Abstract
Over the last 20 years, ecological immunology has provided much insight into how environmental factors shape host immunity and host–parasite interactions. Currently, the application of this thinking to the study of mosquito immunology has been limited. Mechanistic investigations are nearly always conducted under one set of conditions, yet vectors and parasites associate in a variable world. We highlight how environmental temperature shapes cellular and humoral immune responses (melanization, phagocytosis and transcription of immune genes) in the malaria vector, Anopheles stephensi. Nitric oxide synthase expression peaked at 30°C, cecropin expression showed no main effect of temperature and humoral melanization, and phagocytosis and defensin expression peaked around 18°C. Further, immune responses did not simply scale with temperature, but showed complex interactions between temperature, time and nature of immune challenge. Thus, immune patterns observed under one set of conditions provide little basis for predicting patterns under even marginally different conditions. These quantitative and qualitative effects of temperature have largely been overlooked in vector biology but have significant implications for extrapolating natural/transgenic resistance mechanisms from laboratory to field and for the efficacy of various vector control tools.
Keywords: mosquito, innate immunity, temperature, vector, parasite
1. Introduction
During the last decade, considerable effort has been devoted to elucidating the molecular and cellular interactions between mosquitoes and a range of parasites and pathogens [1–5]. This research has advanced general knowledge of innate immune systems [6], and identified key mosquito immune genes, effector molecules and defence pathways that can decrease or block the development of key vector-borne disease agents, providing potential targets for transgenic manipulation [7–11].
Even though the current reductionist paradigm of vector immunology has been extremely insightful, this approach is incomplete. Mosquito resistance to infection is not a static phenotype comprised solely of immune genes involved in standard immune responses measured under customary laboratory conditions [12,13]. Hosts and parasites associate in a variable world. Vector competence involves broad aspects of host physiology and condition, which is shaped by both genetic and environmental variation that often interact in nonlinear ways [14]. From work in other invertebrate–parasite systems, small, realistic changes in temperature can have striking effects on the outcome of invertebrate host–parasite interactions. Ambient temperature profoundly affects overall resistance to a wide diversity of parasites: viruses [15,16], bacteria [17,18], microsporidia [19], fungi [20–22], nematodes [23] and parasitoids [24,25]. Temperature also influences the duration of latency periods [26] and time to host recovery [19].
This evidence suggests that mosquitoes should exhibit diverse resistance phenotypes across different ambient temperatures. Temperature may shape the resistance phenotype and parasite growth in two ways: (i) direct effects of host body temperature on parasite growth (which are independent of the mosquito host), and (ii) the less well studied indirect effects on parasite growth, which are mediated through temperature effects on mosquito innate immune mechanisms. Yet the majority of studies examining aspects of mosquito immune function are conducted under standard laboratory conditions using single temperatures and often single time points for assessing experimental read-outs. Paradoxically, we know more about how environmental variability shapes the immune phenotype in butterflies [27,28], fruitflies [18,29], crickets [30], meal worms [31] and moths [32] than we do in most disease vectors. Given the global health and economic burdens imposed by vector-borne parasites such as malaria, this represents a significant knowledge gap.
If the relative and/or absolute immune response of mosquitoes exhibits thermal sensitivity, the current approach of outlining innate immune responses under standard laboratory conditions is insufficient for understanding vector competence as played out in the field. To test this assertion, we measured humoral and cellular immune responses across a range of different, constant temperatures in the Asian malaria vector, Anopheles stephensi. We demonstrate that temperature can have dramatic and diverse quantitative and qualitative impacts on mosquito immune responses, with potentially complex interactions with factors such as time and nature of immune challenge. That immune responses are affected by temperature is not necessarily surprising. That the effects are complex and unpredictable across different immune measures represents a challenge to current disciplinary convention, where environmental variation is generally ignored.
2. Methods
(a). Mosquito rearing and handling
We reared An. stephensi (Liston) under standard insectary conditions at 27 ± 1°C, 80 per cent humidity and a 12 L : 12 D photo-period. We placed mosquito eggs into plastic trays (25 × 25× 7 cm) filled with 1.5 l of water. To minimize any potential variation in emerging adult mosquito body size, we divided recently hatched larvae to ensure a density of 400 individuals per tray. Larvae were fed Liquifry for the first 5 days post-hatching, and then were fed Tetrafin fish flakes for the duration of the larval period. Pupae were collected from larval trays and placed into experimental cages approximately two weeks after egg hatch. Upon emergence, adults were fed ad libitum on a 6 per cent glucose solution. Mosquitoes used for humoral melanization and immune gene expression experiments were provided a bloodmeal from rats (Wistar, more than six weeks old) at 3 days post-emergence. On day 3–4 post-emergence, mosquitoes were anaesthetized on ice and the immune challenge administered by an intrathoracic injection into the anepisternal cleft [33] with a mouth pipette and microcapillary glass needle or a Nanoject. After immune challenge, mosquitoes were randomly assigned to one of five reach-in incubators with temperatures of 12°C, 18°C, 24°C, 28°C and 34 ± 0.5°C; relative humidity 80 ± 5%. A series of pilot experiments for each immune measure was conducted across a reduced temperature and a sampling time point regime to confirm that the effects of temperature, immune challenge and sampling time point on immune responsiveness were consistent in the full experiment (see electronic supplementary material, text S1).
(b). Melanization: immune challenge with Sephadex beads
Melanization is the product of a series of enzymatic and non-enzymatic reactions beginning with the hydroxylation of tyrosine and ending with the oxidate polymerization of indolequinones [34]. To date, many studies have used total phenoloxidase activity, a key enzyme in the melanization reaction, as a proxy for immunocompetence [27,35–37]. However, because phenoloxidases are involved in a variety of other metabolic functions in addition to innate immunity [34], we chose to measure the melanization response directly. Melanization has been implicated in the defences of refractory Anopheles gambiae (L35) strain against oocysts of the rodent malaria Plasmodium berghei [38–40] and new world Plasmodium falciparum [41], Aedes aegypti against Plasmodium gallinaceum sporozoites [42], Ae. aegypti and Armigeres subalbautus against bacteria [42,43], and Ar. subalbatus against filarial worms [44,45]. To stimulate the melanization response, we injected blood-fed females with one negatively charged CM-25 Sephadex bead. Sephadex beads range in size from 40 to 120 μm in diameter, and only the smallest beads were selected visually for inoculation. Beads were suspended in a DMEM solution (Dulbecco's Modification of Eagle's Modification) and 0.001 per cent methyl green to facilitate bead visualization [46]. We injected one bead in a minimal amount of solution (less than 0.5 μl) and randomly distributed mosquitoes across temperature treatments. At 24 hours post-immune challenge mosquitoes that were able to walk were removed, and beads were dissected out in a phosphate-buffered saline solution stained with 0.01 per cent methyl green.
(c). Phagocytosis: immune challenge with fluospheres
Phagocytosis is a cellular immune response that involves haemocyte recognition, engulfing and destruction of small micro-organisms and apoptotic cells. Phagocytosis is an evolutionarily conserved immune response that plays important roles in antibacterial defence [47]. To stimulate phagocytosis, we injected non-blood-fed females with approximately 50 000 yellow-green carboxylate-modified fluospheres (1 μm diameter) with a Nanoject. After immune challenge, 10 mosquitoes were randomly allocated to a temperature treatment and one of four sampling time points (1, 6, 12 and 24 h). At 1–24 h post-immune challenge, mosquitoes were removed and haemocytes were fluorescently stained in vivo by injecting each mosquito with a solution of Hoescht nucleic acid stain and Vybrant CM-DiI cell-labelling solution (Invitrogen Life Technologies, Carlsbad, CA). Haemolymph was then collected by perfusion from ice-anaesthetized mosquitoes [48] onto a microscope slide. Haemocytes were fixed in 4 per cent paraformaldehyde, washed in phosphate-buffered saline solution (pH 7.4, 0.2 M) and distilled water, and mounted with Aqua-Poly/Mount. For each mosquito, we calculated the phagocytic index and the phagocytic capacity for a total of 50 counted granulocytes [49].
