Abstract
Adaptation in dynamic environments depends on the grain, magnitude and predictability of ecological fluctuations experienced within and across generations. Phenotypic plasticity is a well-studied mechanism in this regard, yet the potentially complex effects of stochastic environmental variation on optimal mean trait values are often overlooked. Using an optimality model inspired by timing of reproduction in great tits, we show that temporal variation affects not only optimal reaction norm slope, but also elevation. With increased environmental variation and an asymmetric relationship between fitness and breeding date, optimal timing shifts away from the side of the fitness curve with the steepest decline. In a relatively constant environment, the timing of the birds is matched with the seasonal food peak, but they become adaptively mismatched in environments with temporal variation in temperature whenever the fitness curve is asymmetric. Various processes affecting the survival of offspring and parents influence this asymmetry, which collectively determine the ‘safest’ strategy, i.e. whether females should breed before, on, or after the food peak in a variable environment. As climate change might affect the (co)variance of environmental variables as well as their averages, risk aversion may influence how species should shift their seasonal timing in a warming world.
Keywords: plasticity, fitness curve, stochasticity, climate, dynamic optimization, bet-hedging
1. Introduction
The question of how populations adapt to environments that are highly variable in time and space has long intrigued evolutionary ecologists. A variety of strategies have evolved that allow organisms to cope with environmental heterogeneity at different scales, including developmental homeostasis, niche specialization, phenotypic plasticity and bet-hedging [1]. In the case of temporal heterogeneity, optimal strategies depend critically on three components: the ‘grain’ of environmental variation (e.g. daily, monthly, interannual, etc.), the magnitude of fluctuations typically experienced across an individual's lifetime, and the predictability of these changes [2,3].
When environments fluctuate predictably, phenotypic plasticity—the ability of a single genotype to produce different phenotypes in response to different environmental conditions—is expected to be at a selective advantage [4–6]. Phenotypic plasticity allows individuals to ‘match’ their phenotype to shifting selective optima, but this match is rarely perfect because the correlations between environmental cues and environment-specific optima are imperfect. At evolutionary equilibrium, the degree of plasticity (in a trait or set of traits) therefore reflects a compromise between the fitness benefits of phenotypically tracking environmental fluctuations and the fitness costs of responding to imperfect cues [7,8], in addition to the costs of being plastic per se [9,10].
In general, the greater the time-lag between when an organism perceives a cue and when the fitness consequences of their responses are determined, the less informative cues are likely to be [11] and thus optimal reaction norms should be less steep than expected based on the fluctuating selection [12]. In the limit where environmental fluctuations are completely unpredictable to organisms, optimal reaction norms should be flat (i.e. no plasticity) and alternative evolutionary outcomes, such as polymorphisms or bet-hedging, are possible [13–15].
In reality, most natural populations experience environmental variation that is only partially predictable and which also might not be constant over time. Moreover, the curve relating expected overall net fitness gain to trait values (hereafter the ‘fitness curve’) might not be symmetrical, with potentially important consequences for optimal trait values in a fluctuating environment [16]. In theoretical studies of adaptation, symmetric fitness curves are typically assumed for analytical convenience; for example, Gaussian stabilizing selection about a fixed or fluctuating optimum [17,18]. For many characters, however, asymmetric fitness curves might be more likely than symmetric curves, given that many ecological and physiological processes affecting fitness are likely to exhibit skewness with respect to key traits [19–21]. For example, frequency-dependent competition for breeding territories in migratory birds can result in asymmetric relationships between reproductive success and arrival date to the breeding grounds, even though breeding resources might exhibit symmetric distributions [22]. In ectothermic insects and lizards, fitness components often exhibit left-skewed distributions in relation to body temperatures, where the fitness consequences of temperatures 5° below the optimum, for example, are more severe than those 5° above it. This asymmetry, in combination with fluctuating environmental temperatures, can lead to optimal trait values (e.g. body temperatures) centred at a temperature below that at which instantaneous fitness is maximal [20].
In this paper, we focus on a phenological trait (one that determines the timing of a particular seasonal activity) and explore whether seasonally fluctuating environmental conditions in combination with potentially asymmetric fitness curves can lead to adaptive mismatches between the phenology of a consumer and that of its resource (cf. [23]). To do so, we develop a model on the timing of avian reproduction and the dynamics of a seasonal resource peak, directly inspired by our work on great tits (Parus major) and caterpillars (Operophtera brumata and other lepidopteran species; reviewed in [24]). Avian timing traits are ideal characters in many ways for understanding how organisms make optimal decisions in variable environments [25]. Individual females often exhibit adaptive phenotypic plasticity in their scheduling of seasonal activities, such as migration, egg-laying and feather-moulting, given that seasonal environments usually provide informative cues that allow phenological adjustment [26,27].
In woodland passerines such as great tits that rely on caterpillars to feed their nestlings, females strive to match the period of maximum nestling energy requirements to the seasonal peak in caterpillar abundance [28]. Caterpillar development is highly temperature-dependent, with the seasonal peak in caterpillar biomass being earlier in warmer years [29]. Female birds must make their ‘decision’ of when to breed several weeks in advance of the food peak, as gonadal development, nest-building, egg-laying and incubation all take time. Photoperiod (day length) acts as the primary environmental cue that ‘sets into motion’ gonadal development and sexual behaviours early in spring. Seasonal changes in photoperiod are the same every year at a given latitude, however, so birds must use supplementary cues such as spring temperatures to fine-tune their laying dates to local, year-specific conditions [30,31]. The timing of avian reproduction and the phenology of prey do not always respond to environmental fluctuations in the same way, however; for example, laying dates of great tits in our Dutch study population respond to temperatures early in spring, whereas caterpillar phenology is sensitive to temperatures over a longer period that includes late spring/early summer, after which great tits have laid their eggs [29]. In highly stochastic environments, risk-averse strategies (e.g. late laying in relation to the seasonal food peak) might be selected if environmental cues such as spring temperature correlate poorly with the fluctuating food peak, particularly if the risks of reproductive failure early in the season are high, as recently suggested for coal tits (Periparus ater) in the UK [32].
