Abstract
The links between fitness, health, sexual signals and mate choice are complex and subject to ongoing study. In 1999, von Schantz et al. made the valuable suggestion that oxidative stress may be an important missing piece of this complex puzzle. Their suggestion has been enthusiastically tested, with over 300 studies citing their paper, but most effort has concerned carotenoid-based (and to a lesser extent melanin-based) visual signals, predominantly in birds and fishes. Today, we know a great deal more about oxidative stress and related physiology, in both a pathological and regulatory sense, than we did in 1999. We revisit von Schantz et al.'s predictions and, more importantly, highlight novel mechanisms that could link oxidative stress with a range of energetically demanding signals, greatly increasing the scope from visual signalling systems that are usually discussed and nearly always tested. In particular, we argue that differences between individuals in their ability to regulate physiology related to oxidative stress may be an important factor influencing the production of sexual signals and the costs that are incurred from investment.
Keywords: oxidative stress, sexual selection, metabolism, energy production, redox regulation
1. Introduction
Sexual signals involved in the attraction of mates show great variety, from the elaborate plumage and complex song of many birds to the odours, mating rituals and acoustic calls employed by mammals and invertebrates. Despite this diversity, a trend predominates; high-quality signallers frequently produce the largest, brightest or most elaborate signals and potential mates typically choose these signallers over those with smaller, duller or less elaborate signals. For such signalling systems to evolve and remain stable, signals must reliably and honestly indicate those signallers that provide direct or genetic benefits as mates [1]. For simplicity's sake, the vast majority of research in this area has concerned male ‘signallers’ and female ‘choosers’.
Honest signalling can be maintained if high- and low-quality individuals differ in some kind of physiological state, usually referred to as ‘condition’, that relates to health, vigour, acquired resources and ultimately reproductive value. Condition-dependence either constrains the ability of low-quality individuals to produce and maintain sexual signals [2] or generates differential costs from signalling investment [3]. Evolutionary biologists have a tendency to treat the physiological factors linking sexual signals to condition as a ‘black box’ [4], or refer to condition purely in relation to resource acquisition and allocation [5].
Thirteen years ago, von Schantz et al. [6] suggested that one particular aspect of physiology—oxidative stress—may link ‘the expression of sexual ornaments to genetic variation in fitness-related traits, thus promoting the evolution of female mate choice and male sexual ornamentation’. More recently, evolutionary biologists have shown even greater interest in oxidative stress, suggesting that it could be a key physiological factor generating life-history trade-offs and constraining investment in a whole range of traits, such as growth, reproduction and lifespan [7–9]. The role of oxidative stress in life-history evolution has gained interest from researchers working on variety of different taxa. However, researchers working on oxidative stress and sexual selection have focused almost entirely on species that produce visual signals, such as birds and fishes, despite von Schantz et al.'s original prediction that oxidative stress could maintain honesty in a range of signalling systems. One reason for this narrow focus relates to the proposed mechanisms that could link oxidative stress with sexual signalling, as some of these pathways are applicable only to species that produce visual sexual signals. Some mechanisms have also been falsified over the past 13 years or shown to be based on an outdated understanding of oxidative stress. In this article, we briefly review these potential mechanisms in the light of modern knowledge of oxidative stress. More importantly, we use this knowledge to highlight other greatly underappreciated physiological pathways that may maintain the honesty of a range of sexual signals, particularly those that require substantial energetic investment.
2. The prediction
Many sexually selected traits depend, for their expression, on the condition of the signaller [3,10,11]. Such signals can be reliable indicators of the signaller's genetic quality because ‘condition’ can be influenced by a whole range of genes segregating throughout the genome [11]. Individuals able to acquire good condition, therefore, are more likely than average individuals to have high breeding values for fitness, including an ability to avoid infection by prevailing parasite and pathogen strains.
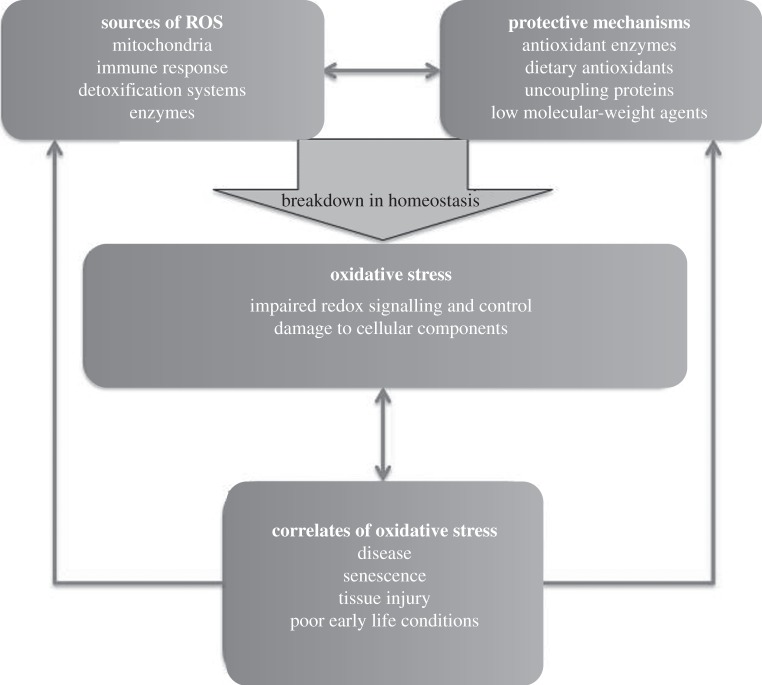
Von Schantz et al. [6] suggested that the links between oxidative stress and organism health, long recognized by physiologists, might provide one important mechanism linking condition to sexual signalling [6]. Oxidative stress is a negative, often pathological process that occurs when there is an overproduction of reactive oxygen species (ROS) in relation to an organism's ability to control their damaging effects ([8]; figure 1). ROS are either free radicals (molecules with one or more unpaired electrons, making them very reactive) or molecules with a high propensity to react with other molecules to produce free radicals. ROS can cause damage to biomolecules (termed oxidative damage), impair cellular function and even cause death [12]. As a consequence, animals have a variety of defence mechanisms that under normal physiological conditions protect against the potentially damaging effects of ROS. These include the superoxide dismutase, catalase and glutathione peroxidase families of antioxidant enzymes, and non-enzymatic antioxidants such as glutathione and dietary derived vitamin E.
Figure 1.
How oxidative stress, an overproduction of ROS that cannot be managed by protective mechanisms, can reveal mate quality. ROS are produced from a number of different sources and their production has the potential to cause oxidative stress. However, animals have a variety of different protective mechanisms that protect against such damaging effects and often work in concert with ROS production to maintain homeostasis. Under some conditions, however, there is a breakdown in homeostasis and oxidative stress results. This can correlate with negative physiological states that in many circumstances would be undesirable in a mate. Arrows indicate causal directions, with the large arrow indicating the key factor influencing oxidative stress.
We stress that there are many other antioxidant defences and protective mechanisms, and a variety of repair mechanisms that help to protect against unwanted oxidation of cellular components and maintain redox homeostasis. However, under certain conditions, these defences are ineffective and oxidative stress can result [8]. Normal cellular metabolism is expected to produce the majority of ROS in biological systems [13], as ROS can be leaked by the electron transport chains during oxidative phosphorylation. However, the immune and detoxification systems also produce ROS when activated, such that individuals with substantial activation of these systems can suffer elevated oxidative stress [6]. Oxidative stress is further correlated with the onset and/or severity of hundreds of diseases [12], increasing its applicability as a marker of condition (figure 1). Because of these links, von Schantz et al. [6] predicted that female choice may have selected for traits in males that reliably reveal low oxidative stress, as this would be a good marker of male quality. How and why might oxidative stress be honestly revealed by sexual signals? Von Schantz et al. [6] suggested a number of signals and mechanisms that could reveal this aspect of physiology, a list that other authors have added to in the intervening 13 years. While keeping in mind a modern understanding of the physiology of oxidative stress, we next very briefly summarize and evaluate those previously suggested mechanisms that have gained the most empirical attention.
