Abstract
Plant-feeding insects have undergone unparalleled diversification among different plant taxa, yet explanations for variation in their diversity lack a quantitative, predictive framework. Island biogeographic theory has been applied to spatially discrete habitats but not to habitats, such as host plants, separated by genetic distance. We show that relationships between the diversity of gall-inducing flies and their host plants meet several fundamental predictions from island biogeographic theory. First, plant-taxon genetic distinctiveness, an integrator for long-term evolutionary history of plant lineages, is a significant predictor of variance in the diversity of gall-inducing flies among host-plant taxa. Second, range size and structural complexity also explain significant proportions of the variance in diversity of gall-inducing flies among different host-plant taxa. Third, as with other island systems, plant-lineage age does not predict species diversity. Island biogeographic theory, applied to habitats defined by genetic distance, provides a novel, comprehensive framework for analysing and explaining the diversity of plant-feeding insects and other host-specific taxa.
Keywords: macroevolution, plant–insect interactions, diversification, island biogeography, plant genetic distinctiveness, insularity
1. Introduction
Disparity in the number of plant-feeding insects inhabiting different types of plants has been attributed, in part, to coevolution of insects with plants [1] and to phylogenetic constraints imposed by plants on insects’ ability to feed upon them [2]. However, a unifying explanation for variation in the number of plant-feeding insects inhabiting different host-plant taxa has proved elusive [3]. Island biogeographic theory has been applied to spatially discrete habitats such as oceanic islands [4,5], mountain tops and lakes [6] but not to habitats where distance is defined by genetic variation. Plants, to the insects feeding upon them, present notable parallels with oceanic islands and island-like habitats [7–9]. Like islands, plant taxa vary in insularity (genetic distance between plants [10]), area (range size and abundance), habitat diversity (plant-structural complexity) and island age (lineage age [7–9]); moreover, speciation may occur on the host plant [11], as within islands [4,5], or between host species (between islands [12]).
As with oceanic islands, the diversity of phytophagous insects inhabiting a given plant taxon may represent a more or less stable equilibrium between colonization, speciation, emigration and extinction [9]. Equilibrium diversity of insects on a plant species or higher plant taxon should be influenced by the distribution and abundance of the host-plant taxon, plant taxon genetic distinctiveness, the number of plant species in the lineage, the age of the plant lineage and the complexity of the habitat provided by the plant [9] (see the electronic supplementary material, figures S1 and S2). This conceptual framework provides clear, testable predictions regarding the causes of variation in species diversity among plant-feeding insects, in relation to characteristics of their hosts (see the electronic supplementary material, table S1).
A useful opportunity for testing and evaluating the predictions of island biogeographic theory in the context of the diversity of phytophagous insects is provided by the greater than 3000 described species of gall-inducing midges (Diptera: Cecidomyiidae) that have well-characterized host-plant associations [13,14]. Gall midge species are highly host-plant-specific [13], disperse easily over large distances [15] and have radiated within a variety of host-plant taxa [13,14]. The species richness of gall-inducing insects on host-plant species is positively associated with the number of species in the plant taxon [16,17] and both colonization and within plant speciation [17] have been shown to be important in their diversification.
Using data on all well-characterized host-plant relationships of gall-inducing midges (Diptera: Cecidomyiidae), plant phylogenies (family-level [18], and genera of Asteraceae [19]), plant family range sizes [20], ages of plant families [21] and plant maximum heights (see the electronic supplementary material, tables S2 and S3), we tested four hypotheses stemming from island biogeographic theory: (i) distantly related (‘more insular’) host-plant taxa should have lower species diversity than closely related plant taxa; (ii) as plant family range size increases, so also should the number of host-specific gall-inducing insects specializing on that plant taxon; (iii) older plant lineages should host a greater proportion of gall-inducing midges than younger lineages; and (iv) plants that are more structurally complex (have more foliar area, more plant parts, and more temporal variation in availability of plant parts, with each of these variables representing indices of ecological opportunity) should harbor a larger gall-inducing midge fauna.