Climate change has added to the impetus to better understand the factors shaping optimal timing in a variable world. Many bird species, including our own study population of Dutch great tits, have become phenologically mismatched with the timing of locally ephemeral food peaks, in some cases intensifying natural selection for earlier breeding [24]. Mismatches can result when cues used by the birds (e.g. temperatures early in spring) no longer accurately predict the peak in food abundance, which responds to temperatures during a different period. Even if cues remain accurate, females might be constrained from tracking advancements in caterpillar phenology by a trade-off: if they lay earlier they reap the benefits, in terms of reproductive success, of being well-matched with the food peak, but they jeopardize their own survival by producing eggs earlier in spring when temperatures are colder and food is potentially less available. This might result in the phenological mismatch (i.e. laying too late) being adaptive [23]. More generally, asymmetric fitness curves combined with temporal environmental fluctuations can lead to strategies that appear to be suboptimal in the short-term, but are in fact optimal in the long run [20,33].
The goal of this paper is therefore to assess how optimal reaction norms for timing of reproduction can be shaped by interactions between the degree of temporal environmental variation and the shape of the fitness curve. This framework also provides insight into when birds might be adaptively mismatched with the timing of their food source.
2. Material and methods
Dynamic programming was used to assess the factors affecting the optimal timing of reproduction. The model was inspired and partially parametrized by our work on great tits. In the model, the optimal decision of when to start egg-laying depends on the state of the bird, the state of the environment and the time of year. To calculate the optimal decision per day, we defined a terminal reward function, i.e. fitness at the end of the season, which depended on whether the female survived, the number of fledglings produced, and their probability of recruitment as determined by the date of fledgling (see §2e). Dynamic programming uses this terminal reward function and backwards iteration from the last to the first day of the season, to calculate the optimal decision for each possible state of the bird and the environment [34]. The optimal decision each day is that with the highest expected fitness at the end of the season.
(a). State variables
A female is characterized by two state variables: its brood size and the age of the brood. Based on these state variables, a female can be in one of four reproductive phases: non-breeding, egg-laying, incubating or caring for dependent young. In the model, females are limited to only one decision: when to start egg-laying. Clutch size is fixed at eight eggs and once they start, they have to continue reproduction (for more details on the model structure, see §2g).
At the start of egg-laying, the date in the season when food availability will peak (hereafter food peak) is uncertain. A female can only use information from the current day and the past when deciding when to lay. Both avian laying date and the time of the seasonal peak in food abundance are affected by temperatures. Females in our model use temperature cues to decide when to start laying. The fluctuating environment is characterized by two state variables: temperature and temperature sum. Temperature influences the energetic costs of egg production and incubation. Temperature sum is used to calculate the food peak. We calculated a profile of daily average temperatures using data from a Dutch weather station (De Bilt, The Netherlands) for the period 1981–2010. This profile represents the typical seasonal progression of temperatures that great tits in The Netherlands are expected to experience across a full year.
(b). Temperature model
Weather states are persistent over short time scales: a warm day is more likely to be followed by another warm day than by a cold day, and vice versa. In other words, day-to-day changes in temperature are dependent on the current temperature. If the current temperature is close to the expected value for that day, there is an approximately equal probability of the temperature the following day being higher or lower. However, if the deviation of the current temperature from the expectation is more extreme, it will have a higher probability to return to the mean the following day. We thus use a ‘regression to the mean’ approach to model daily temperature changes. In this model, q(t) is the current temperature on day t and qavg(t) is the average temperature at day t, given by the temperature profile from 1981–2010. The temperature on the next day is calculated as:
| 2.1 |
with 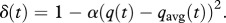
The variable δ controls the strength of the autocorrelation, depending on the deviation from the average temperature, α is a constant, σq is the standard deviation in temperature and rq is a normally distributed random number.
(c). Food availability
Caterpillar hatching and development are temperature-dependent, with the seasonal peak in caterpillar biomass being earlier in warmer years [29]. Based on caterpillar biomass data from 1993–2009 [29], we developed a temperature degree-day model to predict caterpillar biomass:
| 2.2 |
where s is a temperature sum (with temperature threshold Tst), AF adjusts the height of the caterpillar biomass, σF adjust the width of the function and μF is the temperature sum at which the caterpillar biomass is highest.
Note that although the food peak date depends on temperature and the birds in the model base their laying date on temperature, laying takes place about 30 days before the food peak date. Hence, at the time of laying the food peak is to some extent unpredictable.
(d). Sources of mortality
We considered two sources of mortality: predation and starvation. Adult predation is linked to the fraction u of the working day that a bird spends foraging and occurs with probability d(u + u2). If a bird cannot balance its energy expenditure and energy intake for one day, it dies of starvation (for more details on energy balance, see the electronic supplementary material, section A). Daily energy expenditure is dependent on the reproductive state of the bird and the state of the environment (food availability and temperature). Nestling energy need increases with age [35,36] and if parents cannot provide enough energy either the entire brood is lost (scenario 1) or some nestlings die (scenario 2).
(e). Fitness
To calculate the optimal decision, we need to specify the terminal reward function, which here depends on the survival of the female, the number of young that fledge and the fledging date of the young. Empirical studies of Dutch great tits show that offspring recruitment probability decreases over the season [37,38]. Following the findings of these studies, we assume that offspring recruitment probability is highest for offspring fledging at the food peak date.
The terminal reward also depends on the survival of the female until the end of the breeding season, but there could be a trade-off between female survival and the fitness value of the brood [23]. When the costs of reproduction are high early in the season and the food peak date is also early, a female could potentially increase her fitness by fledging her young early, but at the same time potentially decrease her fitness if she is not likely to survive. If she starts to breed later, she increases her own survival chances, but at a cost of reduced fitness benefits from her offspring.
