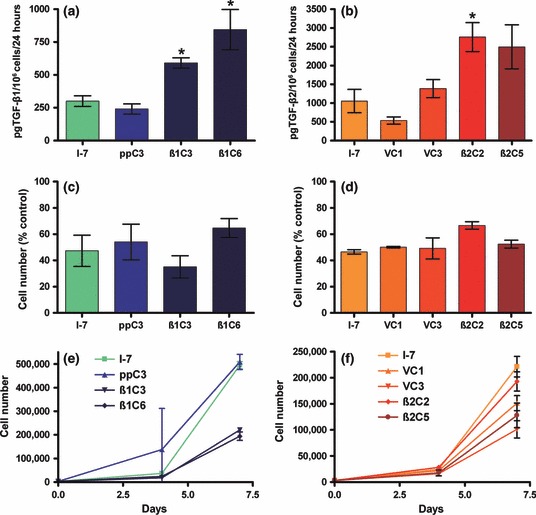
(a,b) production of TGF-β1 or TGF-β2, respectively, as determined by ELISA, total ligand production is expressed as pg produced in 24 h by 1 × 106 cells. (c,d) Growth inhibitory response to TGF-β1 of I-7β1 or I-7β2 clones, respectively. (e,f) Growth characteristics of a monolayer culture of parental, vector and I-7β1 clones or I-7β2 clones, respectively. Cells were counted at 4 and 7 days after seeding. Results are the means of values from conditioned media collected from at least three independent experiments (a,b) or representative of three independent experiments (c–f) ± SD. *Produced significantly (P < 0.05) more ligand than parent or vector controls.
The growth inhibitory response of the transfected cells to TGF-β was retained, and the proliferation of cells overexpressing TGF-β1 or TGF-β2 was inhibited by exogenous TGF-β1 to a similar extent as controls (Figure 1c,d). In growth assays in the absence of exogenous ligand, there was no significant difference between the number of parental I-7 or ppC3 vector control cells at any time point. However, cell lines that overexpressed TGF-β1 grew markedly more slowly than vector controls (Figure 1e). After 7 days, the cell yield for TGF-β1 overexpressing clones was significantly (P < 0.0005) less than that of the yield for either the parental or vector control cell lines. TGF-β2 overexpressing clones grew at a similar rate to control cells (Figure 1f). The differences between the growth rates for the TGF-β1 and TGF-β2 overexpressing cells are consistent with the observation that TGF-β1 is a more potent inhibitor of cell growth than TGF-β2 (Fahey et al. 1996), but may also reflect the fact that we consistently detected some active TGF-β1 in the CM of TGF-β1 overexpressing cells by ELISA, but were unable to detect active TGF-β2 in every experiment undertaken (data not shown).
Exogenous TGF-β1 did not induce an epithelial to mesenchymal transition (EMT) in parent I-7 cells, as determined by the delocalization of E-cadherin from a cell membrane to a cytoplasmic location, and there was no evidence of an EMT in transfected/transduced cells (data not shown).
Invasion in organotypic cultures
To determine whether elevated levels of endogenous TGF-β1 or TGF-β2 altered the invasive capacity of I-7 cells, we used a three-dimensional organotypic model that has been used previously to study tumour cell invasion in a physiologically relevant manner (Nystrom et al. 2005; Yap et al. 2009). I-7 cells that overexpressed either TGF-β1 or TGF-β2 were markedly more invasive than controls, with islands of cells penetrating deeper into the matrix (Figure 2a) for each isoform, both overexpressing clones behaved in a similar way. Tissue sections from the TGF-β1 experiment were stained with Ki67 and the results showed that the percentage of TGF-β1 overexpressing cells that expressed Ki67 was significantly higher than in control cultures (Figure 2b; P < 0.5), indicating that the invading cells were also proliferating at a higher rate than controls.
Figure 2.