2. Material and methods
(a). Host-plant preferences
The host-plant preference database consisted of detailed host-plant preferences for 3044 species from 352 genera of gall-inducing midge species on 141 plant families worldwide (reviewed in [14]) and 456 species from 50 genera of gall-inducing midges inhabiting 53 plant genera within plant family Asteraceae [14]. Cecidomyiidae is a large family of flies and as such, there are undoubtedly species not yet described or recorded. However, in narrowly host-specific groups such as Cecidomyiidae, missing species seem unlikely to be biased towards particular plant taxa in a systematic fashion. It is possible that regional variation may exist in either observer bias (tendency of observers to focus on more conspicuous morphologically diverse plant taxa), or in the distribution and abundance of plant taxa such that in some (i.e. tropical) plant communities certain taxa may cover larger areas and be more abundant leading to potential bias [22]. A detailed table showing the number of gall-inducing midge species by plant family, and by plant genus within Asteraceae can be found in the electronic supplementary material, tables S1 and S2.
(b). Inference of plant insularity and insect diversity
We calculated insularity for each plant family using a phylogeny of angiosperms [18], and for each asterid genus using a composite phylogeny (supertree) of major clades of Asteraceae [19]. We employed the Fair Proportion measure of genetic distinctiveness to calculate insularity values for each taxon in the angiosperm phylogeny [18] for which branch length information was available [23,24]. We also applied the May [25] measure of genetic distinctiveness for the Asteraceae phylogeny, which does not have branch lengths, whereby each plant taxon is assigned the inverse value of the number of nodes connecting it to the root of the plant phylogeny. In comparisons among measures of phylogenetic distinctiveness, the May method has been shown to be the best node-based measure of capturing distinctiveness [24]. Recovered plant taxon insularity values, reported in the electronic supplementary material, tables S2 and S3, were then used in multiple regression analyses.
(c). Estimation of species area relationships
We used published data on the global geographical extent of plant families ([20,26], see the electronic supplementary material, table S1). We used these plant family range values with gall-inducing midge species richness in multivariate generalized linear models with plant insularity and plant-lineage age to control for the influence of other factors. The geographical ranges of plant genera within plant family Asteraceae were not available; so analyses of the influence of range size on cecidomyiid species richness were not conducted at the within-family level.
(d). Plant-lineage age
To test the hypothesis that older plant families would host greater numbers of gall-inducing midges relative to younger taxa, cecidomyiid species richness was compared to plant family ages determined with a combination of molecular data calibrated with available fossil data ([21], see the electronic supplementary material, table S2). Ages of plant genera were not available for Asteraceae; so this variable was not included for genera of Asteraceae.
(e). Plant architectural complexity
To test the hypothesis that plant-structural complexity (niche space and habitat diversity) also contribute to explaining the variance in diversity of phytophagous insects between plant taxa, we compared gall-inducing midge species richness within Asteraceae with maximum average height across species in each genus. For this analysis, plant height was considered a surrogate for habitat complexity [27,28]. Larger plants tend to have a greater variety and persistence of above ground parts, which in turn provides a greater variety of microhabitats for colonization by insects [9].
(f). Plant taxon species richness
We obtained published data on the sizes of North American plant families ([20], see the electronic supplementary material, table S2) and the size of each genus of Asteraceae ([20], see the electronic supplementary material, table S3). We controlled for the influence of the number of species in each plant taxon by including plant taxon species richness in our multivariate models. Results of the multivariate models reported earlier but not including plant taxon species richness are reported in the electronic supplementary material, table S4.
(g). Statistical methods
Count data are well-modelled using a Poisson distribution, but this approach requires modification to account for larger variances in the counts than can be modelled using simple likelihood methods [29]. This is routinely done using quasi-likelihood methods that permit an additional parameter to account for overdispersion in the counts [29]. For analyses at the plant family level and within plant family Asteraceae, we employed generalized linear models (GLM) with quasi-Poisson distributions specifying an extra parameter to account for the inherent overdispersion in the counts of the numbers of gall-inducing midge species. The single GLM model at the plant family level included cecidomyiid species richness as the dependent variable, and plant family range size, plant family insularity and plant-lineage age as independent variables. The GLM model among genera of Asteraceae included cecidomyiid species richness as the dependent variable plant insularity and plant height as independent variables. Since the Akakie Information Criterium (AIC) is not defined in quasi-Poisson models, model selection is best performed using an analysis of deviance approach [29,30]. We thus evaluated our models using analysis of deviance as implemented in R [31].