(f). Scenarios explored
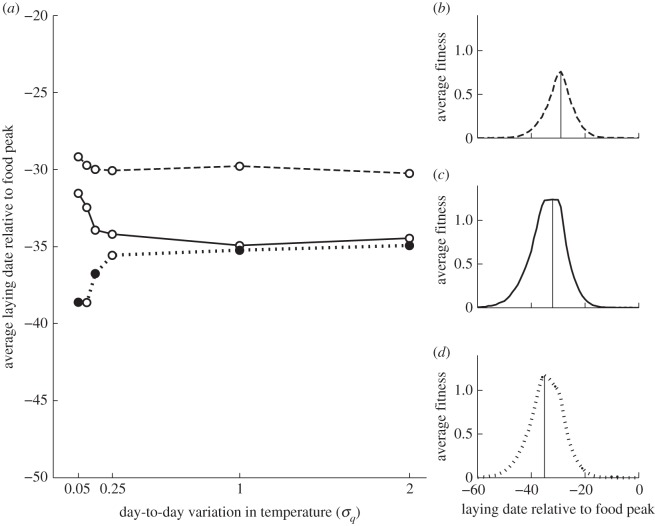
To assess how the shape of the fitness curve and temporal variation of the environment affected optimal timing, we calculated the optimal decision matrix for five values of variability in day-to-day temperatures (equation 2.1; σq = 0.05, 0.1, 0.15, 0.25, 1.0, 2.0). We varied two relationships to assess the effect of the shape of the fitness curve: how nestling energy need depends on nestling age and how offspring recruitment probability depends on fledging date. In the case where nestling energy need is independent of nestling age and offspring recruitment probability is independent of fledging date, the fitness curve is symmetric (curve shape 1, figure 2b). When nestling energy need increases with age the fitness curve becomes asymmetric with a steeper cliff at later dates, which we call ‘left-skewed’ (curve shape 2, figure 2c). If there is also a decline in offspring recruitment probability with fledging date [37] the fitness curve has a steeper cliff for early dates, which we call ‘right-skewed’ (curve shape 3, figure 2d).
Figure 2.
(a) Simulated optimal laying dates relative to the food peak date, plotted against the standard deviation in temperature σq for scenario 1, for each of the three fitness curves. (b) Dashed line denotes fitness curve 1. (c) Solid line denotes fitness curve 2. (d) Dotted line denotes fitness curve 3. (a) Shows the average over 100 000 forward runs under the condition that a female started egg-laying. (b–d) Show the average total fitness for females that were forced to start laying at fixed dates. The solid vertical lines depict the peak in average total fitness.
To assess the potential effects of the costs of different phases of reproduction, we explore two extreme scenarios. In scenario 1, there are no additional costs for egg-laying and incubation, only the costs of self-maintenance. Here, the female cannot abandon the brood during any of the reproductive phases, i.e. she must provision and care for nestlings until fledging. In scenario 2, there are costs for egg-laying and temperature-dependent costs for incubation (see the electronic supplementary material, section A), which potentially also skew the fitness curve. Furthermore, the female can abandon (part) of the brood during the nestling-feeding phase. In both scenarios, there are additional costs for the nestling-rearing phase: on top of her own energy need, a female has to find enough food to feed her nestlings.
(g). Model structure
For each combination of the two reproductive costs scenarios, the three shapes of the fitness curve, and the five values for variation in temperature, we calculated the optimal decision matrix. Each is a five-dimensional matrix that contains the optimal decision for each possible combination of date and the four state variables for that specific combination of reproductive costs scenario, fitness curve shape and inputted temperature variation. To extract the optimal reaction norm for timing of reproduction from the optimal decision matrix, 100 000 forward simulations are run for one breeding season, from the beginning of March to the end of August. Each run starts on 8 March with a randomly drawn deviation from the average temperature, with standard deviation σq and a temperature sum of 0. The same temperature model, which was used to run the optimization, was then used to calculate the temperature and the resulting temperature sum the next day. At the start of each run, the female is not breeding. The decision to start egg-laying or continue not to breed is made daily, until the decision is made to start. From that moment on, the female has to lay eight eggs, incubate 12 days and, under scenario 1, take care of all the nestlings until fledging. Under scenario 2, they have the option of abandoning all or part of the brood during nestling feeding.
For each run, the food peak date, the optimal laying date and the fitness that resulted from breeding at that time (the fitness value of the brood, the survival of the female and the total fitness) was calculated. The difference between the laying date and the food peak date is the synchrony with the food peak.
To express the optimal laying date against a single environmental variable to obtain a simple reaction norm, we averaged (realized model) temperatures across the period 16 March–20 April, the period found to best correlate with laying dates in the wild [29].
To calculate the shapes of the fitness curves (figures 2b–d and 3), for each of the runs we also simulated birds that were forced to start egg-laying on Julian dates 70–170 (calendar dates 11 March–19 June in a non-leap year) and the fitness that resulted from breeding at that time was recorded. To account for the fact that the food peak date varies between runs, we calculated average fitness relative to synchrony with the food peak date, with the average taken over birds with that specific synchrony.
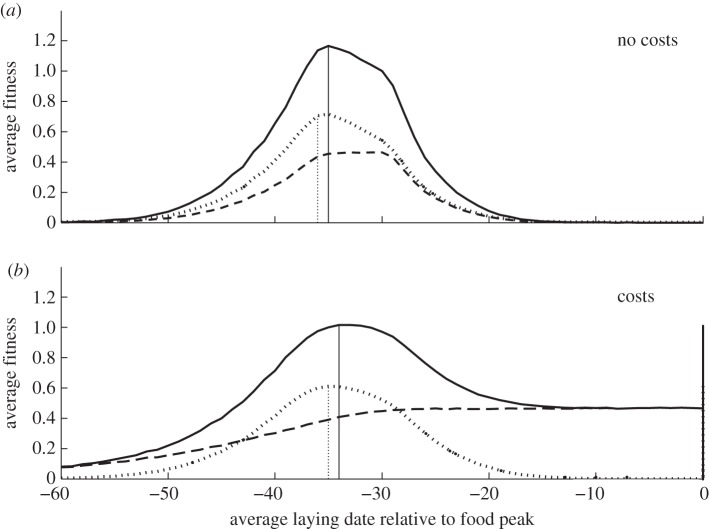
Figure 3.