(a). Oxidative stress and visual sexual signals
The majority of empirical and theoretical research linking oxidative stress to sexual signals has focused on species where males produce colourful carotenoid-based traits that are attractive to females [14–17]. A considerable body of research rests on the idea that because carotenoids cannot be synthesized de novo by animals (but see Kemp et al. [18]), and must be obtained solely from the diet, males with the largest and most colourful signals may be advertising their foraging ability [19,20]. Likewise, if carotenoids also act as immune-enhancers and antioxidants [6,21], then males that can afford to tie carotenoid molecules up in visual signals might be demonstrating their good health, as they do not need to allocate these molecules to health functions. There is evidence that carotenoids are immune-enhancers and females that select males on the basis of carotenoid-based signals gain immune-competent mates [22,23], but there is much less evidence that carotenoids have an antioxidant role in vivo [24,25], which has caused this hypothesis to fall out of favour with evolutionary biologists.
It must also be noted that carotenoids might signal oxidative stress through other pathways. They can be oxidized and lose their coloration, so males with high oxidative stress may be unable to maintain these pigment molecules in their colourful form [26]. Manipulations of other dietary antioxidants have been shown to influence either the size [27] or intensity of [28,29] coloration of several different caroteniod-based visual signals, supporting this theory [27–29]. However, manipulation of ROS levels through treatment with the molecules paraquat or diaquat, which induce an in vivo ROS attack, have produced inconsistent results, with increased ROS production reducing the coloration of carotenoid-based traits in some studies [30] but not others [31]. Although carotenoids have an important role in signalling other information, their ability to signal oxidative stress seems far from ubiquitous.
More recently, the effects of oxidative stress on sexual signalling have focused on changes in melanin-based visual traits. Traditionally, melanin-based signals were expected to be under genetic control and carotenoid signals were expected to reveal condition, although there is little evidence that these signals convey different information [32]. A variety of mechanisms have been proposed that would link the expression of melanin-based traits to oxidative stress, although, as recently highlighted, these pathways and their evolution require further understanding [9]. A full discussion of these is beyond the scope of this article (see [9] and references within), but one hypothesis suggests that individuals are forced to increase their susceptibility to oxidative stress when investing in melanin-based signals [33], possibly because glutathione, an important antioxidant, must be reduced to allow maximal melanization. This handicap could ensure that only males with adequate antioxidant protection can produce these signals while coping with such a cost.
Inhibition of glutathione production in both great tits (Parus major) [33] and greenfinches (Carduelis chloris) [34] has led to increased investment in melanin-based visual sexual signals, illustrating that a reduction in glutathione levels can increase melanization. Furthermore, treatment of developing red-legged partridges (Alectoris rufa) with the pro-oxidant molecule diaquat also led to an increase in the size of melanin trait [35]. Current empirical research thus suggests that investment in some melanin-based traits is related to an individual's level of oxidative stress; somewhat paradoxically, however, these studies show that elevation of oxidative stress leads to an increase in signalling effort. It has been noted that experimental reduction of glutathione levels leads to an increase in the total antioxidant capacity (TAC) of blood [33], supporting the theory of the authors that ‘melanin-based traits are in fact signals of the individual capacity to manage oxidative stress’ [33]. A better understanding of the way individuals differ in their management of oxidative stress would help to reveal the precise mechanisms that maintain honesty in such systems, which is a principle aim of this article.
(b). Testosterone-dependent and energetically costly sexual signals
Another hypothesis that suggests individuals differ in their ability to manage oxidative stress while signalling relates to the dependence of many secondary sexual traits on elevated testosterone levels. If the required increases in testosterone have negative effects such as immune suppression, then only individuals in good health and with few parasites will be able to cope with these effects while investing in signalling [36]. The evidence for an immunosuppressive effect of testosterone is conflicting [37]. Alonso-Alvarez et al. [38] suggested that high levels of testosterone may instead generate oxidative stress and this may be the physiological constraint that maintains honesty. Under this scenario, oxidative stress may maintain the honesty of a range of testosterone-dependent traits, not just visual signals, although the focus of empirical work has been largely on birds and fishes.
Experimental elevation of testosterone levels decreased antioxidant capacity [38] and increased oxidative damage [39] in zebra finches (Taeniopygia guttata) and red grouse (Lagopus lagopus scoticus) respectively. In red-legged partridges, however, elevation of testosterone did not produce oxidative damage but instead caused a decrease in the coloration of a carotenoid-based signal, highlighting that testosterone levels and sexual signalling may not always be correlated. Similarly, in house mice (Mus musculus domesticus), where investment in particular olfactory signals is influenced by castration and subsequent testosterone treatment [40,41], males were able to greatly increase investment in olfactory signalling over a 16-week period, while at the same time reducing both testosterone levels and oxidative damage in liver and serum [42].
Testosterone may inadvertently cause oxidative stress because it stimulates an increase in metabolic rate, which, potentially, could cause greater ROS production [38]. The assumed links between metabolic rate and ROS production require further validation, as the relationship between these is not 1 : 1 and sometimes large increases in metabolism can cause ROS production to decrease [43; discussed later in this article]. Nonetheless, under the assumption that metabolic rate and ROS production are positively correlated in many situations, Metcalfe & Alonso-Alvarez [9] broadened the applicability of oxidative stress in sexual signalling by suggesting that secondary sexual traits ‘whose production requires elevated oxygen consumption should be good candidates to act as signallers of good antioxidant defences’—thus even signals that are not testosterone-dependent may indicate an individual's level of oxidative stress or antioxidant quality. Both of these hypotheses appear based on the premise that an increase in metabolic rate will shift the balance between ROS production and antioxidants.
Von Shantz et al. saw oxidative stress as the outcome of a balance between ROS production and antioxidant defences, and little emphasis was placed on the dynamic regulation of antioxidant defences that occurs in response to a variety of stressors. The ability of organisms to regulate defence mechanisms is beginning to be appreciated by behavioural ecologists [8,44,45], but the precise mechanisms that allow such regulation are rarely discussed and almost never tested. In many empirical studies, TAC is measured as a marker of defence against oxidative stress, which essentially involves assessing the ability of a sample to quench an experimentally generated oxidant [46]. Although these assays are relativity easy to run and require small amounts of sample, they give little indication of the exact mechanisms that individuals use to control ROS production and allow future protection against oxidative stress. It is also a matter of uncertainty as to what these TAC assays actually detect. Such in vitro assays are conducted outside of their usual biological context, which is characterized by a greater enzymatic maintenance of steady state [46]. Furthermore, some measures of TAC preferentially detect the concentration of uric acid over other, arguably more important lines of antioxidant defence [47].
In §3, we highlight how endogenous antioxidant defences are regulated in response to transient increases in ROS production. Moreover, and central to this study, we emphasize that not all individuals have a similar capacity to regulate defences against oxidative stress and this may lead to condition-dependent costs of sexual signalling.