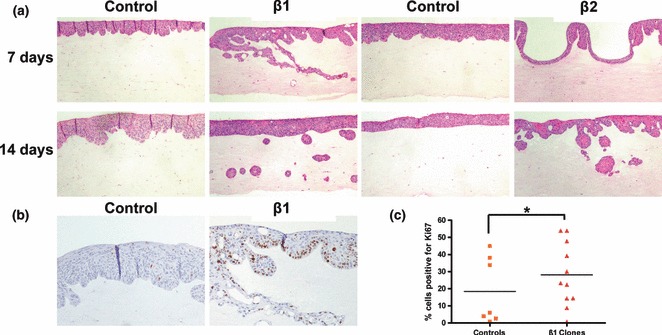
Organotypic co-cultures of I-7β1 and β2 expressing clones and their respective vector controls. (a) H&E staining of co-cultures of I-7β1C3 and I-7β2C2 clones (day 7), I-7β1C6 and I-7β2C5 (day 14) and their respective vector controls. Cells were grown on a 1:1 (v/v) collagen:Matrigel substrate embedded with 1 × 106 ICFR23 fibroblasts. (b) Ki67 expression in organotypic cultures of I-7β1C3 and I-7ppC3. (c) Dot plot of the percentage of cells positive for Ki67 in 7 and 14 days organotypic cultures. Three random fields were counted for each culture. *Significantly fewer (P < 0.05) cells positive for Ki67 were observed in vector as compared to TGF-β1-overexpressing clones.
Functional consequences in vivo
To investigate whether the more aggressive behaviour of the TGF-β overexpressing cells was maintained in vivo, TGF-β1 overexpressing cells were transplanted to the floor of the mouth of athymic mice; tumorigenicity and metastases are shown in Table 3. Tumour uptake was similar in all cases (approx. 100%). There was no difference in the size of the tumours formed by I-7 and I-7ppC3; however, the tumours formed by each clone were significantly bigger than those formed by either I-7 parental cells (P < 0.0001 for both clones) or I-7ppC3 (I-7ppC3 vs. I-7β1C3, P < 0.0001; I-7ppC3 vs. I-7β1C6, P < 0.005) (Figure 3). Whilst metastatic dissemination and local invasion of bone did not occur in animals transplanted with control cells, it was evident in a limited number of animals transplanted with TGF-β1 overexpressing cells. There was no evidence of EMT in any of the tumours, as determined E-cadherin staining in the tumour cells which remained localized to the cell membranes (data not shown).
Table 3.
Tumorigencity of I-7 parental, vector and TGF-β1 overexpressing clones following transplantation to athymic mice
| Size | |||||
|---|---|---|---|---|---|
| Cell line | Tumour | Small | Moderate | Large | Spread |
| I-7 | 9/10 | 9/9 (100%) | 0/9 | ||
| I-7ppC3 | 14/16 | 12/14 (86%) | 2/14 (14%) | 0/16 | |
| I-7β1C3 | 21/21 | 1/21 (5%) | 1/21 (5%) | 19/21 (90%) | 2/10 (lung mets) 1/11 (bone inv) |
| I-7β1C6 | 21/22 | 5/21 (24%) | 11/21 (52%) | 5/21 (24%) | 0/21 |
Animals were sacrificed 50 days after inoculation. Results are expressed as the number of mice with tumours of small, medium or large size or that had evidence of tumour spread per total number of primary tumours. Large tumours occupied more than 50% of the tongue, medium 25–50% and small <25%.
Figure 3.
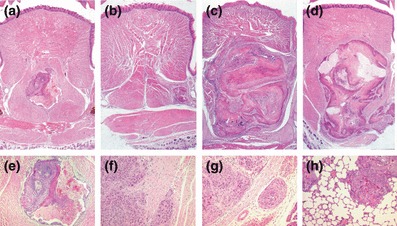
The histological appearance of primary tumours and metastases formed by I-7 cells and transfectants. Primary tumours of (a) I-7 parent, (b) I-7 vector (ppC3), (c) I-7β1C3, (d) I-7β1C6. Examples of (e) I-7, tumour degeneration (f) I-7β1C3, tumour invasion (g) I-7β1C3, vascular spread (h) I-7β1C3, lung metastases. (H&E).