Average fitness for females that were forced to start laying at fixed dates, depicted relative to the peak biomass date. The dashed lines depict average female fitness, the dotted lines depict average fitness of the brood and the solid lines depict the average total fitness. (a) Fitness curve 3 and scenario 1. (b) Fitness curve 3 and scenario 2. Dashed vertical lines show peak in average fitness of the brood. Solid vertical lines show peak in average total fitness.
3. Results
(a). Scenario 1: no costs of egg-laying and incubation
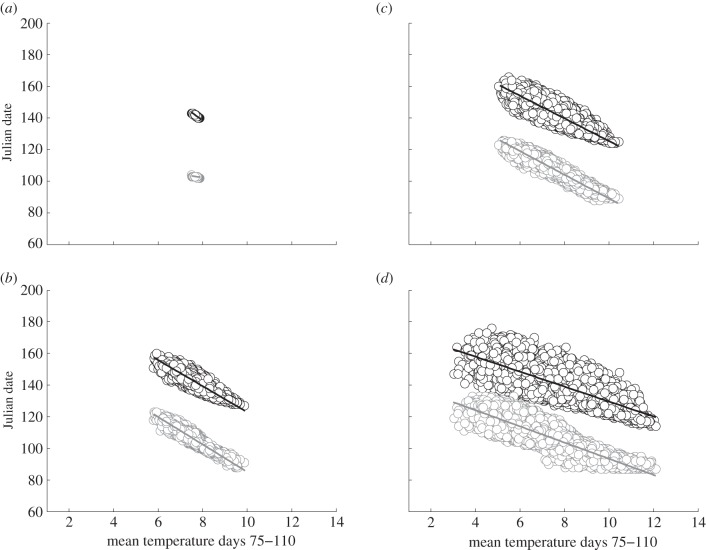
With increasing temperature variation, the slopes of the reaction norm of optimal laying dates against mean temperatures become shallower (figure 1). This matches the theoretical expectation that strong plasticity (i.e. steeper reaction norm slope) is suboptimal when environmental factors determining selection (in this case, temperature-dependent phenology of caterpillars) are less predictable. In the simulations, females respond only to temperatures on the current day and from days previous to that. If stochasticity in these temperatures is high, they correlate less strongly with temperatures during late spring and early summer, which determine the date of maximal caterpillar abundance. Consequently, the higher the variation in daily temperatures, the less predictable the food peak date and the flatter the optimal reaction norm.
Figure 1.
The effect of increasing temperature variation on optimal reaction norms when there are no costs of egg-laying and incubation. Simulated laying dates and food peak dates are plotted against mean temperatures during the reference period 16 March–20 April (Julian dates 75–110). Data points show the results of 10 000 forward simulations based on the optimal decision matrix (grey circles and best-fit regression lines denote lay dates; black circles and lines, food peak dates) for scenario 1, fitness curve 3. Standard deviation in temperature is different in each panel: (a) σq = 0.05, (b) σq = 0.15, (c) σq = 1.0 and (d) σq = 2.0.
That these reactions norms are optimal can be seen by comparing the slopes of the lay date–temperature relationship with the relationship between the food peak date and average temperatures for the same period (figure 1). With low temperature variation, food peak date also varies little and correlates strongly with temperatures during the reference period (figure 1a). By contrast, when temperature variation is high, variation in food peak date is also high and more weakly dependent on the temperatures to which the birds respond (figure 1d). This is because caterpillar biomass continues to develop after the birds have started to lay, so increasing variation reduces the correlation between caterpillar development at the time the birds are laying and caterpillar development after that period until the food peak date (average = 20 May, Julian day 140).
In addition to the changes in optimal slopes in response to increased environmental variation, the average synchrony between lay dates and food peak date (i.e. the difference in elevation between the lines in each panel of figure 1) decreases. In other words, females lay on average later and thus the peak energy demand of their nestlings (nestling day 9) occurs a few days after the food peak date. Although seemingly maladaptive, this in fact maximizes expected fitness in a stochastic environment, as (for the parameters used in figure 1) the fitness curve is right (positively) skewed; i.e. the fitness costs of laying earlier are greater than those of laying later. This asymmetric fitness curve corresponding to the scenario depicted in figure 1d is shown in figure 2d.
The direction and extent of the mismatch for each level of temperature variation explored are shown in figure 2a. This shows average laying date relative to the food peak date for three different types of fitness curve (figure 2b–d), all for scenario 1. In the simplest case (figure 2b), which corresponds to the symmetrical fitness curve 1 in figure 2a, nestling energy need does not depend on nestling age and offspring recruitment probability does not depend on relative fledging date. Here, the best strategy is to time egg-laying such that nestlings are 9 days old at the food peak date (i.e. laying 30 days before the food peak date), ensuring that the nestling–rearing period is centred on the period of maximal caterpillar abundance independent of temperature variation.
In the second case, nestling energy need increases with age, but offspring recruitment probability does not depend on relative fledging date (fitness curve 2, figure 2c). Now, there is a greater penalty for breeding relatively late compared with breeding relatively early (left-skewed fitness curve). With zero variation in temperature, fitness is maximized by laying approximately 31 days before the food peak date, but laying becomes earlier by several days with just a small amount of temperature variation. The higher the variation in temperatures, the greater this ‘adaptive mismatch’.
In the third case, nestling energy need is age-dependent and offspring recruitment probability also depends on relative fledging date, assumed to be highest for nestlings fledging on the food peak date (fitness curve 3, figure 2d). The fitness curve is now right-skewed, with higher costs of breeding too early. At low variation in temperatures, optimal average laying date is 39 days before the food peak date, which results in nestlings fledging close to the food peak date. For higher variation, average laying date is shifted closer to the food peak date (i.e. later), which is a safer strategy because the left-side ‘drop’ of the fitness curve is steeper than the right side.