3. Results
(a). Plant insularity
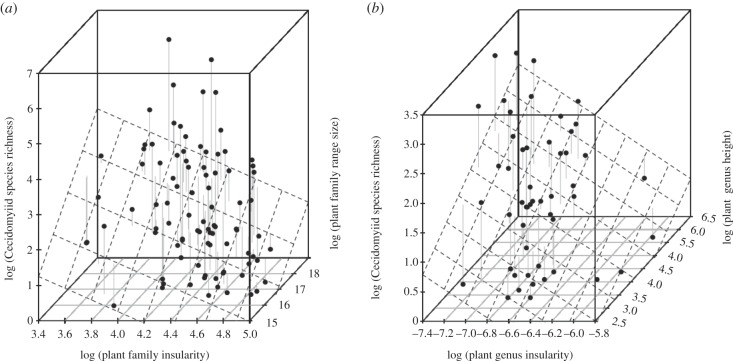
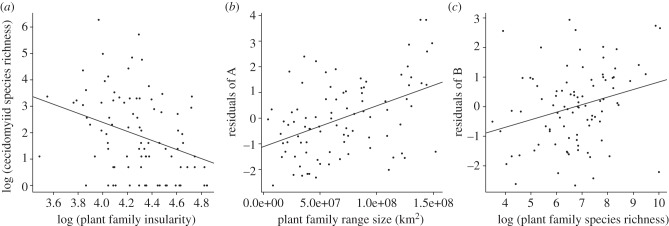
To test hypothesis (i) that plant taxa with higher insularity (that are more genetically distinct) are expected to have lower diversity of gall-inducing insects, we compared the number of species of gall-inducing midges with the insularity of both plant families and among plant genera within Asteraceae. Between plant families, and among genera within the plant family Asteraceae, insularity is a significant predictor of the species richness of gall-inducing midge species between plant taxa (table 1, figures 1a,b and 2a; see the electronic supplementary material, figure S3a). Among plant families, insularity is a significant predictor of gall-inducing midge species richness alone, with the species richness of the plant taxon as a covariate, or in multivariate models including plant family range size and plant family lineage age (table 1 and figures 1a and 2). Within plant family Asteraceae, insularity is also a significant predictor of gall-inducing midge species richness alone and in multivariate models including species richness of the plant taxon and plant genus structural complexity (table 1, figure 1b and electronic supplementary material, figure S3a).
Table 1.
Multivariate generalized linear model results for both plant families and genera of plant family Asteraceae. (The sample size (n), β, β s.e., t-value and p-value are provided for each independent variable in each model.)
| model and response variables | independent variables | n | β | β s.e. | t-value | p-value |
|---|---|---|---|---|---|---|
| plant family insularity alone | ||||||
| Cecidomyiid species richness | plant family insularity | 88 | −1.714 | 0.500 | −3.424 | 0.0009 |
| all plant families | ||||||
| Cecidomyiid species richness | plant family insularity | 88 | −1.440 | 0.591 | −2.435 | 0.01 |
| Cecidomyiid species richness | plant family range size | 88 | 3.95 × 10−8 | 1.65 × 10−8 | 2.398 | 0.01 |
| Cecidomyiid species richness | plant-lineage age | 88 | −0.216 | 0.398 | −0.543 | 0.58 |
| Cecidomyiid species richness | plant family species richness | 88 | 0.571 | 0.145 | 3.922 | <0.01 |
| Asteraceae genus insularity alone | ||||||
| Cecidomyiid species richness | plant genus insularity | 50 | −1.816 | 0.4552 | −3.990 | 0.0002 |
| Asteraceae | ||||||
| Cecidomyiid species richness | plant genus insularity | 50 | −1.631 | 0.401 | −4.067 | <0.0001 |
| Cecidomyiid species richness | plant genus species richness | 50 | 0.204 | 0.079 | 2.568 | <0.05 |
| Cecidomyiid species richness | plant maximum height | 50 | 0.334 | 0.161 | 2.07 | <0.05 |
Figure 1.
Relationship between gall midge diversity (vertical axis) and island geographical processes applied to plant taxa as islands. (a) Relationship between plant family range size (y-axis), plant family insularity (x-axis) decreases in plant family insularity and increases in plant family range size are associated with increases in cecidomyiid species richness. (b) Plant maximum height (y-axis) and plant genus insularity (x-axis) decreases in plant genus insularity and increases in plant genus maximum height are associated with increases in cecidomyiid species richness.