(d). Gene expression: immune challenge with bacteria
We investigated the effects of temperature on defensin 1 (DEF1), cecropin 1 (CEC1) and nitric oxide synthase (NOS) gene expression in response to no manipulation, injury or heat-killed Escherichia coli challenge. DEF1 and CEC1 encode two antimicrobial peptides that are produced in the insect fat body and by local barrier epithelia. DEF1 is active against Gram-positive bacteria and filamentous fungi [50], CEC1 is active against both Gram-positive and -negative bacteria [51], and both peptides have been implicated to some extent with Plasmodium killing [10,52]. NOS encodes nitric oxide, an effector molecule that has been shown to be a ubiquitous killer of a wide diversity of pathogens and parasites [53], and has also been implicated as a major anti-malarial defence in the mosquito midgut epithelia [54–57].
We used heat-killed tetracycline-resistant GFP-expressing E. coli (dh5 alpha strain) as our challenge to avoid temperature-mediated variation in bacterial growth within mosquitoes housed at different mean temperatures. Escherichia coli were grown overnight in Luria-Bertani's rich nutrient medium (LB) in a shaking incubator at 37°C, and a serial dilution was prepared from the overnight culture. To approximate our injection dose of E. coli, we recorded the absorbance (OD600) from each dilution with a NanoDrop (Thermo Scientific, Wilmington, DE). To estimate the dose of E. coli, we compared the absorbance of each dilution to a standard curve of the linear relationship between absorbance and colony-forming units (CFUs) of E. coli that was generated prior to the experiment. The dilution with an absorbance corresponding to approximately 1 × 109 E. coli per millilitre (i.e. 200 000 bacteria per injection) was selected for our injection stock. To further confirm this estimate, we plated our injection stock in triplicate onto LB agar plates, placed them overnight into an incubator at 37°C, and counted the resulting CFUs the next day. We then killed the E. coli stock by autoclaving for 25 min. Ice-anaesthetized mosquitoes were either unmanipulated (control mosquitoes), or received an injection of either 0.2 μl of sterile LB (positive injury control) or 200 000 heat-killed E. coli before being placed into their respective temperature treatment. Fifteen mosquitoes from each immune-challenge group were then allocated to each of five temperatures and four sampling sessions (6, 12, 18 and 24 h).
(e). RNA collection, cDNA synthesis and quantitative PCR
Post-immune challenge, mosquitoes were removed from their temperature treatment, killed with chloroform and immediately stored in RNAlater RNA stabilization reagent at 4°C for future molecular analyses. Immediately after the termination of the experiment, five mosquitoes from each treatment group (n = 300 total) were isolated individually in β-Mercaptoethanol and RLT lysis buffer. Messenger RNA was extracted using the Qiagen RNeasy Mini Kit for animal tissues (as per the manufacturer's protocol). Standards for quantitative polymerase chain reaction (PCR) were prepared by extracting mRNA from a pool of four mosquitoes. The concentration of mRNA in each sample was quantified with a NanoDrop and stored at −80°C. RNA was converted to cDNA with a high-capacity cDNA reverse transcription kit as per the manufacturer's protocol (Applied Biosystems, Foster City, CA) on a Mastercycler Gradient thermal cycler (Eppendorf, Hamburg, Germany).
The expression of ribosomal protein S7, a standard housekeeping gene in mosquito gene expression studies [57–61], was influenced by experimental treatment (see electronic supplementary material, text S2, table S2.2 and figure S2.2). Owing to concerns that the expression of other housekeeping genes may also be influenced by temperature (as reflected by the effects of temperature on total RNA concentration; see electronic supplementary material, text S2, table S2.2 and figure S2.2), we chose to quantify our diluted cDNA from our experimental samples by comparing their threshold cycle numbers against a standard curve generated from 1 : 10 serial dilutions of our standard sample (cDNA from a pool of four mosquitoes; see electronic supplementary material, text S3). Three replicates of each cDNA standard spanning six orders of magnitude were included in each quantitative PCR run. We measured cDNA counts for each gene of interest from individual mosquitoes relative to the standard curve of that assay. DNA contamination in RNA samples was confirmed to be undetectable using quantitative PCR, and primers and probes were designed from An. stephensi and An. gambiae sequences (see electronic supplementary material, text S3).
(f). Statistical analyses
All statistical analyses for these experiments were run in PSAW 18.0 (IBM Corporation, New York, NY). Full models from generalized linear model (GLM) analysis were reduced through backward elimination of non-significant interactions. We assessed goodness of fit of the final models through model deviance, log likelihood values and Akaike information criterion. Covariates included in GLMs were centred on their grand mean.
(i). Humoral melanization: degree of bead melanization
We scored recovered beads for the degree of melanization by assigning each bead to one of three categorical classes: unmelanized, partially melanized (i.e. portions of the bead remained unmelanized) and fully melanized [62–64]. We ran a logistic regression to estimate how the probability of a bead being in a particular class was affected by temperature with total bead area as a covariate.
(ii). Phagocytosis: phagocytic index and capacity
We used GLMs to assess how temperature and sampling time point affected the proportion of phagocytizing granulocytes and the mean number of beads granulocytes can uptake. For both response variables, models included temperature, sampling time point and their interaction as fixed factors. The centred phagocytic index was included in the phagocytic capacity GLM as a covariate to account for a potential relationship between the number of active granulocytes (with beads) and the average number of beads granulocytes consume. We predicted estimated marginal means of phagocytic index and capacity assuming a normal distribution with identity link function and a Poisson distribution with log link function, respectively.
(iii). Gene expression
To compare differences in average gene expression among our treatment groups, we used the cDNA counts generated for each target gene from our standard curve analysis as our expression measure. We analysed all expression data with GLMs assuming a gamma distribution for the dependent variable, which was transformed with a log link function. Full factorial analyses were run for each gene separately to control for any differences in efficiencies among our assays as well as independence among our experimental samples. Temperature, sampling time point and immune challenge were included in all models as fixed factors. We included rpS7 cDNA counts and the total RNA concentration of each sample as covariates in all models to adjust our estimated means of our target gene by any differences in baseline expression among mosquitoes. Inclusion of these covariates improved model fit, but the overall patterns of target gene expression were qualitatively similar without the covariates.
3. Results
We investigated whether the rates of characteristic humoral and cellular immune responses of insects were temperature-sensitive, and especially whether immune responses were influenced in qualitatively consistent ways across different immune challenges and sampling time points. Because temperature has been shown to influence pathogen performance [65–68], we use non-living immune stimuli in the subsequent experiments to disentangle the effects of temperature on immune performance.
(a). Humoral melanization
We recovered 98 per cent of injected beads from mosquitoes housed at all experimental temperatures. Temperature significantly affected the probability of recovering unmelanized, partially melanized or fully melanized beads (figure 1; n = 136; χ2 = 17.468, p = 0.004). We recovered more fully melanized beads than partially melanized beads at 18°C (odds ratio 5.1) than at any other temperature. The proportion of partially melanized beads relative to fully melanized beads increased with temperature and peaked at 28°C (odds ratio 10.1). In contrast, neither temperature nor bead size (area) affected the probability of recovering unmelanized beads, and the size of the injected bead (bead area) did not significantly predict bead status. Peak rate of melanization appears to occur at 18°C and becomes less efficient at warmer temperatures (figure 1).