To investigate why the tumours formed by TGF-β1 overexpressing cells were larger than controls, the expression of pSmad2 and Ki67 was examined in the tumours by immunohistochemistry. Levels of nuclear pSmad2 staining (a marker of active TGF-β signalling) were high in all tumours, but there was no difference the percentage of cells that stained for nuclear pSmad2 (Figure 4). However, similar to the organotypic invasion cultures, the percentage of cells staining positive for Ki67 in the tumours containing TGF-β1 overexpressing cells was significantly (I-7β1C3 vs. ppC3, P < 0.05; I-7β1C3 vs. I-7, P < 0.0001; I-7β1C6 vs. I-7, P < 0.01) or markedly (I-7β1C6 vs. I-7ppC3) higher than in tumours derived from control or vector-only transfected cells (Figure 5).
Figure 4.
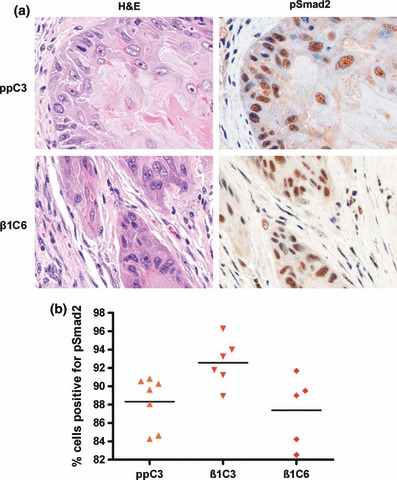
pSmad2 expression in tumours formed by vector and TGF-β1 overexpressing clones following transplantation to athymic mice. (a) Photomicrographs of representative areas of tumours formed by I-7ppC3 and I-7β1C6. Corresponding H&E stained sections are also shown. (b) Dot plot of the percentage of cells positive for pSmad2 in tumours formed by vector and TGF-β1 overexpressing clones. Ten random fields were counted for each tumour scored.
Figure 5.
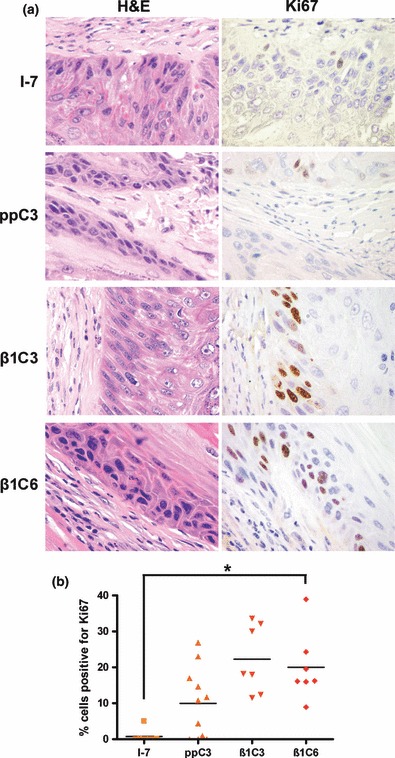
Ki67 expression in tumours formed by vector and TGF-β1 overexpressing clones following transplantation to athymic mice. (a) Photomicrographs of representative areas of tumours formed by I-7ppC3 and I-7β1C6. Corresponding H&E stained sections are also shown. (b) Dot plot of the percentage of cells positive for Ki67 in tumours formed by vector and TGF-β1 overexpressing clones. Ten random fields were counted for each tumour scored. *Expression of Ki67 was significantly (P < 0.5) increased in tumours formed by clones overexpressing TGF-β1 than in those formed by vector or parental cells.