3. Oxidative stress in the twenty-first century
Understanding of the physiology of oxidative stress has changed substantially in the 13 years since the von Schantz et al. review paper. In 1999, oxidative stress was still sometimes considered to be the outcome of a ‘seesaw effect’ between the balance of unwanted ROS production and the quantity of antioxidants an animal has in its system. An increased consumption of dietary antioxidants was hypothesized to decrease oxidative stress and protect against disease and this has been an important component of hypotheses linking oxidative stress to the expression of colourful visual signals. However, the evidence that dietary molecules, particularly carotenoids, function as antioxidants in vivo is slim [24,25]. Furthermore, a number of studies in humans have suggested that an increased consumption of certain antioxidants, such as vitamins A, E and β-carotene, can actually be detrimental to health [48,49]. One possible reason for these detrimental effects is the inhibition of physiological regulatory processes that are mediated by ROS [50].
While ROS can be damaging at high concentrations, at lower levels they have wide-ranging adaptive roles and are produced by a number of enzymes such as NADPH oxidase and nitric oxide synthase [12]. ROS are essential in the adaptive immune response and have a plethora of roles in cellular signalling. These include the control of vascular tone and platelet adhesion, oxygen sensing, cell adhesion and division, contraction and apoptosis [51,52]. Redox signalling, a regulatory process in which signals are delivered through redox chemistry, also involves ROS as essential mediators and plays an important role in protection against oxidative stress [53].
Transient increases in ROS levels can elicit the upregulation of a number of defences that protect against oxidative stress. Increased ROS production from the electron transport chains during energy metabolism can increase the expression and concentrations of specialized uncoupling proteins, which increase proton conductance across the inner mitochondrial membrane in the presence of specific activators [54]. This mild uncoupling of the mitochondria produces heat, which is an active function of one particular uncoupling protein, UCP1. However, uncoupling also reduces the leakage of electrons that give rise to ROS [55] and may be used as an adaptive mechanism to protect against oxidative stress. This may be a principle role of two other uncoupling proteins: UCP2 and UCP3 [54].
Increased ROS production can also cause an upregulation of antioxidant defences and cellular repair mechanisms that protect against oxidative stress. These changes are usually dynamic, with increased ROS causing a shift to a slightly oxidized cellular state. These changes in the redox state influence signalling pathways and gene expression, which help to increase the activities of antioxidant defences and restore redox homeostasis [52]. We give examples of changes to specific antioxidants in the following paragraph; however, it is important to note that there are a variety of discrete pathways that can be modulated to help protect against increased ROS, with upregulation of DNA and protein repair mechanisms also occurring to reduce any damage that has occurred [56]. Upregulation of protective mechanisms may have long-term beneficial effects and it has even been suggested that the known lifespan extending effect of calorie restriction may be a consequence of an increase in mitochondrial metabolism [57]. It has been hypothesized that this increase in metabolism could increase ROS production, which in turn elevates stress resistance and antioxidant defence, facilitating a longer lifespan [58]. This theory, termed mitohormesis, is at odds with the classical, outdated view of ROS as purely negative, damaging molecules.
Some of the best examples of the dynamic regulation of specific antioxidant defences derive from studies of exercise physiology in mice and humans. In mice, a single session of experimentally induced muscle contraction can lead to greater production of ROS and oxidative damage [59,60]. However, healthy individuals respond to such increases by upregulating defences against oxidative stress, such as antioxidant enzymes and heat shock proteins, which protect against ROS production during future exercise bouts [61]. Similar results are seen in humans after moderate exercise [62,63]. Such changes are thought to occur, at least in part, in response to activation of redox-sensitive transcription factors such as activator protein-1 (AP-1) and nuclear factor kappa-B (NF-κB). Consuming dietary antioxidants, previously assumed to facilitate protection against oxidative stress, can actually inhibit the activation of redox-sensitive pathways and limit the adaptive responses to ROS production that occur during exercise [64].
Dysfunctional redox regulation is also a feature of ageing [52] and is present in individuals that are suffering persistent oxidative stress [65]. For example, at rest, the muscles of old mice show chronic activation of transcription factors AP-1 and NF-κB. However, after a demanding bout of forced muscle contraction, there is no change in the activation of these transcription factors and also no change in antioxidant expression, which may limit the ability of old males to protect against further oxidative stress [61]. This lack of redox regulation is expected to be an important cause of the age-related loss of muscle force generation [66].
The increasing recognition of redox dysfunction, particularly in relation to pathology, has lead to the suggestion that oxidative stress should be redefined as ‘a disruption in redox signalling and control’ [67]. Under this hypothesis, the definition of oxidative stress as an imbalance between pro-oxidants and antioxidants is ineffective because of the existence of multiple discrete redox pathways that function outside of a single equilibrium. A shift towards an oxidized redox state is associated with old age, unhealthy lifestyles and many types of diseases and malignancies [53,65]. It has even been suggested that dysfunctional redox regulation could have a greater impact on senescence than the ROS-inflicted damage that occurs to cellular components [52]. Given both the variation that is exhibited in individual redox responses and its apparent ability to influence investment in energetically demanding traits, redox regulation may have a range of roles in the signalling of male quality.
(a). Condition-dependent sexual signalling and impaired redox regulation
Dynamic regulation of antioxidant defences occurs with alteration of redox state, particularly when transient increases in ROS occur [52], and this serves to restore redox balance. This regulation is also likely to result when animals invest in demanding reproductive activities, such as investment in sexual signalling. Female mice increase the concentration of glutathione, an important antioxidant, in the liver when investing in reproduction and territory defence, and this is associated with a reduction in markers of oxidative stress in comparison with non-reproductives [68]. After testosterone levels were increased experimentally in male red-legged partridges, serum levels of glutathione and carotenoids both increased without any observed changes in oxidative damage [69]. Thus, while von Schantz et al. [6] predicted that increased ROS production during signalling could shift the overall balance of ROS production and antioxidant defences to an oxidizing state, current understanding suggests that this balance will simply be restored by an appropriate response from the antioxidant defence system.
Although healthy individuals modulate defences in response to oxidative insults, redox regulation in individuals that are unhealthy or in poor condition has frequently been shown to be dysfunctional, limiting the ability to adapt and protect against oxidative stress [52,65,70,71]. This could have important consequences for the costs that are incurred when individuals invest in sexual signals that shift redox balance to an oxidized state. Individuals in good condition are likely to respond to any transient oxidation incurred from signalling with an upregulation of defence mechanisms that protect against oxidative stress, limiting the viability costs of signalling. However, individuals in poor condition could suffer sustained oxidative stress resulting in greater costs despite an equivalent signalling investment. This falls in line with Zahavi's handicap hypothesis, which predicts that high-quality individuals will pay lower marginal signalling costs when compared with those incurred by poor-quality individuals that make an equivalent investment [3,72,73]. Dysfunctional redox regulation in poor condition individuals may be an important handicap influencing the honesty of condition-dependant signals.
(b). Oxidative stress and the fitness benefits of sexual signalling
There is, however, more to honest signalling than condition-dependent handicaps. Even though differences in the costliness of sexual signals to signallers of differing quality have gathered most theoretic and empirical attention [73], Getty [74] has argued that honesty can also arise in a system where high-quality signallers benefit more from a given level of signalling than low-quality individuals. Getty [74] made this point in the context of parasite-induced costs and showed that honest signalling systems could result in either a positive or a negative relationship between individual signal expression and parasite burden. Oxidative stress and sexual signalling are usually interpreted in the context of the handicap hypothesis, which predicts a negative correlation between the expression of a signal and the handicap it imposes [9,72]. However, there are many possible honest biological signalling systems where this condition will not hold true.
Honesty in a signalling system occurs when it is in the interest of poor-quality individuals to signal at a lower level than high-quality males. Condition-dependent costs can cause signalling strategies to diverge between males that differ in quality, but differences in the fecundity benefits of signalling are equally important [1,75]. If lower quality males get a smaller fecundity return from signalling than higher quality males, it will be in their strategic interest to invest less in signalling (because the benefits are fewer) when other things are equal.