Figure 2.
Two-dimensional plots of relationships between gall midge diversity and island geographical processes applied to plant families. (a) Plant family insularity versus cecidomyiid species richness. (b) Plant family range size versus residuals from (a). (c) Plant family species richness versus residuals from (b).
(b). Plant taxon range size
To test hypothesis (ii) that as plant taxon range size increases, the diversity of cecidomyiid species should also increase, cecidomyiid species richness was compared with plant family range size using the same model framework. Figures 1a, 2b and table 1 show that, plant family range size explains a significant proportion of between plant family variance in the number of species of gall-inducing midges.
(c). Plant-lineage age
To test hypothesis (iii) that plant-lineage age should be positively associated with increases in cecidomyiid species richness, plant family age was compared with cecidomyiid species richness. Plant family age was not a significant predictor of cecidomyiid species richness alone or in multivariate models (table 1). Analysis of deviance model selection (see the electronic supplementary material, tables S6–S9) suggested that our model would be improved through removal of the plant-lineage age term; thus though results are presented, it was not included in our final model.
(d). Plant architectural complexity
Hypothesis (iv) predicts that plant species with higher niche space (more ecological opportunity) will display elevated species richness of plant-feeding insects relative to plant species with fewer niches. Figure 1b, see the electronic supplementary material, figure S3b and table 1 illustrate that plant taxa that are taller (a proxy for plant architectural diversity and nice space) show higher diversity of gall-inducing midge species.
(e). Plant species richness
Plant taxa with higher species richness should also provide more available niche space to plant-feeding insects, also as a result of more ecological opportunity, and thus are expected to display higher numbers of plant-feeding insects relative to more species-poor plant taxa. Figure 2c; see the electronic supplementary material, figure S3c and table 1 show that plant taxa which contain higher numbers of plant species also display greater diversity of gall-inducing midge species.
4. Discussion
In accordance with predictions derived from island biogeographic theory, our results demonstrate that the three island biogeographic variables: (i) insularity, (ii) area and (iii) ecological opportunity, have significant predictive power in explaining variance in the diversity of gall-inducing flies among host-plant taxa. These results suggest that, at least for groups of host-specific taxa, this body of theory can serve as a general quantitative framework for explaining variation in species diversity among taxa.
Previous island biogeographic studies and studies of insect diversity have modelled insularity in spatial terms [5,32]; in this study, our novel consideration of insularity as a function of genetic isolation is a strong predictor of species diversity. Less-insular plant taxa may harbour a higher diversity of insects in large part because shifts between host-plant species are one of the most important drivers of diversification in phytophagous insects [12], and host-plant shifts are more common between plant taxa that are more closely related [17,33]. Closely related host plants are more likely to be ecologically similar relative to distantly related hosts, facilitating colonization of and adaptation to the new host. Consistent with this idea, Nyman et al. [33] showed that in Nematine sawflies most shifts occur between closely related hosts, and when shifts to distantly related host plants do occur they are more frequent between ecologically similar hosts. Diversification of phytophagous beetle species (Coleoptera) has similarly been shown to reflect a complex history of coevolution between beetles and angiosperms in which host-plant relationships structure patterns of host shifts [34]. Host shifts may facilitate diversification in part through specialization on novel host plants, such that specialization proceeds through preference and performance trade-offs between hosts, or via ecological, physiological and behavioural divergence of host-shifted populations. In each case, selection can favour the evolution of reproductive isolation such that incipient species perform better on their own host [35–37]. Such host shifts may also be more likely onto plant taxa covering large geographical areas [11].
The observation that taxon range size explains a significant proportion of between taxon variance in diversity of gall-inducing midges accords well with previous studies showing that plant taxon range size is an important predictor of phytophagous insect diversity in the Palearctic [9,17] and Neotropics [11]. Plant taxa with larger ranges (and higher abundances within those ranges) come into contact with a greater diversity of other plant species and their insect fauna and thus may present larger colonization targets relative to plant taxa with smaller geographical extent [38]. Encounter rate, the frequency with which an insect species will encounter a suitable host plant, is also higher for plant taxa with relatively larger geographical ranges [39]. Further, host-plant taxa that have larger geographical ranges may facilitate diversification of phytophagous insects through geographical isolation of the insect taxa in various parts of the host range [40]. Thus, plant taxa with larger geographical ranges may host larger insect fauna through colonization resulting from increased contact with potential colonizing insects species, and in situ diversification owing to geographical isolation in the large plant range.