The difference in the elevation of the lines in figure 2a for fitness curve 1 versus that of 2 and 3 is that in fitness curve 1 nestling energy need is constant at the highest level of energy need, whereas in fitness curves 2 and 3 young nestlings need less food and thus the female should start earlier, as there is already enough food available earlier in the season.
(b). Scenario 2: costs of egg-laying and incubation
When costs of egg production and incubation are additionally taken into account (scenario 2), fitness curves have even a greater asymmetry (figure 3). With no costs and a standard deviation in temperatures of 2.0, laying ca 35 days before the food peak date is optimal (figure 3a). By contrast, with costs, the fitness penalty of breeding earlier is considerably larger than that of laying later (figure 3b). Hence, laying later is optimal because the increase in female survival (dashed curve figure 3b) is higher than the decrease in offspring recruitment probability (dotted curve figure 3b).
Figure 4 shows the plots of average laying dates (relative to the food peak date) as a function of σq (temperature variation), for each of the three fitness curves. For fitness curve 1, there is little change in laying dates up to σq = 1.0: laying dates are such that females have 9-day-old nestlings at the food peak date. When σq > 1.0, relative laying dates become later, reflecting the fact that declines in fitness are slightly steeper for earlier laying dates. For fitness curve 2, where nestling energy need is age-dependent, there is a steeper cliff for breeding too late and optimal laying dates are earlier with increasing σq up to a value of 1.0. Beyond this, however, earlier laying incurs a risk of reduced female survival, and average relative laying date then becomes later. Finally, for fitness curve 3, where nestling energy need is age-dependent and offspring recruitment probability depends on relative fledging date, there is a steep fitness cliff for breeding too early. At low σq, laying date is close to that which maximizes offspring recruitment probability (i.e. 39 days before the food peak date), but with increasing σq it shifts closer to the food peak date, which avoids the costs for female survival of being too early. Again, the difference in the elevation of the lines for fitness curve 1 versus 2 and 3 is that with fitness curve 1, nestling energy need is constant at the highest level of energy need, whereas in fitness curves 2 and 3, young nestlings need less food and thus the female can start earlier.
Figure 4.
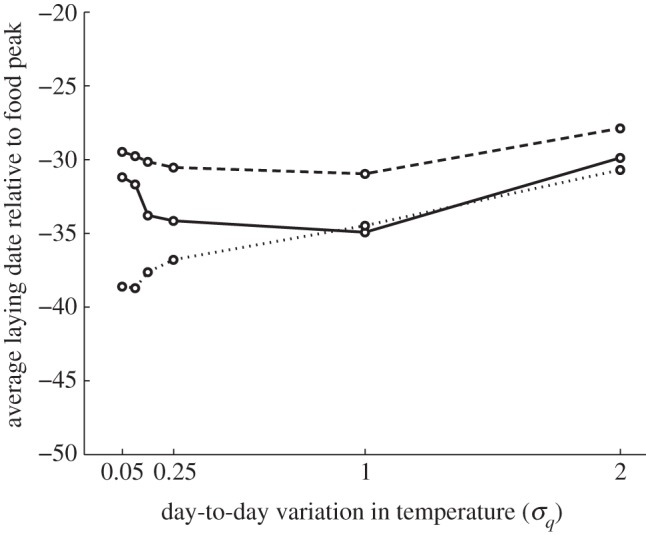
Simulated optimal laying dates relative to the food peak against the standard deviation in temperature σq for scenario 2. The dashed line represents fitness curve 1; solid line, fitness curve 2; dotted line, fitness curve 3.
4. Discussion
Using a model of timing of egg-laying in great tits, we explored the interactive effects of environmental variation and fitness curve shape on optimal breeding time in a variable environment. The results illustrate how seemingly suboptimal phenotypic responses can in fact be optimal when temporal variation in temperature is coupled with asymmetry in fitness curves. Depending on the extent and nature of this asymmetry, our model suggests that ‘adaptive mismatches’ [23] of up to 7 days can result when day-to-day variation in temperature is similar to that experienced by wild great tits in The Netherlands (the actual standard deviation in spring temperature ranges from 1.4 to 2.1, with a mean of 1.8). Timing differences of this magnitude are biologically significant for great tits; the standard deviation of laying dates in our Hoge Veluwe study population is on average 5.4 days within years, and 4.6 days across years (T. E. Reed & M. E. Visser 2012, unpublished data).
We used published empirical relationships and insights from detailed studies of great tit ecology [29,35–38] to characterize the fitness costs and benefits of laying at different dates relative to the seasonal food peak. Different combinations of age-dependent nestling energy need, the dependence of offspring recruitment probability on fledging date and maternal costs of egg-laying and incubation resulted in various types of asymmetry in the overall fitness curve. The functions describing each of these processes were parametrized based on real data from great tits, but we used the model to tell us how they together determine the shape of the overall fitness curve. While we could have used observational data from our long-term study population to directly parametrize the curve relating total fitness to laying dates, this approach is problematic in that many other factors potentially change along the laying-date axis other than date per se, for example, the phenotypic quality of parents [39]. Experimental manipulations of laying dates in both directions (i.e. advancements and delays) would therefore be required to accurately characterize the fitness curve. Moreover, extreme laying dates (e.g. very early laying) are rarely observed in the wild given the associated high survival costs, whereas the model could be used to explore the fitness consequences of a much broader range of laying dates. In the simplest case where the emergent fitness curve was symmetrical (figure 2b), the optimal strategy was to time egg-laying such that the nestling rearing period was centred on the food peak date, independent of temperature variation (figure 2a). When nestling energy need was age-dependent, the resulting fitness curve was asymmetric with a steeper decline at later dates as the costs of rearing offspring at the declining part of the food peak are more severe when large offspring have high energy needs. In this case, the strategy that maximized fitness in the face of day-to-day temperature variation was to lay earlier relative to the seasonal food peak. By contrast, laying relatively later was optimal when the asymmetry was the other way around (a steeper decline at early dates, generated when nestling energy need was age-dependent and offspring recruitment probability depended on fledging date). These contrasting patterns were also evident when maternal costs were present, but only when temperature variation was low; at higher σq, the best strategy was to always lay relatively later given the high survival costs of breeding too early under potentially colder temperatures, when costs of egg production and incubation are high (figure 4).