Oxidative stress can have potentially severe negative impacts on male sperm quality [7,76,77], which may decrease the fecundity benefits of signalling because males are less likely to convert any attraction of females into successful fertilizations. For example, both the percentage of motile sperm and sperm swimming ability can correlate negatively with oxidative stress [78,79], suggesting that oxidative stress may influence the functional characteristics of sperm. Various oxidative insults incurred along the female genital tract could also mean that only individual sperm free from oxidative stress and possessing good antioxidant protection will be able to reach the ovum [77]. Additionally, both these factors could cause males with high oxidative stress to be less likely to successfully fertilize a female's eggs under sperm competition [7,80]. If oxidative stress lowers the probability of a mating resulting in successful fertilization, then males with high oxidative stress may strategically invest less in sexual signalling, as they get fewer benefits from investment in this life-history trait.
(c). Oxidative stress and mitochondrial bioenergetics
Many suggestions that link sexual signalling to oxidative stress are based on the assumption that increasing metabolic rate will increase ROS production. There is now evidence that the relationship between metabolic rate and ROS production is neither linear nor simple and that increases in metabolism can, in some circumstances, even reduce ROS production. As we describe later, large increases in metabolism can cause two substrates that are required for metabolic generation of ROS—electrons and oxygen—to become depleted, limiting ROS production [43].
The biggest metabolic transition that occurs with regards to ROS production is the change from state 4 to state 3 respiration. When mitochondria are in resting state 4 and ADP is absent, electrons flow slowly and tend to accumulate in the respiratory chain, causing particular complexes to be more reduced and increasing their capacity to leak electrons that subsequently produce ROS. In active state 3 respiration, when mitochondria are producing energy, the rate of electron flow strongly increases and decreases reduction of the electron transport chain, greatly reducing the probability that electrons will be leaked and therefore decreasing ROS production. In addition, during periods of high metabolism, oxygen can be depleted from areas surrounding the mitochondria, further limiting ROS production. [43]. Thus, in many situations, the assumption that an increase in metabolic rate will increase ROS production does not hold. For example, increasing the lifelong metabolic rates of short-tailed field voles (Microtus agrestis) by housing subjects in the cold had no effect on oxidative damage and negligible effects on antioxidant defences [81]. Furthermore, in a separate study across a population of mice, those individuals with the highest metabolic rates lived longer and had the greatest uncoupling of their muscle mitochondria [82], which is likely to have reduced ROS production. Much of the evidence linking higher metabolism to greater ROS production is derived from comparative studies across species; but within species, when an individual increases investment in signalling, the associated changes in metabolism will not always produce more ROS.
Oxidative stress may influence the ability of mitochondria to produce energy efficiently, which could have important effects on investment in sexual signalling. Many sexual signals are energetically demanding, from the energy required to generate calls or to display to the demands of carrying large ornaments or weapons around. Animals are typically expected to be limited in their available energy, and these limits may constrain investment in signalling. Indeed, one frequently used definition of ‘condition’ is the amount of resources available for allocation to fitness-enhancing traits [5]. Any changes in mitochondrial efficiency may thus have important effects on the energy available for signalling.
Recent technological advances have allowed measurement of mitochondrial energetics in intact cells [83]. Using these techniques, it has been demonstrated that oxidative stress can influence the basal respiration of mitochondria. Furthermore, mitochondria in cells under oxidative stress have a lower ‘reserve capacity’, that is, a lower ability to increase energy production in response to cellular demands [84,85]. Changes in mitochondrial energetics in response to oxidative stress may occur because damage to cellular biomolecules depletes available cytoplasmic ATP [83]. Additionally, mitochondrial damage may limit the ability of the electron transport chain to support electron transport [83]. While the relevance of these results in vivo requires further validation, if oxidative stress does impact greatly on energy production, then it could severely limit investment in sexual signalling. Direct manipulations of antioxidant expression in vivo demonstrate that oxidative stress can effect mitochondrial energy production and subsequent investment in demanding traits that require ATP. For example, mice with a reduced expression of mitochondrial superoxide dismutase, either globally or in particular muscle fibres, show various impairments of their mitochondria that limit efficient energy production [86–90]. Furthermore, these animals have a lower endurance capacity in energetically demanding activities such as treadmill running [89,91]. Differences in blood lactate and glucose levels between knockout and wild-type mice suggest that dysfunctional mitochondrial metabolism may cause an earlier shift from aerobic to anaerobic respiration, owing to an inability to maintain ATP demand for exercise [89].
(d). Mitochondrial dysfunction and sexual signalling
Given the high energy costs of many sexual signals, surprisingly few studies have investigated the consequences of individual variation in mitochondrial bioenergetics for sexual signalling. Acoustic communication is heavily dependent on the energy generated from metabolism [92] and may be particularly influenced by suboptimal mitochondrial function. A recent comparative study of the metabolic costs of calling revealed that the energy cost of calling in ectotherms averages eight times the resting metabolic rate [93]. In birds, metabolic rate while calling approximately doubled, which is an equivalent increase to that of producing eggs [93].
In mammals, a variety of behaviours involved in competition for mates, including protecting females and scramble competition, have been shown to have substantial energetic costs, sometimes greater than the costs of lactation for females [94]. Andersson [10] in particular highlights that male display behaviour, such as that arising on a lek, may be limited by energy constraints, and that these constraints appear to vary between individuals. Individual differences in the ability to metabolize energy efficiently, possibly arising in part from differences in oxidative stress, could be an important determinant of an individual's ability to invest in energetically costly sexual signals. Of course in some situations, energetic investment will be limited by an animal's food availability; however, it has recently been highlighted that breeding animals are often not limited by food supply [95]. We suggest that empirical studies incorporating mitochondrial energetics may reveal an important physiological constraint on sexual signalling.
4. Conclusions
Over the past 13 years, understanding of oxidative stress has changed fundamentally and this has important implications for predictions implicating oxidative stress in life-history evolution. To date, the vast majority of empirical research in this area has focused on species that signal with visual traits. Here, we highlight that this research field has the potential to be much broader, as oxidative stress could constrain investment in a variety of sexual signals of different sensory modalities.
In order to progress, we believe researchers should attend to the physiological changes that occur with investment in sexual signalling and how these differ between individuals. Instead of measuring TAC, in some circumstances, it would be much more informative to determine changes in the activities of specific antioxidants (for example the superoxide dismutases, catalases and glutathione peroxidases, to name but a few) and repair mechanisms (for example heat shock proteins, DNA excision-repair enzymes). We predict that individuals in poor condition will be less able to regulate some of these defences and protect against oxidative stress when investing in sexual signalling. Furthermore, examination of transcription factors and gene expression may give a better insight into the regulatory responses that occur with sexual signalling and whether these are impaired in individuals of poor condition. Similarly, measurements of mitochondrial energetics, either in isolated mitochondria or ideally mitochondria in situ (for example in permeabilized tissues, where mitochondrial function may be more similar to in vivo conditions; [96]), may help to reveal whether animals that differ in condition and sexual signalling also differ in their ability to produce energy efficiency. Changes in mitochondrial density could also be expected with sexual signalling, which may have important effects on both energy production and the generation of ROS; this can be measured directly (for example, by electron microscopy) and indirectly by the examination of the mitochondrial enzymes citrate synthase and cytochrome c oxidase [97]. Care should be taken when selecting methods to measure factors related to oxidative stress, and we recommend collaborations with physiologists to ensure accurate measurement of the trait of interest.