Figure 1.
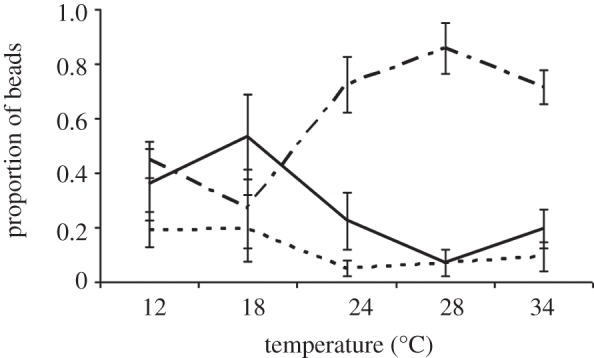
Temperature significantly influences the humoral melanization of Sephadex beads (logistic regression analysis: n = 136; χ2 = 17.468, p = 0.004). Data show mean (±s.e.m.) proportion of unmelanized (dashed line), partially (broken line) and fully melanized beads (solid line) recovered at different temperatures 24 h post-injection. Even though more partially melanized beads were recovered at warmer temperatures, the probability of recovering fully melanized beads was highest at 18°C, suggesting that the rate of melanization is higher at cooler temperatures.
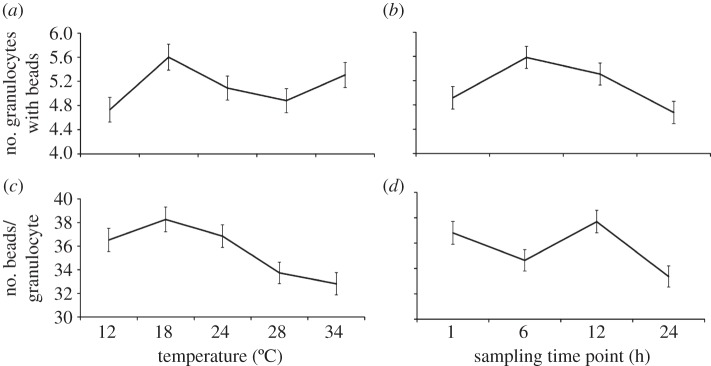
(b). Phagocytosis
A GLM indicated that temperature significantly affected both phagocytic index (Poisson distribution with log link function: Wald 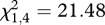 , n = 191, p < 0.0001; figure 2a) and capacity (normal distribution with identity link function: Wald
, n = 191, p < 0.0001; figure 2a) and capacity (normal distribution with identity link function: Wald 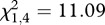 , n = 195, p = 0.026; figure 2b). The mean number of granulocytes phagocytizing fluorescent beads was significantly higher in mosquitoes housed at 18°C relative to mosquitoes housed at 28°C (Bonferroni-adjusted post hoc test: p = 0.010) and 34°C (Bonferroni-adjusted post hoc test: p = 0.001); mosquitoes housed at 34°C had a significantly lower phagocytic index than mosquitoes housed at cooler temperatures (Bonferroni-adjusted post hoc tests: 12°C versus 34°C, p = 0.046; 18°C versus 34°C, p = 0.001; 24°C versus 34°C, p = 0.022; figure 2a). Haemocytes consumed on average more beads in mosquitoes housed at 18°C than 12°C (Bonferroni-adjusted post hoc test: p = 0.032). However, there were no significant differences in the phagocytic capacity of haemocytes in mosquitoes housed at other temperatures.
, n = 195, p = 0.026; figure 2b). The mean number of granulocytes phagocytizing fluorescent beads was significantly higher in mosquitoes housed at 18°C relative to mosquitoes housed at 28°C (Bonferroni-adjusted post hoc test: p = 0.010) and 34°C (Bonferroni-adjusted post hoc test: p = 0.001); mosquitoes housed at 34°C had a significantly lower phagocytic index than mosquitoes housed at cooler temperatures (Bonferroni-adjusted post hoc tests: 12°C versus 34°C, p = 0.046; 18°C versus 34°C, p = 0.001; 24°C versus 34°C, p = 0.022; figure 2a). Haemocytes consumed on average more beads in mosquitoes housed at 18°C than 12°C (Bonferroni-adjusted post hoc test: p = 0.032). However, there were no significant differences in the phagocytic capacity of haemocytes in mosquitoes housed at other temperatures.
Figure 2.
Both temperature and sampling time point significantly influenced the phagocytosis of fluospheres. The effects of temperature on the mean (±s.e.m.) phagocytic index (number of haemocytes containing fluospheres out of 50 counted haemocytes) and capacity (the number of beads per haemocyte) are represented by (a) and (c), respectively (GLMs: index, Poisson distribution with log link function, Wald 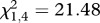 , n = 191, p < 0.0001; capacity, normal distribution with identity link function, Wald
, n = 191, p < 0.0001; capacity, normal distribution with identity link function, Wald 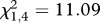 , n = 195, p = 0.026). (b) and (d) depict the effects of sampling time point on the mean (±s.e.) phagocytic index and capacity, respectively (GLMs: index, Poisson distribution with log link function, Wald
, n = 195, p = 0.026). (b) and (d) depict the effects of sampling time point on the mean (±s.e.) phagocytic index and capacity, respectively (GLMs: index, Poisson distribution with log link function, Wald 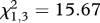 , n = 191, p = 0.001; capacity, normal distribution with identity link function, Wald
, n = 191, p = 0.001; capacity, normal distribution with identity link function, Wald 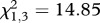 , n = 195, p = 0.002).
, n = 195, p = 0.002).
Both the phagocytic index (GLM, Poisson distribution with log link function: Wald 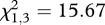 , n = 191, p = 0.001) and capacity (GLM, normal distribution with identity link function: Wald
, n = 191, p = 0.001) and capacity (GLM, normal distribution with identity link function: Wald 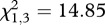 , n = 195, p = 0.002) were significantly influenced by sampling time point (figure 2). The mean number of granulocytes with beads varied across sampling time points (figure 2c), while the mean number of beads granulocytes consumed was highest 6–12 h post-immune challenge (Bonferroni-adjusted post hoc test: p = 0.002; figure 2d). There was a strong positive relationship between the phagocytic index and mean phagocytic capacity (Wald
, n = 195, p = 0.002) were significantly influenced by sampling time point (figure 2). The mean number of granulocytes with beads varied across sampling time points (figure 2c), while the mean number of beads granulocytes consumed was highest 6–12 h post-immune challenge (Bonferroni-adjusted post hoc test: p = 0.002; figure 2d). There was a strong positive relationship between the phagocytic index and mean phagocytic capacity (Wald 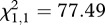 , n = 195, p < 0.0001; regression analysis controlling for the effects of temperature and sampling time:
, n = 195, p < 0.0001; regression analysis controlling for the effects of temperature and sampling time: 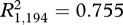 , F = 593.79, p < 0.0001). This suggests that immune stimulation of phagocytosis increased granulocyte efficiency, as well as overall activity, within the haemolymph. There was no significant interaction between temperature and sampling time point for either measure of phagocytosis.
, F = 593.79, p < 0.0001). This suggests that immune stimulation of phagocytosis increased granulocyte efficiency, as well as overall activity, within the haemolymph. There was no significant interaction between temperature and sampling time point for either measure of phagocytosis.