Collectively, these results show that variation in the environment coupled with asymmetric fitness curves leads not only to shallower reaction norm slopes, but also adaptive mismatches in reaction norm elevation. In our example of laying dates in great tits, various processes influenced the asymmetry in the overall fitness function, but the specific forms of these relationships are unimportant. The novel general insight is that any process which leads to asymmetrical fitness curves will lead to adaptive phenological mismatch when environments vary through time. Temporal environmental heterogeneity is a ubiquitous feature of natural populations and we argue, on first principles, that asymmetric fitness curves are also probably common given that many ecological and physiological processes affecting fitness are likely to exhibit skewness, particularly with respect to temperature [19–21]. Martin and Huey [20] discussed a similar phenomenon apparent in ectotherms, where average body temperatures are typically observed to be lower than those that maximize instantaneous fitness. Using a simple optimality model, they showed that this apparent mismatch could be understood in terms of Jensen's inequality (a mathematical property of nonlinear functions) [33], and the variance and skew inherent in ectotherm body temperatures and fitness curves, respectively [20]. In their example, the reason for the apparent departure from optimality is that ectotherms are imperfect thermoregulators in the face of fluctuating environmental temperatures, and body temperature deviations to the right of the fitness peak (higher temperatures) reduce fitness more than equivalent deviations to the left (lower temperatures) do. Our focus was very different in terms of the trait and taxon considered (i.e. timing of breeding in an endothermic bird), but we show that similar reasoning can be employed to understand the selective factors shaping timing decisions. Indeed, the phenomenon might be particularly relevant for predicting optimal phenology in seasonal environments, given that environmental factors affecting reproductive success and parental survival (e.g. temperature and precipitation) themselves exhibit seasonal profiles that are often nonlinear and asymmetric. Few empirical studies have characterized the true shape of individual-level fitness curves in natural populations [40,41], and experimental manipulations of phenotypes are required to test the intuition that fitness scales asymmetrically with phenology and other key life-history traits.
We note also some parallels between our modelling results regarding adaptive mismatch and the concept of conservative bet-hedging: the idea that ‘safe’ life-history strategies maximize geometric mean fitness in a variable environment [13,14]. Conservative bet-hedging has frequently been invoked in the evolution of timing traits in animals and plants, for example, the timing of bolting in monocarpic perennials [42], diapause in copepods [43], parturition in viviparous lizards [44], and laying dates in woodland passerines [32]. Variance in fitness reduces the geometric mean relative to the arithmetic mean, and thus bet-hedging is typically assumed to involve processes that minimize fitness variance across generations, potentially at the expense of reduced arithmetic mean fitness [13,45]. Our dynamic programming model, based purely on the maximization of arithmetic mean fitness, shows that nonlinear averaging processes alone are sufficient to select for adaptive mismatches in the face of temporal environmental heterogeneity. Inferring a role for conservative bet-hedging would require the optimization model to be couched in terms of geometric mean fitness, which is a non-trivial problem in dynamic programming [46]. We nonetheless speculate that there might be additional benefits of adaptive mismatch in terms of reductions in fitness variance, if temperature deviations one side of the mean produce more variable fitness outcomes than deviations in the other direction, owing to an asymmetric fitness curve. Indeed, our forward simulations suggest that the variation in fitness often exhibited a minimum close to the observed optimal laying dates (see the electronic supplementary material, section B)
In conclusion, we show that the degree of temporal environmental variability affects not only the optimal level of plasticity, but also the optimal mean timing of reproduction, whenever fitness curves are asymmetric. Our model of avian timing of reproduction illustrates how various processes can result in asymmetric fitness curves, and how this can select for adaptively mismatched reproduction with respect to a fluctuating seasonal food peak. The results add to a growing number of studies which show that, under certain circumstances, phenological mismatch between consumers and their resources might be adaptive [22,23,47]. Such mismatches are sometimes taken uncritically as maladaptive symptoms of adverse impacts of climate change, yet they might have been present prior to the current warming. Climate data and models suggest that greenhouse gas forcing can increase the frequency of extreme weather events [48,49]. Our results suggest that in addition to the complex population effects of changing climatic variability [50], such changes coupled with asymmetric fitness curves could also influence how species should shift their seasonal timing in a warming world.
Acknowledgements
M.E.V. is supported by a NWO-VICI grant. We thank the associate editor and three anonymous reviewers for constructive comments on the manuscript.