The finding that plant-lineage age is not significantly associated with the diversity of phytophagous insects is consistent with previous studies of island age and diversity on oceanic islands [5] or plant-lineages [16]. However, other studies of phytophagous insects in relation to plant-lineage age have shown that on woody host species, the number of species of some groups, such as Lepidoptera and Auchenorrhyncha, has increased over time [40]. Furthermore, Farrell & Mitter [41] showed that in accordance with predictions from Erhlich & Raven [1], much of the diversity of phytophagous insects can be attributed to the effects of plant-lineage age and ecological opportunity. The feeding mode of phytophagous insects may, in part, determine the likelihood of a relationship with host-plant-lineage age, for any particular insect taxon. The feeding mode of phytophagous insects may also determine the likelihood of a relationship with plant architectural diversity.
Plant architectural diversity probably promotes insect diversification through increases in available niche space [11,42], whereby plants that are more architecturally diverse exhibit more variation in types and positions of plant parts [43], which in turn allow increased specialization such that the result is the finer division of a host plant into discrete niches associated with different plant parts [11,43,44]. Diversification of beetles has also been associated with the utilization of the niches provided by different plant parts (roots, stems, fruits, flowers and leaves [45]). Complementary work on Blephanoneura fruitflies [11] and some cecidomyiidflies [46] has shown that diversification in flies may often accompany specialization on different plant parts. Similarly, in the oak gall wasps diversification has been associated with divergence in plant organ use [44]. Metrics of niche space that are more detailed than plant height are expected to yield even stronger associations with insect diversity.
Plant taxon species richness also probably promotes insect diversification through increases in ecological opportunity [9,16,47,48]. Since most plant-feeding insects display some level of specialization towards particular host taxa as plant taxon species richness increases so does the ecological opportunity available to host-associated insects in the form of niches on novel host taxa [47,49]. This is supported, especially, by evidence from many studies of highly host-specific gall-inducing taxa illustrating that increases in plant species richness are associated with significant increases in the species richness of the gall-inducing fauna [16,50,51].
Considered together, the causal processes underlying host-plant genetic distinctiveness, range size and architectural complexity explain substantial proportions of the variance in diversity of cecidomyid flies among host plant taxa, providing an integrative, statistical approach to explicating and quantifying the causes of variation in biodiversity within this group. When similar plant attribute data are comprehensively available for plant species, it will prove informative to perform these analyses at lower taxonomic scales. The analyses conducted here show that the island biogeographic theory provides an integrative framework for integrating the evolutionary processes that have determined the remarkable diversity of phytophagous insects. This framework is especially relevant to phytophagous insects that are host-plant-specific, but should also be applicable to other taxa for which ecological interactions strongly mediate diversification.
Acknowledgements
We thank Arne O. Mooers, Felix Breden and Brian Farrell for comments on the manuscript. We especially thank Tanja Schwander, Christine Parent, Arne Mooers, Will Stein and Laura Weir for helpful discussion and encouragement. Ruth Joy and Pete Hurd provided invaluable statistical advice. We are also grateful to the Simon Fraser University FAB* Laboratory. This work was supported by NSERC operating grants to B.J.C. and Arne O. Mooers.