(c). Defensin expression
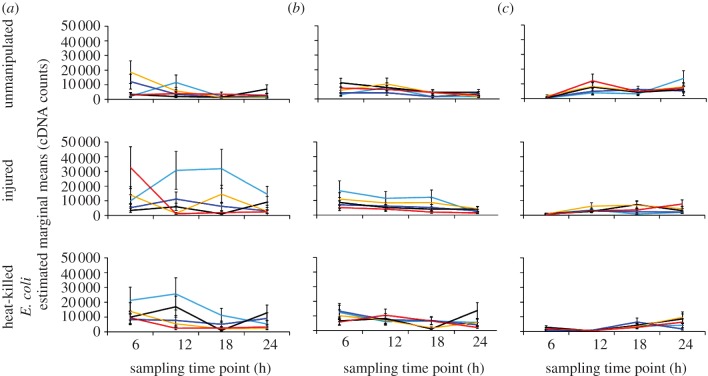
Temperature significantly influenced the expression of DEF1; these effects were strongly shaped by sampling time point and immune challenge (table 1). For example, mosquitoes housed at 26°C experienced increased DEF1 expression within the first 6–12 h and at 24 h post-immune challenge with either an injury or injection of heat-killed E. coli. However, this pattern is not maintained in mosquitoes housed at different temperatures that received the same immune challenge. In injured mosquitoes housed at 18°C, DEF1 expression peaks 12–18 h post-immune challenge, while DEF1 expression peaks within the first 6 h and rapidly declines at subsequent sampling time points in mosquitoes housed at 34°C (figure 3a). Alternatively, for mosquitoes treated with heat-killed E. coli, DEF1 expression is elevated within the first 6 h for mosquitoes housed at warmer temperatures (30°C and 34°C), while DEF1 expression is elevated in the first 6–12 h and declines thereafter in mosquitoes housed at 18°C. In addition to the interacting effects of temperature, sampling time point and immune challenge, there was a significant main effect of temperature on DEF1 expression (table 1); mosquitoes housed at 18°C expressed considerably more DEF1, overall, relative to mosquitoes housed at warmer temperatures (Bonferroni-adjusted post hoc tests: 18°C versus 22°C, p = 0.037; 18°C versus 26°C, p = 0.001; 18°C versus 30°C, p = 0.002; and 18°C versus 34°C, p < 0.0001).
Table 1.
Final model results for DEF1, CEC1 and NOS from GLM analysis. A gamma distribution and log link function were assumed for all models. Dashes indicate higher order interactions backward eliminated from the full model. Omnibus tests confirmed that each fitted model was significantly different from its null model (DEF1: likelihood ratio 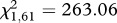 , p < 0.0001; CEC1: likelihood ratio
, p < 0.0001; CEC1: likelihood ratio 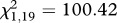 , p < 0.0001; NOS1: likelihood ratio
, p < 0.0001; NOS1: likelihood ratio 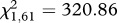 , p < 0.0001). Goodness of fit was assessed by evaluating potential overdispersion through model deviance scores (DEF1: deviance value/d.f. = 1.25; CEC1: deviance value/d.f. = 1.11; NOS: deviance value/d.f. = 1.00). p-values are significant (in bold) if they were below a 0.05 probability of committing a Type I error.)
, p < 0.0001). Goodness of fit was assessed by evaluating potential overdispersion through model deviance scores (DEF1: deviance value/d.f. = 1.25; CEC1: deviance value/d.f. = 1.11; NOS: deviance value/d.f. = 1.00). p-values are significant (in bold) if they were below a 0.05 probability of committing a Type I error.)
|
DEF1 |
CEC1 |
NOS |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| factors (n = 299) | d.f. | Wald χ2 | p-value | d.f. | Wald χ2 | p-value | d.f. | Wald χ2 | p-value |
| intercept | 1 | 24954.69 | <0.0001 | 1 | 24468.06 | <0.0001 | 1 | 25699.21 | <0.0001 |
| temperature | 4 | 34.84 | <0.0001 | 4 | 8.17 | 0.085 | 4 | 20.04 | <0.0001 |
| sampling time point | 3 | 42.31 | <0.0001 | 3 | 31.68 | <0.0001 | 3 | 142.27 | <0.0001 |
| immune challenge | 2 | 32.12 | <0.0001 | 2 | 11.12 | 0.004 | 2 | 18.14 | <0.0001 |
| centred rpS7 cDNA counts | 1 | 4.76 | 0.029 | 1 | 6.76 | 0.009 | 1 | 4.88 | 0.027 |
| total RNA concentration | 1 | 4.91 | 0.027 | 1 | 5.41 | 0.020 | 1 | 145.54 | <0.0001 |
| temperature × sampling time point | 12 | 76.66 | <0.0001 | — | — | — | 12 | 17.47 | 0.133 |
| sampling time point × immune challenge | 6 | 10.88 | 0.092 | — | — | — | 6 | 4.87 | 0.772 |
| temperature × immune challenge | 8 | 26.59 | 0.001 | 8 | 22.45 | 0.004 | 8 | 111.01 | <0.0001 |
| temperature × sampling time point × immune challenge | 24 | 68.56 | <0.0001 | — | — | — | 24 | 47.23 | 0.003 |
Figure 3.
The effects of temperature, sampling time point and immune challenge on the expression of immune genes. The relationship between mean gene expression (cDNA counts ± s.e.m.) for (a) defensin (DEF1) and (c) nitric oxide synthase (NOS) and temperature varied significantly among mosquitoes sampled at different time points post-challenge and treated with different immune stimuli (GLM, gamma distribution with log link function: DEF1 Wald 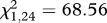 , n = 299, p < 0.0001; NOS Wald
, n = 299, p < 0.0001; NOS Wald 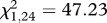 , n = 299, p = 0.003). The relationship between (b) cecropin (CEC1) expression and temperature varied significantly only among mosquitoes receiving different immune challenges (GLM, gamma distribution with log link function: Wald
, n = 299, p = 0.003). The relationship between (b) cecropin (CEC1) expression and temperature varied significantly only among mosquitoes receiving different immune challenges (GLM, gamma distribution with log link function: Wald 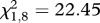 , n = 299, p = 0.004). Light blue lines, 18°C; dark blue, 22°C; black, 26°C; yellow, 30°C; red, 34°C.
, n = 299, p = 0.004). Light blue lines, 18°C; dark blue, 22°C; black, 26°C; yellow, 30°C; red, 34°C.
(d). Cecropin expression
Temperature also significantly influenced the expression of CEC1, and this occurred in a manner which depended on the nature of the immune challenge (table 1). Unlike DEF1 expression, the effects of temperature did not depend on the time of sampling. Generally, CEC1 expression was highest in unmanipulated mosquitoes housed at optimal to warmer temperatures (26°C, 30°C and 34°C), injured mosquitoes housed at 18°C and 30°C, and heat-killed E. coli-treated mosquitoes housed at cooler to optimal temperatures (18°C, 22°C and 26°C; figure 3b).
(e). Nitric oxide synthase expression
Similar to DEF1 expression, the effects of temperature on NOS expression varied significantly with both sampling time point and immune challenge (table 1). In unmanipulated mosquitoes, NOS expression peaked at later sampling time points in mosquitoes housed at cooler temperatures (18°C: 24 h; 22°C: 18 h) than in mosquitoes housed at optimal or warmer temperatures (26–34°C: 12 h; figure 3c). Mosquitoes challenged with heat-killed E. coli generally experienced increased NOS expression 24 h post-immune challenge (with the exception of mosquitoes housed at 22°C; figure 3c). The relationship between sampling time and NOS expression is much more variable in injured mosquitoes housed at different temperatures (figure 3c). There also was a main effect of temperature on NOS expression (table 1); mosquitoes housed at 30°C had on average higher NOS expression than mosquitoes from other temperatures (Bonferroni-adjusted post hoc tests: 18°C versus 30°C, p = 0.002; 22°C versus 30°C, p = 0.016).