Discussion
The role of TGF-β in carcinogenesis is extremely complex, and this complexity arises from the fact that the actions of the ligand are context dependent (Meulmeester & ten Dijke 2011) and also because differences between the TGF-β isoforms have been documented (Laverty et al. 2009). A number of strategies aimed at targeting the TGF-β pathway are currently being developed as cancer therapeutics (Korpal & Kang 2010), but, before these approaches can be fully utilized, a more comprehensive understanding of how TGF-β regulates tumour behaviour in common human cancer types is required. The present study examined the effects of overexpressing TGF-β1 or TGF-β2 in cells representing the early stages of human epidermal SCC.
To study the effects of TGF-β on early stage human epidermal SCC, we used the I-7 cell line which was one of a series of cell lines generated following transfection of HaCaT cells with mutant Ha-Ras and has been shown to be representative of non-invasive, preneoplastic stages of human SCC (Fusenig & Boukamp 1998; Gold et al. 2000). To ectopically express TGF-β1, I-7 cells were transfected with latent TGF-β1 cDNA in the plasmid pIRESpuro2. For TGF-β2, we needed to use a more efficient retroviral transduction approach, as we did not achieve sufficient expression with pIRESpuro2. Nevertheless, both expression systems resulted in clones that produced approximately twice as much ligand as control (vector only) cells. Levels of bioactive ligand in vitro were measured in non-activated samples of CM and the levels varied from low to moderate, although generally higher in the TGF-β1 compared to the TGF-β2 expressing cells (data not shown). We used latent TGF-β to reflect the fact that cells secrete latent ligand in vivo that must be activated. We show that I-7 cells retain a partial response to the growth inhibitory effects of TGF-β and cells that overexpressed either TGF-β1 or TGF-β2 retained this response, but showed no evidence of EMT. However, both the TGF-β1 and the TGF-β2 overexpressing cells were more invasive in organotypic assays, compared to control cell lines. Additionally, when the TGF-β1 overexpressing cells were transplanted to the floor of the mouth of athymic mice, the tumours formed were larger than controls and showed some evidence of metastasis and local bone invasion, an effect that was absent in controls. Collectively, these data demonstrate that overexpression of either TGF-β1 or TGF-β2 in I-7 early stage human SCC cells results in a more invasive and aggressive phenotype. Further, Ki67 staining in tissue sections from the organotypic invasion assays and in vivo tumours containing the TGF-β1 overexpressing cells was higher than those containing control cells, showing that these cells were not only more invasive but, also, had a higher rate of proliferation.
Few studies to date have investigated the role of TGF-β2 in carcinogenesis, and none have examined its expression in human clinical SCC, but this isoform has been shown to be overexpressed in gastric and colon carcinomas with increasing expression correlating with stage and poor prognosis, respectively, (Tsamandas et al. 2004; Vagenas et al. 2007). The increase in TGFβ2 expression seen from stage I – II in gastric cancer supports a role for this isoform in early stage carcinogenesis. In addition, a polymorphism in the promoter of the TGBβ2 gene that increases the expression of the isoform and correlates with lymph node metastasis in breast cancer patients has been identified (Beisner et al. 2006), supporting a role for TGF-β2 in inducing local invasion. Levels of expression of TGF-β1 in human clinical SCC have been examined previously, and similar to TGF-β2 in gastric cancer, it has been demonstrated that expression of the ligand increases progressively during carcinogenesis from normal skin to SCC (normal < Actinic Keratoses < Carcinoma in situ < SCC; Han et al. 2005). These authors also observed an inverse correlation between TGF-β1 expression and both nuclear pSmad2 and TβRII levels and suggested that overexpression of TGF-β1 enhances tumorigenicity in SCC by down-regulating TβRII, and subsequently pSmad2, resulting in an escape from ligand-mediated growth control. Previous studies in our laboratory, however, have demonstrated that levels of pSmad2 are high in clinical samples of well-differentiated human SCCs (Ganapathy et al. 2010), and in the present study, levels of pSmad2 remained high in tumours formed by TGF-β1 overexpressing clones. Our data suggest that proliferation in these tumours is not enhanced as a result of decreased signalling because of a down-regulation of TβRII, although the mechanisms to account for the increased