We have highlighted several novel pathways that could link oxidative stress to investment in a range of sexual signals associated with high-energy demands. Impaired redox regulation and mitochondrial energetics have been well studied with regard to exercise physiology and are important factors limiting endurance and muscle strength. Acoustic calling is energetically costly and investment in these signals is somewhat constrained by the size of the sound producing muscle [93]; thus, our examples from exercise physiology may be especially relevant. In mammals, investment in olfactory signalling, aggressive behaviours and displaying can all entail energetic costs and could also be influenced by oxidative stress. Only after empirical tests in more diverse animal groups, will we understand whether oxidative stress poses a general constraint on sexual signalling, as hypothesized [6,9], or whether it applies to a limited number of visual signals.
Acknowledgements
Thanks to Michael Kasumovic, Jean-François Lemaitre and Nicolas Pichaud for valuable comments on a version of the manuscript. M.G. thanks members of the Pathophysiology Research Group and Mammalian Behaviour and Evolution Group at the University of Liverpool, UK, for many interesting discussions, which increased his understanding of this area and in doing so helped him to formulate some of the ideas presented here.
References
- 1.Getty T. 2006. Sexually selected signals are not similar to sports handicaps. Trends Ecol. Evol. 21, 83–88 10.1016/j.tree.2005.10.016 (doi:10.1016/j.tree.2005.10.016) [DOI] [PubMed] [Google Scholar]
- 2.Maynard Smith J., Harper D. 2003. Animal signals. Oxford, UK: Oxford University Press [Google Scholar]
- 3.Cotton S., Fowler K., Pomiankowski A. 2004. Do sexual ornaments demonstrate heightened condition-dependent expression as predicted by the handicap hypothesis? Proc. R. Soc. Lond. B 271, 771–783 10.1098/rspb.2004.2688 (doi:10.1098/rspb.2004.2688) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lailvaux S. P., Irschick D. J. 2006. A functional perspective on sexual selection: insights and future prospects. Anim. Behav. 72, 263–273 10.1016/j.anbehav.2006.02.003 (doi:10.1016/j.anbehav.2006.02.003) [DOI] [Google Scholar]
- 5.Tomkins J. L., Radwan J., Kotiaho J. S., Tregenza T. 2004. Genic capture and resolving the lek paradox. Trends Ecol. Evol. 19, 323–328 10.1016/j.tree.2004.03.029 (doi:10.1016/j.tree.2004.03.029) [DOI] [PubMed] [Google Scholar]
- 6.von Schantz T., Bensch S., Grahn M., Hasselquist D., Wittzell H. 1999. Good genes, oxidative stress and condition-dependent sexual signals. Proc. R. Soc. Lond. B 266, 1–12 10.1098/rspb.1999.0597 (doi:10.1098/rspb.1999.0597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dowling D. K., Simmons L. W. 2009. Reactive oxygen species as universal constraints in life-history evolution. Proc. R. Soc. B 276, 1737–1745 10.1098/rspb.2008.1791 (doi:10.1098/rspb.2008.1791) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monaghan P., Metcalfe N. B., Torres R. 2009. Oxidative stress as a mediator of life history trade-offs: mechanisms, measurements and interpretation. Ecol. Lett. 12, 75–92 10.1111/j.1461-0248.2008.01258.x (doi:10.1111/j.1461-0248.2008.01258.x) [DOI] [PubMed] [Google Scholar]
- 9.Metcalfe N. B., Alonso-Alvarez C. 2010. Oxidative stress as a life-history constraint: the role of reactive oxygen species in shaping phenotypes from conception to death. Funct. Ecol. 24, 984–996 10.1111/j.1365-2435.2010.01750.x (doi:10.1111/j.1365-2435.2010.01750.x) [DOI] [Google Scholar]
- 10.Andersson M. 1994. Sexual selection. New York, NY: Princeton University Press [Google Scholar]
- 11.Rowe L., Houle D. 1996. The lek paradox and the capture of genetic variance by condition dependent traits. Proc. R. Soc. Lond. B 263, 1415–1421 10.1098/rspb.1996.0207 (doi:10.1098/rspb.1996.0207) [DOI] [Google Scholar]
- 12.Halliwell B., Gutteridge J. M. 1999. Free radicals in biology and medicine. Oxford, UK: Oxford University Press [Google Scholar]
- 13.Balaban R. S., Nemoto S., Finkel T. 2005. Mitochondria, oxidants, and aging. Cell 120, 483–495 10.1016/j.cell.2005.02.001 (doi:10.1016/j.cell.2005.02.001) [DOI] [PubMed] [Google Scholar]
- 14.Olson V. A., Owens I. P. F. 1998. Costly sexual signals: are carotenoids rare, risky or required? Trends Ecol. Evol. 13, 510–514 10.1016/s0169-5347(98)01484-0 (doi:10.1016/s0169-5347(98)01484-0) [DOI] [PubMed] [Google Scholar]
- 15.Zuk M., Thornhill R., Ligon J. D., Johnson K., Austad S., Ligon S. H., Thornhill N. W., Costin C. 1990. The role of male ornaments and courtship behavior in female mate choice of red jungle fowl. Am. Nat. 136, 459–473 10.1086/285107 (doi:10.1086/285107) [DOI] [Google Scholar]
- 16.Milinski M., Bakker T. C. M. 1990. Female sticklebacks use male coloration in mate choice and hence avoid parasitized males. Nature 344, 330–333 10.1038/344330a0 (doi:10.1038/344330a0) [DOI] [Google Scholar]
- 17.Burley N., Cooper-smith C. B. 1987. Bill color preferences of zebra finches. Ethology 76, 133–151 10.1111/j.1439-0310.1987.tb00679.x (doi:10.1111/j.1439-0310.1987.tb00679.x) [DOI] [Google Scholar]
- 18.Kemp D. J., Herberstein M. E., Grether G. F. 2012. Unraveling the true complexity of costly color signaling. Behav. Ecol. 23, 233–236 10.1093/beheco/arr153 (doi:10.1093/beheco/arr153) [DOI] [Google Scholar]
- 19.Endler J. A. 1980. Natural selection on color patterns in Poecilia reticulata. Evolution 34, 76–91 10.2307/2408316 (doi:10.2307/2408316) [DOI] [PubMed] [Google Scholar]
- 20.Hill G. E. 1992. Proximate basis of variation in carotenoid pigmentation in male house finches. Auk 109, U1–U12 [Google Scholar]
- 21.Lozano G. A. 1994. Carotenoids, parasites, and sexual selection. Oikos 70, 309–311 10.2307/3545643 (doi:10.2307/3545643) [DOI] [Google Scholar]
- 22.Faivre B., Gregoire A., Preault M., Cezilly F., Sorci G. 2003. Immune activation rapidly mirrored in a secondary sexual trait. Science 300, 103. 10.1126/science.1081802 (doi:10.1126/science.1081802) [DOI] [PubMed] [Google Scholar]
- 23.Blount J. D., Metcalfe N. B., Birkhead T. R., Surai P. F. 2003. Carotenoid modulation of immune function and sexual attractiveness in zebra finches. Science 300, 125–127 10.1126/science.1082142 (doi:10.1126/science.1082142) [DOI] [PubMed] [Google Scholar]
- 24.Costantini D., Moller A. P. 2008. Carotenoids are minor antioxidants for birds. Funct. Ecol. 22, 367–370 10.1111/j.1365-2435.2007.01366.x (doi:10.1111/j.1365-2435.2007.01366.x) [DOI] [Google Scholar]