References
- 1.Ehrlich P. R., Raven P. H. 1964. Butterflies and plants: a study in coevolution. Evolution 18, 586–608 10.2307/2406212 (doi:10.2307/2406212) [DOI] [Google Scholar]
- 2.Price P. W. 1994. Phylogenetic constraints, adaptive syndromes, and emergent properties: from individuals to population dynamics. Res. Popul. Ecol. 36, 3–14 10.1007/BF02515079 (doi:10.1007/BF02515079) [DOI] [Google Scholar]
- 3.Janz N., Nylin S., Wahlberg N. 2006. Diversity begets diversity: host expansions and the diversification of plant-feeding insects. BMC Evol. Biol. 6, 4. 10.1186/1471-2148-6-4 (doi:10.1186/1471-2148-6-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Losos J., Schluter D. 2000. Analysis of an evolutionary species–area relationship. Nature 408, 847–850 10.1038/35048558 (doi:10.1038/35048558) [DOI] [PubMed] [Google Scholar]
- 5.Parent C. E., Crespi B. J. 2006. Sequential colonization and diversification of Galapagos endemic land snail genus Bulimulus (Gastropoda, Stylommatophora). Evolution 60, 2311–2328 [PubMed] [Google Scholar]
- 6.MacArthur R. H., Wilson E. O. 1967. The theory of island biogeography. Princeton, NJ: Princeton University Press [Google Scholar]
- 7.Janzen D. 1968. Host plants as islands in evolutionary and contemporary time. Am. Nat. 102, 592–595 10.1086/282574 (doi:10.1086/282574) [DOI] [Google Scholar]
- 8.Lawton J. H., Schroeder D. 1977. Effects of plant type, size of geographical range and taxonomic isolation on number of insect species associated with British plants. Nature 265, 137–140 10.1038/265137a0 (doi:10.1038/265137a0) [DOI] [Google Scholar]
- 9.Strong D. R., Lawton J. H., Southwood T. R. E. 1984. Insects on plants: community patterns and mechanisms. Oxford, UK: Blackwell Scientific Publiations [Google Scholar]
- 10.Weiblen G. D., Webb C. O., Novotny V., Basset Y., Miller S. E. 2006. Phylogenetic dispersion of host use in a tropical insect herbivore community. Ecology 87, S62–S75 10.1890/0012-9658(2006)87[62:PDOHUI]2.0.CO;2 (doi:10.1890/0012-9658(2006)87[62:PDOHUI]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 11.Condon M. A., Scheffer S. J., Lewis M. L., Swenson S. M. 2008. Hidden neotropical diversity: greater than the sum of its parts. Science 320, 928–931 10.1126/science.1155832 (doi:10.1126/science.1155832) [DOI] [PubMed] [Google Scholar]
- 12.Dres M., Mallet J. 2002. Host races in plant-feeding insects and their importance in sympatric speciation. Phil. Trans. R. Soc. Lond. B 357, 471–492 10.1098/rstb.2002.1059 (doi:10.1098/rstb.2002.1059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gagne R. J. 1989. The plant-feeding gall midges of North America. Ithaca, NY: Cornell University Press [Google Scholar]
- 14.Gagne R. J. 2010. A catalog of the Cecidomyiidae (Diptera) of the world. Digital version 1. See: http://www.ars.usda.gov/SP2UserFiles/Place/12754100/Gagne_2010_World_Catalog_Cecidomyiidae.pdf [Google Scholar]
- 15.Mamaev B. M., Krivosheina N. P. 1993. The larvae of the gall midges (Diptera, Cecidomyiidae). Rotterdam, The Netherlands: A.A. Balkema [Google Scholar]
- 16.Fernandes G. W. 1993. Plant family size and age effects on insular gall-forming species richness. Glob. Ecol. Biogeogr. Lett. 2, 71–74 10.2307/2997508 (doi:10.2307/2997508) [DOI] [Google Scholar]
- 17.Roskam J. C. 1985. Evolutionary patterns in gall midge–host plant associations (Diptera: Cecidomyiidae). Tijdschrift voor Entomologie 128, 193–213 [Google Scholar]