growth rate in TGF-β overexpressing tumours in vivo are unclear. In addition to activating the canonical Smad pathway, TGF-β can also activate Smad-independent pathways, such as the activation of Erk via a mechanism that involves Shc (Lee et al., 2007; Zhang 2009). However, it is not known whether the overexpression of TGF-β in I-7 cells in the present study can enhance signalling downstream of oncogenic Ras. It is also possible that the enhanced proliferation of the TGF-β1 overexpressing cells seen in the organotypic assays and in vivo could be due to indirect effects, such as TGF-β-induced expression of mitogenic growth factors in the fibroblasts or tumour microenvironment. In support of our observations, elevated rates of proliferation have been reported in tumours formed by breast cancer cells that overexpressed TGF-β1 (Tobin et al. 2002), and these cells also grew more slowly than controls in vitro. In addition, Tobin et al. (2002) showed that expression of a dominant-negative TβRII (dnTβRII) construct in the TGF-β overexpressing breast cancer cells reduced the rate of proliferation of tumours, demonstrating that in these tumours, TβRII expression was required to maintain enhanced growth rates in vivo. Collectively, these data highlight the importance of using cell systems that closely reflect the in vivo situation when examining the effect of overexpressing TGF-β on tumour cell behaviour.
There is accumulating evidence for a tumour promoting role for TGF-β in the later stages of cancer, but there is little evidence to suggest that TGF-β switches from tumour suppressor to tumour promoter early in disease progression. Epithelial to mesenchymal transition is known to be associated with a more invasive and aggressive phenotype (Thiery et al. 2009), and we have shown previously that exogenous TGF-β1 can cooperate with mutant Ras to induce an EMT in human epidermal keratinocytes (Davies et al. 2005). However, in the present study, overexpression of either TGF-β1 or TGF-β2 in cells that represent early stage human SCC resulted in a more aggressive/invasive phenotype without evidence of EMT, either in organotypic cultures or tumours formed in athymic mice. So, the question remains as to the mechanism(s) responsible for our observations. The molecular mechanisms that underlie the switch of TGF-β from tumour suppressor to promoter are still largely unclear. Hannigan et al. (2010) recently reported that methylation of the DAB2 promoter enabled TGF-β to promote cell motility in vitro and enhance tumour growth in vitro in SCC cells, an effect that was associated with elevated levels of TGF-β2. The status of the DAB2 promoter in I-7 cells is unknown, but it seems unlikely that it is methylated because I-7 was derived from HaCaT, which was derived from normal skin (Fusenig & Boukamp 1998). Possibly, a more likely explanation comes from the observation that TGF-β acts in concert with oncogenic Ras and mutant p53 to antagonize the function of p63, resulting in an increase in TGF-β-induced migration, invasion and metastasis (Adorno et al. 2009). More specifically, Adorno et al. (2009) used HaCaT cells (which contain mutant p53) to show the formation of a TGF-β-induced ternary complex between endogenous receptor Smads (R-Smads), mutant p53 and p63. Further, they showed that the formation of this complex is dependent upon oncogenic Ras and results in tumour promotion. In our study, we used I-7 cells, which contain oncogenic Ras, which in TGF-β overexpressing cells would result in ternary complex formation and inhibition of p63 function, giving rise to a more aggressive phenotype. The formation of a TGF-β-induced ternary complex between endogenous R-Smads, mutant p53 and p63 might also explain our previous findings that expression of a dnTβRII in late stage SCC cells results in tumour promotion (Ganapathy et al. 2010), results that seemingly contrast with the results of the present study. However, in a situation where TGF-β signalling is attenuated, normal ligand levels would likely be insufficient to promote the formation this complex, leaving the function of p63 unopposed, such that ligand induction of metastasis would be inhibited.
In summary, the results of the present study demonstrate that elevated expression of either TGF-β1 or TGF-β2 in cells that represent the early stages in the development of human SCC results in a more aggressive phenotype. These findings highlight the complexity of the role played by TGF-β in SCC and carcinogenesis in general. A full understanding of the mechanisms underlying how TGF-β influences tumour development and progression will be required before therapeutic targeting of the TGF-β pathway can be used.