4. Discussion
Research on a range of insects and other ectotherms clearly demonstrates impacts of temperature on host resistance and parasite virulence. Here, we extend this research to show that ambient temperature can profoundly influence the rates of both humoral and cellular immune responses in a major malaria vector. Surprisingly, the effects of temperature do not simply scale quantitatively, nor are they consistent across immune measures. Accordingly, the standard approach of exploring immune function and mosquito–pathogen interactions under a very narrow range of temperatures in the laboratory fails to describe much of the immune phenotype relevant to more diverse field conditions. Further, for several of the measures, there were significant time or rate effects, which varied depending on the nature of immune challenge and/or complex interactions among factors (figure 3). The standard approaches that constrain such experimental complexity will miss these relevant intricacies.
A null hypothesis is that temperature effects on immune function should scale simply with temperature-related changes in general physiology and baseline gene expression. This is what we found for CEC expression, where there was no main effect of temperature above the background effects on housekeeping gene expression. However, CEC expression did vary with temperature depending on whether an injury or heat-killed E. coli were administered. Thus, while temperature initially appeared to be insignificant, interactions with other sources of ‘environmental’ variability can yield unpredictable and complex responses. Recent results from another insect system reinforce this finding, with temperature effects on innate immune measures manifesting only through complex interactions with other environmental variables, like density of conspecifics and quality of food resources [32].
NOS expression peaked slightly above the assumed temperature optimum for the mosquito; colonies are typically maintained at around 27°C, which is the optimum for other anophelines [69]. Nitric oxide functions as a cell signalling and cytotoxic effector molecule, and has been implicated as a major anti-malarial defence in the midgut of An. stephensi, contributing to the parasite bottleneck associated with ookinete migration through the midgut epithelium [54,70]. Further, it may also be a late-stage line of defence against Plasmodium parasites [55,56], with elevated activity being detected in the fat body as well as circulating granulocytes in response to infection [33]. Recent theoretical temperature models predict that the temperature optima for development of P. falciparum [71,72] and P. vivax [71] is around 30–31°C. Thus, increased expression of NOS at warmer temperatures may be an important mosquito defence that counters and limits optimal parasite development.
Unexpectedly, several of the immune responses studied were more robust at 18°C. However, evidence from studies in a range of other systems suggests that divergent temperature optima for different life-history/immune traits are not uncommon [32]. For example, research on butterflies and isopods demonstrated that overall baseline phenoloxidase activity was higher at cooler temperatures (butterflies: 10°C or 17.7°C; isopod: 19°C) than warmer temperatures (butterflies: 27°C or 34°C; isopod: 26°C) [27,28]. Further, Suwanchaichinda & Paskewitz [73] showed that An. gambiae melanization of Sephadex beads was highest at 24°C relative to 27°C and 30°C. The production of melanin is essential for many other physiological processes in addition to innate immunity, such as egg hardening and cuticular tanning [34], which may be an explanation for why the rate of humoral melanization is faster at lower temperatures.
Similarly, in immune responses of the mosquito Ae. aegypti against E. coli, both the defensin peptide and phenoloxidase colocalize at the sites of melanin deposition. In addition, they are often present in the same melanotic capsules [74], potentially explaining why DEF1 expression follows the pattern of melanization. Linder et al. [29] demonstrated that overall expression of a diversity of immune genes (Pgrp-LC, Cactus, Spatzle) in D. melanogaster were upregulated in response to heat-killed bacterial challenge at 17°C relative to flies housed at 25°C and/or 29°C. Additionally, expression of heat-shock protein Hsp83 was upregulated at both 17°C and 29°C relative to flies housed at 25°C, suggesting that heat-shock proteins may boost enzymatic efficiency at cooler temperatures in addition to high temperatures [29,75].
Phagocytic index and capacity were also higher in mosquitoes maintained at 18–24°C relative to warmer temperatures. So far as we are aware, there has been very little research examining temperature influences on phagocytosis in general. In monarch butterflies, the number of circulating haemocytes was greater at 10°C compared with warmer temperatures (27°C and 34°C). In ectothermic vertebrates, non-specific defences might play an important role in offsetting immune suppression at low environmental temperatures, while the specific immune system adapts. The rate of phagocytosis significantly increased with low environmental temperatures in tench (Tinca tinca) [76], channel catfish (Ictalurus punctatus) [77] and rainbow trout (Oncorhynchus mykiss) [78].
As with numerous other transcriptional studies, we have not linked temperature-induced variation in gene expression with functional resistance or vector competence, and it is possible that temperature might significantly modify post-transcriptional regulation. Thus, the effects of temperature on antimicrobial peptide production, nitric oxide enzyme activity and pathogen clearance should be investigated. Equally, we do not know how much melanin is required for pathogen killing, and hence whether the functional temperature optimum for melanization is at 18°C or 28°C (i.e. where we found the highest proportion of beads showing any level of melanization). Nonetheless, the interactions among temperature, the type of immune challenge and the time point at which mosquitoes are evaluated post-immune challenge clearly complicate interpretation of the many studies conducted under one set of conditions.
For instance, it is commonplace to infer importance of different elements of immune function by measuring fold differences in expression relative to some control baseline (e.g. [2,3,11]). In our study, it is clear that fold differences would differ substantially depending on the individual immune measure, nature of the controls, temperature and time point, yet the vast majority of expression/transcriptional studies ignore such complexities. Similarly, it is generally accepted that the immune gene families and pathways, and the associated mosquito immune responses implicated in resistance to the rodent malaria parasite (P. berghei) are different from those involved in defence against the human malaria parasite (P. falciparum) [1,58,79]. However, experiments on P. berghei are typically run at 19–21°C, whereas experiments on P. falciparum are run at around 27°C. Given the differential effects of temperature on immune responses, such as melanization and nitric oxide synthase across this range, it is unclear whether the reported differences in mosquito responses are actually parasite-derived, environment-derived or some combination of both. Further, it is unclear how temperature mediates interacting immune responses that experience diverse temperature optima, such as the potential reactivity of nitric oxide with components of the melanization response and phagocytosis [33]. It is quite possible that the relative importance of different immune mechanisms for controlling the same pathogen species varies with temperature. More broadly, with aspects of mosquito resistance being important for the success of insecticides [80,81], fungal biopesticides [82,83], biological larvicides [84] and prospective transgenesis, and paratransgenesis and transinfection tools in the field [85,86], the implications of complex temperature–immune interactions could be far-reaching. Our results highlight the need to begin framing vector immunity in the context of the ecologically variable world in which mosquitoes and parasites/pathogens interact.
Acknowledgements
We thank members of the Thomas and Read laboratory groups for discussion and D. Kroczynski for insectary support. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences, the National Institute of Allergy and Infectious Diseases, and the National Institutes of Health. This project is funded, in part, under a grant with the Pennsylvania Department of Health using Tobacco Settlement Funds. The Department specifically disclaims responsibility for any analyses, interpretations or conclusions. This research was funded by the following grants: NSF-NIH EID (EF-0914384), NIH-R21 (AI096036-01) and NSF (IOS-1051636).