- 25.Perez-Rodriguez L. 2009. Carotenoids in evolutionary ecology: re-evaluating the antioxidant role. Bioessays 31, 1116–1126 10.1002/bies.200900070 (doi:10.1002/bies.200900070) [DOI] [PubMed] [Google Scholar]
- 26.Hartley R. C., Kennedy M. W. 2004. Are carotenoids a red herring in sexual display? Trends Ecol. Evol. 19, 353–354 10.1016/j.tree.2004.04.002 (doi:10.1016/j.tree.2004.04.002) [DOI] [PubMed] [Google Scholar]
- 27.Perez C., Lores M., Velando A. 2008. Availability of nonpigmentary antioxidant affects red coloration in gulls. Behav. Ecol. 19, 967–973 10.1093/beheco/arn053 (doi:10.1093/beheco/arn053) [DOI] [Google Scholar]
- 28.Pike T. W., Blount J. D., Lindstrom J., Metcalfe N. B. 2007. Availability of non-carotenoid antioxidants affects the expression of a carotenoid-based sexual ornament. Biol. Lett. 3, 353–356 10.1098/rsbl.2007.0072 (doi:10.1098/rsbl.2007.0072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bertrand S., Faivre B., Sorci G. 2006. Do carotenoid-based sexual traits signal the availability of non-pigmentary antioxidants? J. Exp. Biol. 209, 4414–4419 10.1242/jeb.02540 (doi:10.1242/jeb.02540) [DOI] [PubMed] [Google Scholar]
- 30.Alonso-Alvarez C., Galvan I. 2011. Free radical exposure creates paler carotenoid-based ornaments: a possible interaction in the expression of black and red traits. PLoS ONE 6, e19403. 10.1371/journal.pone.0019403 (doi:10.1371/journal.pone.0019403) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Isaksson C., Andersson S. 2008. Oxidative stress does not influence carotenoid mobilization and plumage pigmentation. Proc. R. Soc. B 275, 309–314 10.1098/rspb.2007.1474 (doi:10.1098/rspb.2007.1474) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Griffith S. C., Parker T. H., Olson V. A. 2006. Melanin-versus carotenoid-based sexual signals: is the difference really so black and red? Anim. Behav. 71, 749–763 10.1016/j.anbehav.2005.07.016 (doi:10.1016/j.anbehav.2005.07.016) [DOI] [Google Scholar]
- 33.Galvan I., Alonso-Alvarez C. 2008. An intracellular antioxidant determines the expression of a melanin-based signal in a bird. PLoS ONE 3, e3335. 10.1371/journal.pone.0003335 (doi:10.1371/journal.pone.0003335) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horak P., Sild E., Soomets U., Sepp T., Kilk K. 2010. Oxidative stress and information content of black and yellow plumage coloration: an experiment with greenfinches. J. Exp. Biol. 213, 2225–2233 10.1242/jeb.042085 (doi:10.1242/jeb.042085) [DOI] [PubMed] [Google Scholar]
- 35.Galvan I., Alonso-Alvarez C. 2009. The expression of melanin-based plumage is separately modulated by exogenous oxidative stress and a melanocortin. Proc. R. Soc. B 276, 3089–3097 10.1098/rspb.2009.0774 (doi:10.1098/rspb.2009.0774) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Folstad I., Karter A. J. 1992. Parasites, bright males, and the immunocompetence handicap. Am. Nat. 139, 603–622 10.1086/285346 (doi:10.1086/285346) [DOI] [Google Scholar]
- 37.Roberts M. L., Buchanan K. L., Evans M. R. 2004. Testing the immunocompetence handicap hypothesis: a review of the evidence. Anim. Behav. 68, 227–239 10.1016/j.anbehav.2004.05.001 (doi:10.1016/j.anbehav.2004.05.001) [DOI] [Google Scholar]
- 38.Alonso-Alvarez C., Bertrand S., Faivre B., Chastel O., Sorci G. 2007. Testosterone and oxidative stress: the oxidation handicap hypothesis. Proc. R. Soc. B 274, 819–825 10.1098/rspb.2006.3764 (doi:10.1098/rspb.2006.3764) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mougeot F., Martinez-Padilla J., Webster L. M. I., Blount J. D., Perez-Rodriguez L., Piertney S. B. 2009. Honest sexual signalling mediated by parasite and testosterone effects on oxidative balance. Proc. R. Soc. B 276, 1093–1100 10.1098/rspb.2008.1570 (doi:10.1098/rspb.2008.1570) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kimura T., Hagiwara Y. 1985. Regulation of urine marking in male and female mice: effects of sex steroids. Horm. Behav. 19, 64–70 10.1016/0018-506X(85)90006-6 (doi:10.1016/0018-506X(85)90006-6) [DOI] [PubMed] [Google Scholar]
- 41.Clissold P. M., Hainey S., Bishop J. O. 1984. Messenger-RNAs coding for mouse major urinary proteins are differentially induced by testosterone. Biochem. Genet. 22, 379–387 10.1007/BF00484236 (doi:10.1007/BF00484236) [DOI] [PubMed] [Google Scholar]
- 42.Garratt M., McArdle F., Stockley P., Vasilaki A., Beynon R. J., Jackson M. J., Hurst J. L. 2011. Tissue-dependent changes in oxidative damage with male reproductive effort in house mice. Funct. Ecol. 26, 423–433 10.1111/j.1365-2435.2011.01952.x (doi:10.1111/j.1365-2435.2011.01952.x) [DOI] [Google Scholar]
- 43.Barja G. 2007. Mitochondrial oxygen consumption and reactive oxygen species production are independently modulated: implications for aging studies. Rejuvenation Res. 10, 215–223 10.1089/rej.2006.0516 (doi:10.1089/rej.2006.0516) [DOI] [PubMed] [Google Scholar]
- 44.Costantini D. 2010. Redox physiology in animal function: the struggle of living in an oxidant environment. Curr. Zool. 56, 687–702 [Google Scholar]
- 45.Hill G. E. 2011. Condition-dependent traits as signals of the functionality of vital cellular processes. Ecol. Lett. 14, 625–634 10.1111/j.1461-0248.2011.01622.x (doi:10.1111/j.1461-0248.2011.01622.x) [DOI] [PubMed] [Google Scholar]
- 46.Sies H. 2007. Total antioxidant capacity: appraisal of a concept. J. Nutr. 137, 1493–1495 [DOI] [PubMed] [Google Scholar]
- 47.Costantini D. 2011. On the measurement of circulating antioxidant capacity and the nightmare of uric acid. Methods Ecol. Evol. 2, 321–325 10.1111/j.2041-210X.2010.00080.x (doi:10.1111/j.2041-210X.2010.00080.x) [DOI] [Google Scholar]
- 48.Bjelakovic G., Nikolova D., Simonetti R. G., Gluud C. 2004. Antioxidant supplements for prevention of gastrointestinal cancers: a systematic review and meta-analysis. Lancet 364, 1219–1228 10.1016/S0140-6736(04)17138-9 (doi:10.1016/S0140-6736(04)17138-9) [DOI] [PubMed] [Google Scholar]
- 49.Seifried H. E., Anderson D. E., Fisher E. I., Milner J. A. 2007. A review of the interaction among dietary antioxidants and reactive oxygen species. J. Nutr. Biochem. 18, 567–579 10.1016/j.jnutbio.2006.10.007 (doi:10.1016/j.jnutbio.2006.10.007) [DOI] [PubMed] [Google Scholar]
- 50.Bjelakovic G., Gluud C. 2007. Surviving antioxidant supplements. J. Natl Cancer Inst. 99, 742–743 10.1093/jnci/djk211 (doi:10.1093/jnci/djk211) [DOI] [PubMed] [Google Scholar]