Acknowledgments
This work was supported by the University of Bristol Cancer Research Fund.
References
- Adorno M, Cordenonsi M, Montagner M, et al. A mutant-p53/Smad complex opposes p63 to empower TGFbeta-induced metastasis. Cell. 2009;137:87–98. doi: 10.1016/j.cell.2009.01.039. [DOI] [PubMed] [Google Scholar]
- Beisner J, Buck MB, Fritz P, et al. A novel functional polymorphism in the transforming growth factor-β2 gene promoter and tumour progression in breast cancer. Cancer Res. 2006;66:7554–7561. doi: 10.1158/0008-5472.CAN-06-0634. [DOI] [PubMed] [Google Scholar]
- Cui W, Fowlis DJ, Bryson S, et al. TGFbeta1 inhibits the formation of benign skin tumors, but enhances progression to invasive spindle carcinomas in transgenic mice. Cell. 1996;86:531–542. doi: 10.1016/s0092-8674(00)80127-0. [DOI] [PubMed] [Google Scholar]
- Davies M, Prime SS, Stone AM, et al. Overexpression of autocrine TGF-β1 suppresses the growth of spindle epithelial cells in vitro and in vivo in the rat 4-NQO model of oral carcinogenesis. Int. J. Cancer. 1997;73:68–74. doi: 10.1002/(sici)1097-0215(19970926)73:1<68::aid-ijc12>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Davies M, Robinson M, Smith E, Huntley S, Prime S, Paterson I. Induction of an epithelial to mesenchymal transition in human immortal and malignant keratinocytes by TGF-β1 involves MAPK, Smad and AP-1 signalling pathways. J. Cell. Biochem. 2005;95:918–931. doi: 10.1002/jcb.20458. [DOI] [PubMed] [Google Scholar]
- Fahey MS, Paterson IC, Stone A, et al. Dysregulation of autocrine TGF-β isoform production and ligand responses in tumour-derived and Ha-ras-transfected keratinocytes and fibroblasts. Br. J. Cancer. 1996;74:1074–1080. doi: 10.1038/bjc.1996.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusenig NE, Boukamp P. Multiple stages and genetic alterations in immortalization, malignant transformation, and tumour progression of human skin keratinocytes. Mol. Carcinog. 1998;23:144–158. doi: 10.1002/(sici)1098-2744(199811)23:3<144::aid-mc3>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Ganapathy A, Paterson IC, Prime SS, et al. TGF-β inhibits metastasis in late stage human squamous cell carcinoma of the skin by a mechanism that does not involve Id1. Cancer Lett. 2010;298:107–118. doi: 10.1016/j.canlet.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Gold LI, Jussila T, Fusenig NE, Stenbäck F. TGF-beta isoforms are differentially expressed in increasing malignant grades of HaCaT keratinocytes, suggesting separate roles in skin carcinogenesis. J. Pathol. 2000;190:579–588. doi: 10.1002/(SICI)1096-9896(200004)190:5<579::AID-PATH548>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Graycar JL, Miller DA, Arrick BA, Lyons RM, Moses HL, Derynck R. Human transforming growth factor-beta 3: recombinant expression, purification, and biological activities in comparison with transforming growth factors-beta 1 and -beta 2. Mol. Endocrinol. 1989;3:1977–1986. doi: 10.1210/mend-3-12-1977. [DOI] [PubMed] [Google Scholar]
- Han G, Lu S-L, Li AG, et al. Distinct mechanisms of TGF-β1-mediated epithelial-to-mesenchymal transition and metastasis during skin carcinogenesis. J. Clin. Invest. 2005;115:1714–1723. doi: 10.1172/JCI24399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannigan A, Smith P, Kalna G, et al. Epigenetic downregulation of human disabled homolog 2 switches TGF-beta from a tumor suppressor to a tumor promoter. J. Clin. Invest. 2010;120:2842–2857. doi: 10.1172/JCI36125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin CH, Landstrom M, Moustaks A. Mechanisms of TGF-β signaling to growth arrest, apoptosis and epithelial-mesenchymal transition. Curr. Opin. Cell Biol. 2009;21:166–176. doi: 10.1016/j.ceb.2009.01.021. [DOI] [PubMed] [Google Scholar]