References
- 1.Levins R. 1968. Evolution in changing environments. Princeton, NJ: Princeton University Press [Google Scholar]
- 2.Via S., Gomulkiewicz R., De Jong G., Scheiner S. M., Schlichting C. D., van Tienderen P. H. 1995. Adaptive phenotypic plasticity: consensus and controversy. Trends Ecol. Evol. 10, 212–217 10.1016/S0169-5347(00)89061-8 (doi:10.1016/S0169-5347(00)89061-8) [DOI] [PubMed] [Google Scholar]
- 3.Meyers L. A., Bull J. J. 2002. Fighting change with change: adaptive variation in an uncertain world. Trends Ecol. Evol. 17, 551–557 10.1016/s0169-5347(02)02633-2 (doi:10.1016/s0169-5347(02)02633-2) [DOI] [Google Scholar]
- 4.Bradshaw A. D. 1965. Evolutionary significance of phenotypic plasticity in plants. Adv. Genet. 13, 115–155 10.1016/S0065-2660(08)60048-6 (doi:10.1016/S0065-2660(08)60048-6) [DOI] [Google Scholar]
- 5.Schlichting C. D. 1986. The evolution of phenotypic plasticity in plants. Annu. Rev. Ecol. Syst. 17, 667–693 10.1146/annurev.es.17.110186.003315 (doi:10.1146/annurev.es.17.110186.003315) [DOI] [Google Scholar]
- 6.Stearns S. C. 1989. The evolutionary significance of phenotypic plasticity: phenotypic sources of variation among organisms can be described by developmental switches and reaction norms. Bioscience 39, 436–445 10.2307/1311135 (doi:10.2307/1311135) [DOI] [Google Scholar]
- 7.Moran N. A. 1992. The evolutionary maintenance of alternative phenotypes. Am. Nat. 139, 971–989 10.1086/285369 (doi:10.1086/285369) [DOI] [Google Scholar]
- 8.Reed T. E., Waples R. S., Schindler D. E., Hard J. J., Kinnison M. T. 2010. Phenotypic plasticity and population viability: the importance of environmental predictability. Proc. R. Soc. B 277, 3391–3400 10.1098/rspb.2010.0771 (doi:10.1098/rspb.2010.0771) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeWitt T. J., Sih A., Wilson D. S. 1998. Costs and limits of phenotypic plasticity. Trends Ecol. Evol. 13, 77–81 10.1016/S0169-5347(97)01274-3 (doi:10.1016/S0169-5347(97)01274-3) [DOI] [PubMed] [Google Scholar]
- 10.Auld J. R., Agrawal A. A., Relyea R. A. 2010. Re-evaluating the costs and limits of adaptive phenotypic plasticity. Proc. R. Soc. B 277, 503–511 10.1098/rspb.2009.1355 (doi:10.1098/rspb.2009.1355) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Padilla D. K., Adolph S. C. 1996. Plastic inducible morphologies are not always adaptive: the importance of time delays in a stochastic environment. Evol. Ecol. 10, 105–117 10.1007/BF01239351 (doi:10.1007/BF01239351) [DOI] [Google Scholar]
- 12.Lande R. 2009. Adaptation to an extraordinary environment by evolution of phenotypic plasticity and genetic assimilation. J. Evol. Biol. 22, 1435–1446 10.1111/j.1420-9101.2009.01754.x (doi:10.1111/j.1420-9101.2009.01754.x) [DOI] [PubMed] [Google Scholar]
- 13.Philippi T., Seger J. 1989. Hedging ones evolutionary bets, revisited. Trends Ecol. Evol. 4, 41–44 10.1016/0169-5347(89)90138-9 (doi:10.1016/0169-5347(89)90138-9) [DOI] [PubMed] [Google Scholar]
- 14.Simons A. M. 2011. Modes of response to environmental change and the elusive empirical evidence for bet hedging. Proc. R. Soc. B 278, 1601–1609 10.1098/rspb.2011.0176 (doi:10.1098/rspb.2011.0176) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leimar O. 2009. Environmental and genetic cues in the evolution of phenotypic polymorphism. Evol. Ecol. 23, 125–135 10.1007/s10682-007-9194-4 (doi:10.1007/s10682-007-9194-4) [DOI] [Google Scholar]
- 16.Collins E. J., McNamara J. M., Ramsey D. M. 2006. Learning rules for optimal selection in a varying environment: mate choice revisited. Behav. Ecol. 17, 799–809 10.1093/beheco/arl008 (doi:10.1093/beheco/arl008) [DOI] [Google Scholar]
- 17.Lande R., Shannon S. 1996. The role of genetic variation in adaptation and population persistence in a changing environment. Evolution 50, 434–437 10.2307/2410812 (doi:10.2307/2410812) [DOI] [PubMed] [Google Scholar]
- 18.Bürger R., Krall C. 2004. Quantitive-genetic models and changing environments. In Evolutionary conservation biology (eds Ferriere R., Dieckmann U., Couvet D.), pp. 171–187 Cambridge, UK: Cambridge University Press [Google Scholar]
- 19.Gilchrist G. W. 1995. Specialists and generalists in changing environments. 1. Fitness landscapes of thermal sensitivity. Am. Nat. 146, 252–270 10.1086/285797 (doi:10.1086/285797) [DOI] [Google Scholar]
- 20.Martin T. L., Huey R. B. 2008. Why ‘suboptimal’ is optimal: Jensen's inequality and ectotherm thermal preferences. Am. Nat. 171, E102–E118 10.1086/527502 (doi:10.1086/527502) [DOI] [PubMed] [Google Scholar]
- 21.Dell A. I., Pawar S., Savage V. M. 2011. Systematic variation in the temperature dependence of physiological and ecological traits. Proc. Natl Acad. Sci. USA 108, 10 591–10 596 10.1073/pnas.1015178108 (doi:10.1073/pnas.1015178108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johansson J., Jonzén N. 2012. Effects of territory competition and climate change on timing of arrival to breeding grounds: a game–theory approach. Am. Nat. 179, 463–474 10.1086/664624 (doi:10.1086/664624) [DOI] [PubMed] [Google Scholar]
- 23.Visser M. E., te Marvelde L., Lof M. E. 2011. Adaptive phenological mismatches of birds and their food in a warming world. J. Ornithol. (doi:10.1007/s10336-011-770-6) [Google Scholar]