References
- 1.Jaramillo-Gutierrez G., Rodrigues J., Ndikuyeze G., Povelones M., Molina-Cruz A., Barillas-Mury C. 2009. Mosquito immune responses and compatibility between Plasmodium parasites and anopheline mosquitoes. BMC Microbiol. 9, 154. 10.1186/1471-2180-9-154 (doi:10.1186/1471-2180-9-154) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cirimotich C. M., Dong Y. M., Garver L. S., Sim S. Z., Dimopoulos G. 2010. Mosquito immune defenses against Plasmodium infection. Dev. Comp. Immunol. 34, 387–395 10.1016/j.dci.2009.12.005 (doi:10.1016/j.dci.2009.12.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aguilar R., Jedlicka A. E., Mintz M., Mahairaki V., Scott A. L., Dimopoulos G. 2005. Global gene expression analysis of Anopheles gambiae responses to microbial challenge. Insect Biochem. Mol. Biol. 35, 709–719 10.1016/j.ibmb.2005.02.019 (doi:10.1016/j.ibmb.2005.02.019) [DOI] [PubMed] [Google Scholar]
- 4.Magalhaes T., Oliveira I. F., Melo-Santos M. A. V., Oliveira C. M. F., Lima C. A., Ayres C. F. J. 2008. Expression of defensin, cecropin, and transferrin in Aedes aegypti (Diptera: Culicidae) infected with Wuchereria bancrofti (Spirurida: Onchocercidae), and the abnormal development of nematodes in the mosquito. Exp. Parasitol. 120, 364–371 10.1016/j.exppara.2008.09.003 (doi:10.1016/j.exppara.2008.09.003) [DOI] [PubMed] [Google Scholar]
- 5.Steinert S., Levashina E. A. 2011. Intracellular immune responses of dipteran insects. Immunol. Rev. 240, 129–140 10.1111/j.1600-065X.2010.00985.x (doi:10.1111/j.1600-065X.2010.00985.x) [DOI] [PubMed] [Google Scholar]
- 6.Christophides G. K., Vlachou D., Kafatos F. C. 2004. Comparative and functional genomics of the innate immune system in the malaria vector Anopheles gambiae. Immunol. Rev. 198, 127–148 10.1111/j.0105-2896.2004.0127.x (doi:10.1111/j.0105-2896.2004.0127.x) [DOI] [PubMed] [Google Scholar]
- 7.Moreira L. A., et al. 2009. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, chikungunya, and Plasmodium. Cell 139, 1268–1278 10.1016/j.cell.2009.11.042 (doi:10.1016/j.cell.2009.11.042) [DOI] [PubMed] [Google Scholar]
- 8.Crampton J. M. 1994. Approaches to vector control: new and trusted prospects for genetic manipulation of insect vectors. Trans. R. Soc. Trop. Med. Hyg. 88, 141–143 10.1016/0035-9203(94)90266-6 (doi:10.1016/0035-9203(94)90266-6) [DOI] [PubMed] [Google Scholar]
- 9.Speranca M. A., Capurro M. L. 2007. Perspectives in the control of infectious diseases by transgenic mosquitoes in the post-genomic era: a review. Mem. Inst. Oswaldo Cruz 102, 425–433 10.1590/S0074-02762007005000054 (doi:10.1590/S0074-02762007005000054) [DOI] [PubMed] [Google Scholar]
- 10.Kim W., Koo H., Richman A. M., Seeley D., Vizioli J., Klocko A. D., O'Brochta D. A. 2004. Ectopic expression of a cecropin transgene in the human malaria vector mosquito Anopheles gambiae (Diptera: Culicidae): effects on susceptibility to Plasmodium. J. Med. Entomol. 41, 447–455 10.1603/0022-2585-41.3.447 (doi:10.1603/0022-2585-41.3.447) [DOI] [PubMed] [Google Scholar]
- 11.Hughes G. L., Koga R., Xue P., Fukatsu T., Rasgon J. L. 2011. Wolbachia infections are virulent and inhibit the human malaria parasite Plasmodium falciparum in Anopheles gambiae. Plos Pathogens 7, e1002043. 10.1371/journal.ppat.1002043 (doi:10.1371/journal.ppat.1002043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Little T. J., Hultmark D., Read A. F. 2005. Invertebrate immunity and the limits of mechanistic immunology. Nat. Immunol. 6, 651–654 10.1038/ni1219 (doi:10.1038/ni1219) [DOI] [PubMed] [Google Scholar]
- 13.Lazzaro B. P., Little T. J. 2009. Immunity in a variable world. Phil. Trans. R. Soc. B 364, 15–26 10.1098/rstb.2008.0141 (doi:10.1098/rstb.2008.0141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schulenburg H., Kurtz J., Moret Y., Siva-Jothy M. T. 2009. Introduction. Ecological immunology. Phil. Trans. R. Soc. B 364, 3–14 10.1098/rstb.2008.0249 (doi:10.1098/rstb.2008.0249) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frid L., Myers J. H. 2002. Thermal ecology of western tent caterpillars Malacosoma californicum pluviale and infection by nucleopolyhedrovirus. Ecol. Entomol. 27, 665–673 10.1046/j.1365-2311.2002.00460.x (doi:10.1046/j.1365-2311.2002.00460.x) [DOI] [Google Scholar]
- 16.Kobayashi M., Inagaki S., Kawase S. 1981. Effect of high temperature on the development of nuclear polyhedrosis virus in the silkworm, Bombyx mori. J. Invertebr. Pathol. 38, 386–394 10.1016/0022-2011(81)90106-3 (doi:10.1016/0022-2011(81)90106-3) [DOI] [Google Scholar]
- 17.Mitchell S. E., Rogers E. S., Little T. J., Read A. F. 2005. Host–parasite and genotype-by-environment interactions: temperature modifies potential for selection by a sterilizing pathogen. Evolution 59, 70–80 [PubMed] [Google Scholar]
- 18.Lazzaro B. P., Flores H. A., Lorigan J. G., Yourth C. P. 2008. Genotype-by-environment interactions and adaptation to local temperature affect immunity and fecundity in Drosophila melanogaster. PLoS Pathogens 4, e1000025. 10.1371/journal.ppat.1000025 (doi:10.1371/journal.ppat.1000025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olsen L. E., Hoy M. A. 2002. Heat curing Metaseiulus occidentalis (Nesbitt) (Acari, Phytoseiidae) of a fitness-reducing microsporidium. J. Invertebr. Pathol. 79, 173–178 10.1016/S0022-2011(02)00015-0 (doi:10.1016/S0022-2011(02)00015-0) [DOI] [PubMed] [Google Scholar]
- 20.Blanford S., Thomas M. B. 2000. Thermal behavior of two acridid species: effects of habitat and season on body temperature and the potential impact of biocontrol with pathogens. Environ. Entomol. 29, 1060–1069 10.1603/0046-225X-29.5.1060 (doi:10.1603/0046-225X-29.5.1060) [DOI] [Google Scholar]
- 21.Carruthers R. I., Larkin T. S., Firstencel H., Feng Z. D. 1992. Influence of thermal ecology on the mycosis of a rangeland grasshopper. Ecology 73, 190–204 10.2307/1938731 (doi:10.2307/1938731) [DOI] [Google Scholar]
- 22.Stacey D. A., Thomas M. B., Blanford S., Pell J. K., Pugh C., Fellows M. D. 2003. Genotype and temperature influences pea aphid resistance to a fungal entomopathogen. Physiol. Entomol. 28, 75–81 10.1046/j.1365-3032.2003.00309.x (doi:10.1046/j.1365-3032.2003.00309.x) [DOI] [Google Scholar]