- Korpal M, Kang Y. Targeting the transforming growth factor-beta signalling pathway in metastatic cancer. Eur. J. Cancer. 2010;46:1232–1240. doi: 10.1016/j.ejca.2010.02.040. [DOI] [PubMed] [Google Scholar]
- Laverty HG, Wakefield LM, Occleston NL, Ferguson MWJ. TGF-β3 and cancer: a review. Cytokine Growth Factor Rev. 2009;20:305–317. doi: 10.1016/j.cytogfr.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MK, Pardoux C, Hall MC, et al. TGF-beta activates Erk MAP kinase signalling through direct phosphorylation of ShcA. EMBO J. 2007;26:3957–3967. doi: 10.1038/sj.emboj.7601818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letterio JJ, Bottinger EP. TGF-beta knockout and dominant-negative transgenic mice. Miner. Electrolyte Metab. 1998;24:161–167. doi: 10.1159/000057365. [DOI] [PubMed] [Google Scholar]
- Massague J. TGF-β in cancer. Cell. 2008;134:220–227. [Google Scholar]
- Meulmeester E, ten Dijke P. The dynamic roles of TGF-β in cancer. J. Pathol. 2011;223:205–218. doi: 10.1002/path.2785. [DOI] [PubMed] [Google Scholar]
- Nystrom ML, Thomas GJ, Hart IR, Stone M, McKenzie IC, Marshall JF. Development of a quantitative method to analyse tumorcell invasion in organotypic culture. J. Pathol. 2005;205:468–475. doi: 10.1002/path.1716. [DOI] [PubMed] [Google Scholar]
- Pelton RW, Saxena B, Jones M, Moses HL, Gold LI. Immunohistochemical localization of TGF-β1, TGF-β2 and TGF-β3 in the mouse embryo: expression patterns suggest multiple roles during embryonic development. J. Cell Biol. 1991;115:1091–1105. doi: 10.1083/jcb.115.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portella G, Cumming SA, Liddell J, et al. Transforming growth factor beta is essential for spindle cell conversion of mouse skin carcinoma in vivo: implications for tumor invasion. Cell Growth Differ. 1998;9:393–404. [PubMed] [Google Scholar]
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;39:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Tobin SW, Douville K, Benbow U, Brinckerhoff CE, Memoli VA, Arrick BA. Consequences of altered TGF-β expression and responsiveness in breast cancer: evidence for autocrine and paracrine effects. Oncogene. 2002;21:108–118. doi: 10.1038/sj.onc.1205026. [DOI] [PubMed] [Google Scholar]
- Tsamandas AC, Kardamakis D, Ravazoula P, et al. The potential role of TGFβ1, TGFβ2, and TGFβ3 protein expression in colorectal carcinomas. Strahlenther. Onkol. 2004;180:201–208. doi: 10.1007/s00066-004-1149-x. [DOI] [PubMed] [Google Scholar]
- Vagenas K, Spyropoulos C, Gavala V, Tsmandas AC. TGF-β1, TGF-β2, and TGFβ3 protein expression in gastric ccarcinomas: correlation with prognostic factors and patient survival. J. Surg. Res. 2007;139:182–188. doi: 10.1016/j.jss.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Yap LF, Jenei V, Robinson CM, et al. Upregulation of Eps8 in oral squamous cell carcinoma promotes cell migration and invasion through integrin-dependent Rac1 activation. Oncogene. 2009;28:2524–2534. doi: 10.1038/onc.2009.105. [DOI] [PubMed] [Google Scholar]
- Zhang YE. Non Smad pathways in TGF-β signalling. Cell Res. 2009;19:128–139. doi: 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]