- 18.Davies T. J., Barraclough T. G., Chase M. W., Soltis D. E., Savolainen V. 2004. Darwin's abominable mystery: insights from a supertree of the angiosperms. Proc. Natl Acad. Sci. USA 101, 1904–1909 10.1073/pnas.0308127100 (doi:10.1073/pnas.0308127100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Funk V. A., et al. 2005. Everywhere but Antarctica: using a supertree to understand the diversity and distribution of the Compositae. Biol. Skr. 55, 343–374 [Google Scholar]
- 20.Stevens P. F. 2010. Angiosperm phylogeny. See: www.mobot.org/MOBOT/research/APweb [Google Scholar]
- 21.Bell C. D., Soltis D. E., Soltis P. S. 2010. The age and diversification of angiosperms revisited. Am. J. Bot. 97, 1296–1303 10.3732/ajb.0900346 (doi:10.3732/ajb.0900346) [DOI] [PubMed] [Google Scholar]
- 22.Novotny V., Basset Y., Miller S. E., Weiblen G. D., Bremer B., Cizek L., Drozd P. 2002. Low host specificity of herbivorous insects in a tropical forest. Nature 416, 841–844 10.1038/416841a (doi:10.1038/416841a) [DOI] [PubMed] [Google Scholar]
- 23.Issac N. J. B., Turvey S. T., Collen B., Waterman C., Baillie J. E. M. 2007. Mammals on the EDGE: conservation priorities based on threat and phylogeny. PLoS ONE 23, e296. 10.1371/journal.pone.0000296 (doi:10.1371/journal.pone.0000296) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Redding D. W., Hartmann K., Mimoto A., Bokal D., Devos M., Mooers A. O. 2008. Evolutionary distinctive species often capture more phylogenetic diversity than expected. J. Theor. Biol. 251, 606–15 10.1016/j.jtbi.2007.12.006 (doi:10.1016/j.jtbi.2007.12.006) [DOI] [PubMed] [Google Scholar]
- 25.May R. M. 1990. Taxonomy as destiny. Nature 347, 129–130 10.1038/347129a0 (doi:10.1038/347129a0) [DOI] [Google Scholar]
- 26.Vamosi J. C., Vamosi S. M. 2010. Key innovations within a geographical context in flowering plants: towards resolving Darwin's abominable mystery. Ecol. Lett. 13, 1270–1279 10.1111/j.1461-0248.2010.01521.x (doi:10.1111/j.1461-0248.2010.01521.x) [DOI] [PubMed] [Google Scholar]
- 27.Cytrynowicz M. 1991. Resource size and predictability, and local herbivore richness in a subtropical Brazilian cerrado. In Plant–animal interactions: evolutionary ecology in tropical and temperate regions (eds Price P. W., Lewinsohn T. M., Fernandes G. W., Benson W. W.), pp. 639 New York, NY: John Riley [Google Scholar]
- 28.Marquis R. J. 1991. Herbivore fauna of Piper (Piperaceae) in a Costa Rican wet forest: diversity, specificity and impact. In Plant–animal interactions: evolutionary ecology in tropical and temperate regions (eds Price P., Lewinsohn T. M., Fernandes G. W., Benson W. W.), pp. 179–208 New York, NY: John Wiley [Google Scholar]
- 29.McCullogh P., Nelder J. A. 1989. Generalized linear models. London, UK: Chapman and Hall [Google Scholar]
- 30.Zuur A. F., Ieno E. N., Walker N., Saveliev A. A., Smith G. M. 2009. Mixed effects models and extensions in ecology with R. New York, NY: Springer [Google Scholar]
- 31.R Development Core Team 2011. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 32.Whittaker R. J., Fernandes-Palacios J. M. 2007. Island biogeography: ecology, evolution and conservation. Oxford, UK: Oxford University Press [Google Scholar]
- 33.Nyman T., Farrell B. D., Zinovjev A. G., Vikberg V. 2006. Larval habits, host–plant associations, and speciation in nematine sawflies (Hymenoptera: Tenthredinidae). Evolution 60, 1622–1637 10.1554/05-674.1 (doi:10.1554/05-674.1) [DOI] [PubMed] [Google Scholar]
- 34.McKenna D. D., Sequeira A. S., Marvaldi A. E., Farrell B. D. 2009. Temporal lags and overlap in the diversification of weevils and flowering plants. Proc. Natl Acad. Sci. USA 106, 7083–7088 10.1073/pnas.0810618106 (doi:10.1073/pnas.0810618106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feder J. L., Filchak K. E. 1999. It's about time: the evidence for host plant-mediated selection in the apple maggot fly, Rhagoletis pomonella, and its implications for fitness trade-offs in phytophagous insects. Entomologia Experimentalis Et Applicata 91, 211–225 10.1046/j.1570-7458.1999.00486.x (doi:10.1046/j.1570-7458.1999.00486.x) [DOI] [Google Scholar]