- 24.Visser M. E. 2008. Keeping up with a warming world; assessing the rate of adaptation to climate change. Proc. R. Soc. B 275, 649–659 10.1098/rspb.2007.0997 (doi:10.1098/rspb.2007.0997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McNamara J., Barta Z., Klassen M., Bauer S. 2011. Cues and the optimal timing of activities under environmental changes. Ecol. Lett. 14, 1183–1190 10.1111/j.1461-0248.2011.01686.x (doi:10.1111/j.1461-0248.2011.01686.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dawson A. 2008. Control of the annual cycle in birds: endocrine constraints and plasticity in response to ecological variability. Phil. Trans. R. Soc. B 363, 1621–1633 10.1098/rstb.2007.0004 (doi:10.1098/rstb.2007.0004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shine R., Brown G. P. 2008. Adapting to the unpredictable: reproductive biology of vertebrates in the Australian wet-dry tropics. Phil. Trans. R. Soc. B 363, 363–373 10.1098/rstb.2007.2144 (doi:10.1098/rstb.2007.2144) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lack D. 1968. Ecological adaptations for breeding in birds. London, UK: Methuen [Google Scholar]
- 29.Visser M. E., Holleman L. J. M., Gienapp P. 2006. Shifts in caterpillar biomass phenology due to climate change and its impact on the breeding biology of an insectivorous bird. Oecologia 147, 164–172 10.1007/s00442-005-0299-6 (doi:10.1007/s00442-005-0299-6) [DOI] [PubMed] [Google Scholar]
- 30.Visser M. E., Holleman L. J. M., Caro S. P. 2009. Temperature has a causal effect on avian timing of reproduction. Proc. R. Soc. B 276, 2323–2331 10.1098/rspb.2009.0213 (doi:10.1098/rspb.2009.0213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schaper S. V., Dawson A., Sharp P. J., Gienapp P., Caro S. P., Visser M. E. 2012. Increasing temperature, not mean temperature, is a cue for avian timing of reproduction. Am. Nat. 179, E55–E69 10.1086/663675 (doi:10.1086/663675) [DOI] [PubMed] [Google Scholar]
- 32.Goodenough A. E., Hart A. G., Stafford R. 2010. Is adjustment of breeding phenology keeping pace with the need for change? Linking observed response in woodland birds to changes in temperature and selection pressure. Clim. Change 102, 687–697 10.1007/s10584-010-9932-4 (doi:10.1007/s10584-010-9932-4) [DOI] [Google Scholar]
- 33.Ruel J. J., Ayres M. P. 1999. Jensen's inequality predicts effects of environmental variation. Trends Ecol. Evol. 14, 361–366 10.1016/s0169-5347(99)01664-x (doi:10.1016/s0169-5347(99)01664-x) [DOI] [PubMed] [Google Scholar]
- 34.Houston A. I., McNamara J. M. 1999. Models of adaptive behavior. Cambridge, UK: Cambridge University Press [Google Scholar]
- 35.Royama T. 1966. Factors governing feeding rate, food requirement and brood size of nestling great tits Parus major. Ibis 108, 313–315 10.1111/j.1474-919X.1966.tb07348.x (doi:10.1111/j.1474-919X.1966.tb07348.x) [DOI] [Google Scholar]
- 36.Mols C. M. M., van Noordwijk A. J., Visser M. E. 2005. Assessing the reduction of caterpillar numbers by great tits Parus major breeding in apple orchards. Ardea 93, 259–269 [Google Scholar]
- 37.Verboven N., Visser M. E. 1998. Seasonal variation in local recruitment of great tits: the importance of being early. Oikos 81, 511–524 10.2307/3546771 (doi:10.2307/3546771) [DOI] [Google Scholar]
- 38.Monros J. S., Belda E. J., Barba E. 2002. Post-fledging survival of individual great tits: the effect of hatching date and fledging mass. Oikos 99, 481–488 10.1034/j.1600-0706.2002.11909.x (doi:10.1034/j.1600-0706.2002.11909.x) [DOI] [Google Scholar]
- 39.Verhulst S., Nilsson J. A. 2008. The timing of birds’ breeding seasons: a review of experiments that manipulated timing of breeding. Phil. Trans. R. Soc. B 363, 399–410 10.1098/rstb.2007.2146 (doi:10.1098/rstb.2007.2146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kingsolver J. G., Pfennig D. W. 2007. Patterns and power of phenotypic selection in nature. Bioscience 57, 561–572 10.1641/B570706 (doi:10.1641/B570706) [DOI] [Google Scholar]
- 41.Fear K. K., Price T. 1998. The adaptive surface in ecology. Oikos 82, 440–448 10.2307/3546365 (doi:10.2307/3546365) [DOI] [Google Scholar]
- 42.Simons A. M., Johnston M. O. 2003. Suboptimal timing of reproduction in Lobelia inflata may be a conservative bet-hedging strategy. J. Evol. Biol. 16, 233–243 10.1046/j.1420-9101.2003.00530.x (doi:10.1046/j.1420-9101.2003.00530.x) [DOI] [PubMed] [Google Scholar]
- 43.Hairston N. G., Munns W. R. 1984. The timing of copepod diapause as an evolutionarily stable strategy. Am. Nat. 123, 733–751 10.1086/284236 (doi:10.1086/284236) [DOI] [Google Scholar]
- 44.Rock J. 2006. Delayed parturition: constraint or coping mechanism in a viviparous gekkonid? J. Zool. 268, 355–360 10.1111/j.1469-7998.2006.00065.x (doi:10.1111/j.1469-7998.2006.00065.x) [DOI] [Google Scholar]
- 45.Gillespie J. 1974. Natural selection for within-generation variance in offspring number. Genetics 76, 601–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McNamara J. M. 2000. A classification of dynamic optimization problems in fluctuating environments. Evol. Ecol. Res. 2, 457–471 [Google Scholar]
- 47.Singer M. C., Parmesan C. 2010. Phenological asynchrony between herbivorous insects and their hosts: signal of climate change or pre-existing adaptive strategy? Phil. Trans. R. Soc. B 365, 3161–3176 10.1098/rstb.2010.0144 (doi:10.1098/rstb.2010.0144) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Easterling D. R., Evans J. L., Groisman P. Y., Karl T. R., Kunkel K. E., Ambenje P. 2000. Observed variability and trends in extreme climate events: a brief review. Bull. Am. Meteorol. Soc. 81, 417–426 (doi:10.1175/1520-0477(2000)081<0417:OVATIE>2.3.CO;2) [DOI] [Google Scholar]
- 49.Easterling D. R., Meehl G. A., Parmesan C., Changnon S. A., Karl T. R., Mearns L. O. 2000. Climate extremes: observations, modeling, and impacts. Science 289, 2068–2074 10.1126/science.289.5487.2068 (doi:10.1126/science.289.5487.2068) [DOI] [PubMed] [Google Scholar]
- 50.Drake J. M. 2005. Population effects of increased climate variation. Proc. R. Soc. B 272, 1823–1827 10.1098/rspb.2005.3148 (doi:10.1098/rspb.2005.3148) [DOI] [PMC free article] [PubMed] [Google Scholar]