- 51.Janssen-Heininger Y. M. W., Mossman B. T., Heintz N. H., Forman H. J., Kalyanaraman B., Finkel T., Stamler J. S., Rhee S. G., van der Vliet A. 2008. Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radical Biol. Med. 45, 1–17 10.1016/j.freeradbiomed.2008.03.011 (doi:10.1016/j.freeradbiomed.2008.03.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Droge W. 2002. Free radicals in the physiological control of cell function. Physiol. Rev. 82, 47–95 [DOI] [PubMed] [Google Scholar]
- 53.Valko M., Leibfritz D., Moncol J., Cronin M. T. D., Mazur M., Telser J. 2007. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 39, 44–84 10.1016/j.biocel.2006.07.001 (doi:10.1016/j.biocel.2006.07.001) [DOI] [PubMed] [Google Scholar]
- 54.Brand M. D., Esteves T. C. 2005. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab. 2, 85–93 10.1016/j.cmet.2005.06.002 (doi:10.1016/j.cmet.2005.06.002) [DOI] [PubMed] [Google Scholar]
- 55.Brand M. D., Affourtit C., Esteves T. C., Green K., Lambert A. J., Miwa S., Pakay J. L., Parker N. 2004. Mitochondrial superoxide: production, biological effects, and activation of uncoupling proteins. Free Radical Biol. Med. 37, 755–767 10.1016/j.freeradbiomed.2004.05.034 (doi:10.1016/j.freeradbiomed.2004.05.034) [DOI] [PubMed] [Google Scholar]
- 56.Davies K. J. A. 2000. Oxidative stress, antioxidant defenses, and damage removal, repair, and replacement systems. IUBMB Life 50, 279–289 10.1080/15216540051081010 (doi:10.1080/15216540051081010) [DOI] [PubMed] [Google Scholar]
- 57.Nisoli E., et al. 2005. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science 310, 314–317 10.1126/science.1117728 (doi:10.1126/science.1117728) [DOI] [PubMed] [Google Scholar]
- 58.Ristow M., Zarse K. 2010. How increased oxidative stress promotes longevity and metabolic health: the concept of mitochondrial hormesis (mitohormesis). Exp. Gerontol. 45, 410–418 10.1016/j.exger.2010.03.014 (doi:10.1016/j.exger.2010.03.014) [DOI] [PubMed] [Google Scholar]
- 59.Close G. L., Kayani A. C., Ashton T., McArdle A., Jackson M. J. 2007. Release of superoxide from skeletal muscle of adult and old mice: an experimental test of the reductive hotspot hypothesis. Aging Cell 6, 189–195 10.1111/j.1474-9726.2007.00277.x (doi:10.1111/j.1474-9726.2007.00277.x) [DOI] [PubMed] [Google Scholar]
- 60.Vasilaki A., Mansouri A., Van Remmen H., van der Meulen J. H., Larkin L., Richardson A. G., McArdle A., Faulkner J. A., Jackson M. J. 2006. Free radical generation by skeletal muscle of adult and old mice: effect of contractile activity. Aging Cell 5, 109–117 10.1111/j.1474-9726.2006.00198.x (doi:10.1111/j.1474-9726.2006.00198.x) [DOI] [PubMed] [Google Scholar]
- 61.Vasilaki A., McArdle F., Iwanejko L. M., McArdle A. 2006. Adaptive responses of mouse skeletal muscle to contractile activity: the effect of age. Mech. Ageing Dev. 127, 830–839 10.1016/j.mad.2006.08.004 (doi:10.1016/j.mad.2006.08.004) [DOI] [PubMed] [Google Scholar]
- 62.Gomez-Cabrera M. C., Domenech E., Vina J. 2008. Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free Radical Biol. Med. 44, 126–131 10.1016/j.freeradbiomed.2007.02.001 (doi:10.1016/j.freeradbiomed.2007.02.001) [DOI] [PubMed] [Google Scholar]
- 63.Khassaf M., Child R. B., McArdle A., Brodie D. A., Esanu C., Jackson M. J. 2001. Time course of responses of human skeletal muscle to oxidative stress induced by nondamaging exercise. J. Appl. Physiol. 90, 1031–1035 [DOI] [PubMed] [Google Scholar]
- 64.Ristow M., Zarse K., Oberbach A., Kloting N., Birringer M., Kiehntopf M., Stumvoll M., Kahn C. R., Bluher M. 2009. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc. Natl Acad. Sci. USA 106, 8665–8670 10.1073/pnas.0903485106 (doi:10.1073/pnas.0903485106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vasilaki A., et al. 2010. The age-related failure of adaptive responses to contractile activity in skeletal muscle is mimicked in young mice by deletion of Cu, Zn superoxide dismutase. Aging Cell 9, 979–990 10.1111/j.1474-9726.2010.00635.x (doi:10.1111/j.1474-9726.2010.00635.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Broome C. S., Kayani A. C., Palomero J., Dillmann W. H., Mestril R., Jackson M. J., McArdle A. 2006. Effect of lifelong overexpression of HSP70 in skeletal muscle on age-related oxidative stress and adaptation after nondamaging contractile activity. FASEB J. 20, 1549–1551 10.1096/fj.05-4935fje (doi:10.1096/fj.05-4935fje) [DOI] [PubMed] [Google Scholar]
- 67.Jones D. P. 2006. Redefining oxidative stress. Antioxidants Redox Signal. 8, 1865–1879 10.1089/ars.2006.8.1865 (doi:10.1089/ars.2006.8.1865) [DOI] [PubMed] [Google Scholar]
- 68.Garratt M., Vasilaki A., Stockley P., McArdle F., Jackson M., Hurst J. L. 2011. Is oxidative stress a physiological cost of reproduction? An experimental test in house mice. Proc. R. Soc. B 278, 1098–1106 10.1098/rspb.2010.1818 (doi:10.1098/rspb.2010.1818) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alonso-Alvarez C., Perez-Rodriguez L., Mateo R., Chastel O., Vinuela J. 2008. The oxidation handicap hypothesis and the carotenoid allocation trade-off. J. Evol. Biol. 21, 1789–1797 10.1111/j.1420-9101.2008.01591.x (doi:10.1111/j.1420-9101.2008.01591.x) [DOI] [PubMed] [Google Scholar]
- 70.Jones D. P., Go Y. M. 2010. Redox compartmentalization and cellular stress. Diabetes Obes. Metab. 12, 116–125 10.1111/j.1463-1326.2010.01266.x (doi:10.1111/j.1463-1326.2010.01266.x). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Heo J. Y. 2011. Redox control of GTPases: from molecular mechanisms to functional significance in health and disease. Antioxidants Redox Signal. 14, 689–724 10.1089/ars.2009.2984 (doi:10.1089/ars.2009.2984) [DOI] [PubMed] [Google Scholar]
- 72.Zahavi A. 1975. Mate selection: selection for a handicap. J. Theor. Biol. 53, 205–214 10.1016/0022-5193(75)90111-3 (doi:10.1016/0022-5193(75)90111-3) [DOI] [PubMed] [Google Scholar]
- 73.Grafen A. 1990. Biological signals as handicaps. J. Theor. Biol. 144, 517–546 10.1016/S0022-5193(05)80088-8 (doi:10.1016/S0022-5193(05)80088-8) [DOI] [PubMed] [Google Scholar]
- 74.Getty T. 2002. Signaling health versus parasites. Am. Nat. 159, 363–371 10.1086/338992 (doi:10.1086/338992) [DOI] [PubMed] [Google Scholar]
- 75.Getty T. 1998. Handicap signalling: when fecundity and viability do not add up. Anim. Behav. 56, 127–130 10.1006/anbe.1998.0744 (doi:10.1006/anbe.1998.0744) [DOI] [PubMed] [Google Scholar]
- 76.Blount J. D., Moller A. P., Houston D. C. 2001. Antioxidants, showy males and sperm quality. Ecol. Lett. 4, 393–396 10.1046/j.1461-0248.2001.00255.x (doi:10.1046/j.1461-0248.2001.00255.x) [DOI] [Google Scholar]