- 36.Hawthorne D. J., Via S. 2001. Genetic linkage of ecological specialization and reproductive isolation in pea aphids. Nature 412, 904–907 10.1038/35091062 (doi:10.1038/35091062) [DOI] [PubMed] [Google Scholar]
- 37.Nosil P., Crespi B. J., Sandoval C. P. 2002. Host–plant adaptation drives the parallel evolution of reproductive isolation. Nature 417, 440–443 10.1038/417440a (doi:10.1038/417440a) [DOI] [PubMed] [Google Scholar]
- 38.Williams C. B. 1964. Patterns in the balance of nature. London, UK: Academic Press [Google Scholar]
- 39.Southwood T. R. E. 1961. The number of species of insect associated with various trees. J. Anim. Ecol. 30, 1–8 10.2307/2109 (doi:10.2307/2109) [DOI] [Google Scholar]
- 40.Brandle M., Brandl R. 2001. Species richness of insects and mites on trees: expanding Southwood. J. Anim. Ecol. 70, 491–504 10.1046/j.1365-2656.2001.00506.x (doi:10.1046/j.1365-2656.2001.00506.x) [DOI] [Google Scholar]
- 41.Farrell B. D., Mitter C. 1994. Adaptive radiation in insects and plants: time and opportunity. Am. Zool. 34, 57–69 10.1093/icb/34.1.57 (doi:10.1093/icb/34.1.57) [DOI] [Google Scholar]
- 42.Basset Y. 1996. Local communities of arboreal herbivores in Papua New Guinea: predictors of insect variables. Ecology 77, 1906–1919 10.2307/2265793 (doi:10.2307/2265793) [DOI] [Google Scholar]
- 43.Després L., Pettex E., Plaisance V., Pompanon F. 2002. Speciation in the globeflower fly Chiastocheta spp. (Diptera: Anthomyiidae) in relation to host plant species, biogeography, and morphology. Mol. Phylogenet. Evol. 22, 258–68 10.1006/mpev.2001.1047 (doi:10.1006/mpev.2001.1047) [DOI] [PubMed] [Google Scholar]
- 44.Cook R. J., Rokas A., Pagel M., Stone G. N. 2002. Evolutionary shifts between host oak sections and host-plant organs in Andricus gallwasps. Evolution 56, 1821–1830 [DOI] [PubMed] [Google Scholar]
- 45.Hunt T., et al. 2007. A comprehensive phylogeny of beetles reveals the evolutionary origins of a super radiation. Science 318, 1913–1916 10.1126/science.1146954 (doi:10.1126/science.1146954) [DOI] [PubMed] [Google Scholar]
- 46.Joy J. B., Crespi B. J. 2007. Adaptive radiation of gall-inducing insects within a single host-plant species. Evolution 61, 784–795 10.1111/j.1558-5646.2007.00069.x (doi:10.1111/j.1558-5646.2007.00069.x) [DOI] [PubMed] [Google Scholar]
- 47.Cruitsinger G. M., Collins M. D., Fordyce J. A., Gompert Z., Nice C. C., Sanders N. J. 2006. Plant genotypic diversity predicts community structure and governs an ecosystem process. Science 313, 966–968 10.1126/science.1128326 (doi:10.1126/science.1128326) [DOI] [PubMed] [Google Scholar]
- 48.Lewinsohn T. M., Novotny V., Basset Y. 2005. Insects on plants: diversity of herbivore assemblages revisited. Annu. Rev. Ecol. Evol. Syst. 35, 597–620 10.1146/annurev.ecolsys.36.091704.175520 (doi:10.1146/annurev.ecolsys.36.091704.175520) [DOI] [Google Scholar]
- 49.Bernays E., Graham M. 1988. On the evolution of host specificity in phytophagous arthropods. Ecology 69, 886–892 10.2307/1941237 (doi:10.2307/1941237) [DOI] [Google Scholar]
- 50.Price P. W. 2005. Adaptive radiation of gall-inducing insects. Basic Appl. Ecol. 6, 413–421 10.1016/j.baae.2005.07.002 (doi:10.1016/j.baae.2005.07.002) [DOI] [Google Scholar]
- 51.Yukawa J., Uechi N., Tokuda M., Sato S. 2005. Radiation of gall midges (Diptera: Cecidomyiidae) in Japan. Basic Appl. Ecol. 6, 453–461 10.1016/j.baae.2005.07.004 (doi:10.1016/j.baae.2005.07.004) [DOI] [Google Scholar]