- 23.Menti H., Wright D. J., Perry R. N. 2000. Infectivity of populations of the entomopathogenic nematodes Steinernema feltiae and Heterorhabditis megidis in relation to temperature, age, and lipid content. Nematology 2, 515–521 10.1163/156854100509439 (doi:10.1163/156854100509439) [DOI] [Google Scholar]
- 24.Adamo S. A. 1998. The specificity of behavioral fever in the cricket Acheta domesticus. J. Parasitol. 84, 529–533 10.2307/3284717 (doi:10.2307/3284717) [DOI] [PubMed] [Google Scholar]
- 25.Geden C. J. 1997. Development models for the filth fly parasitoids Spalangia gemina, S. cameroni, and Muscidifurax raptor (Hymenoptera: Pteromalidae) under constant and variable temperatures. Biol. Control 9, 185–192 10.1006/bcon.1997.0532 (doi:10.1006/bcon.1997.0532) [DOI] [Google Scholar]
- 26.Blanford S., Thomas M. B. 1999. Host thermal biology: the key to understanding insect–pathogen interactions and microbial pest control. Agric. Forest Entomol. 1, 195–202 10.1046/j.1461-9563.1999.00027.x (doi:10.1046/j.1461-9563.1999.00027.x) [DOI] [Google Scholar]
- 27.Karl I., Stoks R., De Block M., Janowitz S. A., Fischer K. 2010. Temperature extremes and butterfly fitness: conflicting evidence from life history and immune function. Global Change Biol. 17, 676–687 10.1111/j.1365-2486.2010.02277.x (doi:10.1111/j.1365-2486.2010.02277.x) [DOI] [Google Scholar]
- 28.Fischer K., Koelzow N., Hoeltje H., Karl I. 2011. Assay conditions in laboratory experiments: is the use of constant rather than fluctuating temperatures justified when investigating temperature-induced plasticity. Oecologia 166, 23–33 10.1007/s00442-011-1917-0 (doi:10.1007/s00442-011-1917-0) [DOI] [PubMed] [Google Scholar]
- 29.Linder J. E., Owers K. A., Promislow D. E. L. 2008. The effects of temperature on host–pathogen interactions in D. melanogaster: who benefits? J. Insect. Physiol. 54, 297–308 10.1016/j.jinsphys.2007.10.001 (doi:10.1016/j.jinsphys.2007.10.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adamo S. A., Lovett M. M. E. 2011. Some like it hot: the effects of climate change on reproduction, immune function and disease resistance in the cricket Gryllus texensis. J. Exp. Biol. 214, 1997–2004 10.1242/jeb.056531 (doi:10.1242/jeb.056531) [DOI] [PubMed] [Google Scholar]
- 31.Catalan T., Wozniak A., Niemeyer H. M., Kalergis A. M., Bozinovis F. 2011. Interplay between thermal and immune ecology: effect of environmental temperature on insect immune response and energetic costs after an immune challenge. J. Insect Physiol. 58, 310–317 10.1016/j.jinsphys.2011.10.001 (doi:10.1016/j.jinsphys.2011.10.001) [DOI] [PubMed] [Google Scholar]
- 32.Triggs A., Knell R. J. 2012. Interactions between environmental variable determine immunity in the Indian meal moth Plodia interpunctella. J. Anim. Ecol. 81, 386–394 10.1111/j.1365-2656.2011.01920.x (doi:10.1111/j.1365-2656.2011.01920.x) [DOI] [PubMed] [Google Scholar]
- 33.Hillyer J. F., Estevez-Lao T. Y. 2010. Nitric oxide is an essential component of the hemocyte-mediated mosquito immune response against bacteria. Dev. Comp. Immunol. 34, 141–149 10.1016/j.dci.2009.08.014 (doi:10.1016/j.dci.2009.08.014) [DOI] [PubMed] [Google Scholar]
- 34.Christensen B. M., Li J. Y., Chen C. C., Nappim A. J. 2005. Melanization immune responses in mosquito vectors. Trends Parasitol. 21, 192–199 10.1016/j.pt.2005.02.007 (doi:10.1016/j.pt.2005.02.007) [DOI] [PubMed] [Google Scholar]
- 35.Adamo S. A. 2004. Estimating disease resistance in insects: phenoloxidase and lysozyme-like activity and disease resistance in the cricket Gryllus texensis. J. Insect Physiol. 50, 209–216 10.1016/j.jinsphys.2003.11.011 (doi:10.1016/j.jinsphys.2003.11.011) [DOI] [PubMed] [Google Scholar]
- 36.Alaux C., Ducloz F., Crauser D., Le Conte Y. 2010. Diet effects on honeybee immunocompetence. Biol. Lett. 6, 562–565 10.1098/rsbl.2009.0986 (doi:10.1098/rsbl.2009.0986) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Block M., Stoks R. 2008. Short-term larval food stress and associated compensatory growth reduce adult immune function in a damselfly. Ecol. Entomol. 33, 796–801 10.1111/j.1365-2311.2008.01024.x (doi:10.1111/j.1365-2311.2008.01024.x) [DOI] [Google Scholar]
- 38.Blandin S., Levashina E. A. 2004. Mosquito immune responses against malaria parasites. Curr. Opin. Immunol. 16, 16–20 10.1016/j.coi.2003.11.010 (doi:10.1016/j.coi.2003.11.010) [DOI] [PubMed] [Google Scholar]
- 39.Osta M. A., Christophides G. K., Kafatos F. C. 2004. Effects of mosquito genes on Plasmodium development. Science 303, 2030–2032 10.1126/science.1091789 (doi:10.1126/science.1091789) [DOI] [PubMed] [Google Scholar]
- 40.Michel K., Budd A., Pinto S., Gibson T. J., Kafatos F. C. 2005. Anopheles gambiae SRPN2 facilitates midgut invasion by the malaria parasite Plasmodium berghei. EMBO Rep. 6, 891–897 10.1038/sj.embor.7400478 (doi:10.1038/sj.embor.7400478) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collins F. H., Sakai R. K., Vernick K. D., Paskewitz S., Seeley D. C., Miller L. H., Collins W. E., Campbell C. C., Gwadz R. W. 1986. Genetic selection of a Plasmodium-refractory strain of the malaria vector Anopheles gambiae. Science 234, 607–610 10.1126/science.3532325 (doi:10.1126/science.3532325) [DOI] [PubMed] [Google Scholar]
- 42.Hillyer J. F., Schmidt S. L., Christensen B. M. 2003. Rapid phagocytosis and melanization of bacteria and Plasmodium sporozoites by hemocytes of the mosquito Aedes aegypti. J. Parasitol. 89, 62–69 10.1645/0022-3395(2003)089[0062:RPAMOB]2.0.CO;2 (doi:10.1645/0022-3395(2003)089[0062:RPAMOB]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 43.Hillyer J. F., Schmidt S. L., Christensen B. M. 2003. Hemocyte-mediated phagocytosis and melanization in the mosquito Armigeres subalbatus following immune challenge by bacteria. Cell Tissue Res. 313, 117–127 10.1007/s00441-003-0744-y (doi:10.1007/s00441-003-0744-y) [DOI] [PubMed] [Google Scholar]
- 44.Huang C. Y., Christensen B. M., Chen C. C. 2005. Role of dopachrome conversion enzyme in the melanization of filarial worms in mosquitoes. Insect Mol. Biol. 14, 675–682 10.1111/j.1365-2583.2005.00597.x (doi:10.1111/j.1365-2583.2005.00597.x) [DOI] [PubMed] [Google Scholar]