- 77.Velando A., Torres R., Alonso-Alvarez C. 2008. Avoiding bad genes: oxidatively damaged DNA in germ line and mate choice. Bioessays 30, 1212–1219 10.1002/bies.20838 (doi:10.1002/bies.20838) [DOI] [PubMed] [Google Scholar]
- 78.Jones R., Mann T., Sherins R. 1979. Peroxidative breakdown of phospholipids in human spermatozoa, spermicidal properties of fatty acid peroxides, and protective action of seminal plasma. Fertil. Steril. 31, 531–537 [DOI] [PubMed] [Google Scholar]
- 79.Helfenstein F., Losdat S., Moller A. P., Blount J. D., Richner H. 2010. Sperm of colourful males are better protected against oxidative stress. Ecol. Lett. 13, 213–222 10.1111/j.1461-0248.2009.01419.x (doi:10.1111/j.1461-0248.2009.01419.x) [DOI] [PubMed] [Google Scholar]
- 80.Parker G. A. 1970. Sperm competition and its evolutionary consequences in insects. Biol. Rev. Camb. Phil. Soc. 45, 525–567 10.1111/j.1469-185X.1970.tb01176.x (doi:10.1111/j.1469-185X.1970.tb01176.x) [DOI] [Google Scholar]
- 81.Selman C., McLaren J. S., Collins A. R., Duthie G. G., Speakman J. R. 2008. The impact of experimentally elevated energy expenditure on oxidative stress and lifespan in the short-tailed field vole Microtus agrestis. Proc. R. Soc. B 275, 1907–1916 10.1098/rspb.2008.0355 (doi:10.1098/rspb.2008.0355) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Speakman J. R., et al. 2004. Uncoupled and surviving: individual mice with high metabolism have greater mitochondrial uncoupling and live longer. Aging Cell 3, 87–95 10.1111/j.1474-9728.2004.00097.x (doi:10.1111/j.1474-9728.2004.00097.x) [DOI] [PubMed] [Google Scholar]
- 83.Sansbury B. E., Jones S. P., Riggs D. W., Darley-Usmar V. M., Hill B. G. 2011. Bioenergetic function in cardiovascular cells: the importance of the reserve capacity and its biological regulation. Chem. Biol. Interact. 191, 288–295 10.1016/j.cbi.2010.12.002 (doi:10.1016/j.cbi.2010.12.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hill B. G., Dranka B. P., Zou L. Y., Chatham J. C., Darley-Usmar V. M. 2009. Importance of the bioenergetic reserve capacity in response to cardiomyocyte stress induced by 4-hydroxynonenal. Biochem. J. 424, 99–107 10.1042/bj20090934 (doi:10.1042/bj20090934) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dranka B. P., Hill B. G., Darley-Usmar V. M. 2010. Mitochondrial reserve capacity in endothelial cells: the impact of nitric oxide and reactive oxygen species. Free Rad. Biol. Med. 48, 905–914 10.1016/j.freeradbiomed.2010.01.015 (doi:10.1016/j.freeradbiomed.2010.01.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kokoszka J. E., Coskun P., Esposito L. A., Wallace D. C. 2001. Increased mitochondrial oxidative stress in the Sod2 (+/−) mouse results in the age-related decline of mitochondrial function culminating in increased apoptosis. Proc. Natl Acad. Sci. USA 98, 2278–2283 10.1073/pnas.051627098 (doi:10.1073/pnas.051627098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.van Remmen H., Williams M. D., Guo Z. M., Estlack L., Yang H., Carlson E. J., Epstein C. J., Huang T. T., Richardson A. 2001. Knockout mice heterozygous for Sod2 show alterations in cardiac mitochondrial function and apoptosis. Am. J. Physiol. Heart Circul. Physiol. 281, H1422–H1432 [DOI] [PubMed] [Google Scholar]
- 88.Williams M. D., Van Remmen H., Conrad C. C., Huang T. T., Epstein C. J., Richardson A. 1998. Increased oxidative damage is correlated to altered mitochondrial function in heterozygous manganese superoxide dismutase knockout mice. J. Biol. Chem. 273, 28 510–28 515 10.1074/jbc.273.43.28510 (doi:10.1074/jbc.273.43.28510) [DOI] [PubMed] [Google Scholar]
- 89.Lustgarten M. S., et al. 2009. Conditional knockout of Mn-SOD targeted to type IIB skeletal muscle fibers increases oxidative stress and is sufficient to alter aerobic exercise capacity. Am. J. Physiol. Cell Physiol. 297, C1520–C1532 10.1152/ajpcell.00372.2009 (doi:10.1152/ajpcell.00372.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lustgarten M. S., et al. 2011. MnSOD deficiency results in elevated oxidative stress and decreased mitochondrial function but does not lead to muscle atrophy during aging. Aging Cell 10, 493–505 10.1111/j.1474-9726.2011.00695.x (doi:10.1111/j.1474-9726.2011.00695.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kinugawa S., Wang Z. P., Kaminski P. M., Wolin M. S., Edwards J. G., Kaley G., Hintze T. H. 2005. Limited exercise capacity in heterozygous manganese superoxide dismutase gene-knockout mice: roles of superoxide anion and nitric oxide. Circulation 111, 1480–1486 10.1161/01.cir.0000159261.11520.63 (doi:10.1161/01.cir.0000159261.11520.63) [DOI] [PubMed] [Google Scholar]
- 92.Gillooly J. F., Ophir A. G. 2010. The energetic basis of acoustic communication. Proc. R. Soc. B 277, 1325–1331 10.1098/rspb.2009.2134 (doi:10.1098/rspb.2009.2134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ophir A. G., Schrader S. B., Gillooly J. F. 2010. Energetic cost of calling: general constraints and species-specific differences. J. Evol. Biol. 23, 1564–1569 10.1111/j.1420-9101.2010.02005.x (doi:10.1111/j.1420-9101.2010.02005.x) [DOI] [PubMed] [Google Scholar]
- 94.Lane J. E., Boutin S., Speakman J. R., Humphries M. M. 2010. Energetic costs of male reproduction in a scramble competition mating system. J. Anim. Ecol. 79, 27–34 10.1111/j.1365-2656.2009.01592.x (doi:10.1111/j.1365-2656.2009.01592.x) [DOI] [PubMed] [Google Scholar]
- 95.Speakman J. R., Krol E. 2010. The heat dissipation limit theory and evolution of life histories in endotherms: time to dispose of the disposable soma theory? Integr. Comp. Biol. 50, 793–807 10.1093/icb/icq049 (doi:10.1093/icb/icq049) [DOI] [PubMed] [Google Scholar]
- 96.Picard M., Ritchie D., Wright K. J., Romestaing C., Thomas M. M., Rowan S. L., Taivassalo T., Hepple R. T. 2010. Mitochondrial functional impairment with aging is exaggerated in isolated mitochondria compared to permeabilized myofibers. Aging Cell 9, 1032–1046 10.1111/j.1474-9726.2010.00628.x (doi:10.1111/j.1474-9726.2010.00628.x) [DOI] [PubMed] [Google Scholar]
- 97.Magwere T., Goodall S., Skepper J., Mair W., Brand M. D., Partridge L. 2006. The effect of dietary restriction on mitochondrial protein density and flight muscle mitochondrial morphology in Drosophila. J. Gerontol. A Biol. Sci. Med. Sci. 61, 36–47 10.1093/gerona/61.1.36 (doi:10.1093/gerona/61.1.36) [DOI] [PubMed] [Google Scholar]