- 45.Ferdig M. T., Beerntsen B. T., Spray F. J., Li J. Y., Christensen B. M. 1993. Reproductive costs associated with resistance in a mosquito-filarial worm system. Am. J. Trop. Med. Hyg. 49, 756–762 [DOI] [PubMed] [Google Scholar]
- 46.Paskewitz S., Riehle M. A. 1994. Response of Plasmodium-refractory and susceptible strains of Anopheles gambiae to inocoluated Sephadex beads. Dev. Comp. Immunol. 18, 369–375 10.1016/0145-305X(94)90002-7 (doi:10.1016/0145-305X(94)90002-7) [DOI] [PubMed] [Google Scholar]
- 47.Moita L. F., Wang-Sattler R., Michel K., Zimmermann T., Blandin S., Levashina E. A., Kafatos F. C. 2005. In vivo identification of novel regulators and conserved pathways of phagocytosis in Anopheles gambiae. Immunity 23, 65–73 10.1016/j.immuni.2005.05.006 (doi:10.1016/j.immuni.2005.05.006) [DOI] [PubMed] [Google Scholar]
- 48.Hillyer J. F., Christensen B. M. 2002. Characterization of hemocytes from the yellow fever mosquito, Aedes aegypti. Histochem. Cell Biol. 117, 431–440 10.1007/s00418-002-0408-0 (doi:10.1007/s00418-002-0408-0) [DOI] [PubMed] [Google Scholar]
- 49.Hillyer J. F., Schmidt S. L., Fuchs J. F., Boyle J. P., Christensen B. M. 2005. Age-associated mortality in immune challenged mosquitoes (Aedes aegypti) correlates with a decrease in haemocyte numbers. Cell Microbiol. 7, 39–51 10.1111/j.1462-5822.2004.00430.x (doi:10.1111/j.1462-5822.2004.00430.x) [DOI] [PubMed] [Google Scholar]
- 50.Vizioli J., Richman A. M., Uttenweiler-Joseph S., Blass C., Bulet P. 2001. The defensin peptide of the malaria vector mosquito Anopheles gambiae: antimicrobial activities and expression in adult mosquitoes. Insect. Biochem. Mol. Biol. 31, 241–248 10.1016/S0965-1748(00)00143-0 (doi:10.1016/S0965-1748(00)00143-0) [DOI] [PubMed] [Google Scholar]
- 51.Vizioli J., et al. 2000. Cloning and analysis of a cecropin gene from the malaria vector mosquito, Anopheles gambiae. Insect Mol. Biol. 9, 75–84 10.1046/j.1365-2583.2000.00164.x (doi:10.1046/j.1365-2583.2000.00164.x) [DOI] [PubMed] [Google Scholar]
- 52.Kokoza V., Ahmed A., Shin S. W., Okafor N., Zou Z., Raikhel A. S. 2010. Blocking of Plasmodium transmission by cooperative action of cecropin A and defensin A in transgenic Aedes aegypti mosquitoes. Proc. Natl Acad. Sci. USA 107, 8111–8116 10.1073/pnas.1003056107 (doi:10.1073/pnas.1003056107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rivero A. 2006. Nitric oxide: an antiparasitic molecule of invertebrates. Trends Parasitol. 22, 219–225 10.1016/j.pt.2006.02.014 (doi:10.1016/j.pt.2006.02.014) [DOI] [PubMed] [Google Scholar]
- 54.Luckhart S., Vodovotz Y., Cui L. W., Rosenberg R. 1998. The mosquito Anopheles stephensi limits malaria parasite development with inducible synthesis of nitric oxide. Proc. Natl Acad. Sci. USA 95, 5700–5705 10.1073/pnas.95.10.5700 (doi:10.1073/pnas.95.10.5700) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gupta L., Molina-Cruz A., Kumar S., Rodrigues J., Dixit R., Zamora R. E., Barillas-Mury C. 2009. The STAT pathway mediates late-phase immunity against Plasmodium in the mosquito Anopheles gambiae. Cell Host Microbe 5, 498–507 10.1016/j.chom.2009.04.003 (doi:10.1016/j.chom.2009.04.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Surachetpong W., Singh N., Cheung K. W., Luckhart S. 2009. MAPK ERK signaling regulates the TGF-beta 1-dependent mosquito response to Plasmodium falciparum. PLoS Pathog. 5, 1–11 10.1371/journal.ppat.1000366 (doi:10.1371/journal.ppat.1000366) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oliveira G., Lieberman J., Barillas-Mury C. 2012. Epithelial nitration by a peroxidase/NOX5 system mediates mosquito antiplasmodial immunity. Science 335, 856–859 10.1126/science.1209678 (doi:10.1126/science.1209678) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Garver L. S., Dong Y. M., Dimopoulos G. 2009. Casper controls resistance to Plasmodium falciparum in diverse anopheline species. PLoS Pathog. 5, e1000335. 10.1371/journal.ppat.1000335 (doi:10.1371/journal.ppat.1000335) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arrighi R. B. G., Debierre-Grockiege F., Schwarz R. T., Faye I. 2009. The immunogenic properties of protozoan glycosylphosphatidylinositols in the mosquito Anopheles gambiae. Dev. Comp. Immunol. 33, 216–223 10.1016/j.dci.2008.08.009 (doi:10.1016/j.dci.2008.08.009) [DOI] [PubMed] [Google Scholar]
- 60.Coggins S. A., Estevez-Lao T. Y., Hillyer J. F. In press. Increased survivorship following bacterial infection by the mosquito Aedes aegypti as compared to Anopheles gambiae correlates with increased transcriptional induction of antimicrobial peptides. Dev. Comp. Immunol . [DOI] [PubMed] [Google Scholar]
- 61.Dong Y., Das S., Cirimotich C., Souza-Neto J. A., McLean K. J., Dimopoulos G. 2011. Engineered Anopheles immunity to Plasmodium infection. PLoS Pathog. 7, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lambrechts L., Vulule J. M., Koella J. C. 2004. Genetic correlation between melanization and antibacterial immune responses in a natural population of the malaria vector Anopheles gambiae. Evolution 58, 2377–2381 [DOI] [PubMed] [Google Scholar]
- 63.Lambrechts L., Morlais I., Awono-Ambene P. H., Cohuet A., Simard F., Jacques J. C., Bourgouin C., Koella J. C. 2007. Effect of infection by Plasmodium falciparum on the melanization immune response of Anopheles gambiae. Am. J. Trop. Med. Hyg. 76, 475–480 [PubMed] [Google Scholar]
- 64.Boete C., Paul R. E. L., Koella J. C. 2004. Direct and indirect immunosuppression by a malaria parasite in its mosquito vector. Proc. R. Soc. Lond. B 271, 1611–1615 10.1098/rspb.2004.2762 (doi:10.1098/rspb.2004.2762) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kilpatrick A. M., Meola M. A., Moudy R. M., Kramer L. D. 2008. Temperature, viral genetics, and the transmission of West Nile virus by Culex pipiens mosquitoes. PLoS Pathog. 4, 1–7 10.1371/journal.ppat.1000092 (doi:10.1371/journal.ppat.1000092) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Noden B. H., Kent M. D., Beier J. C. 1995. The impact of variations in temperature on early Plasmodium falciparum development in Anopheles stephensi. Parasitology 111, 539–545 10.1017/S0031182000077003 (doi:10.1017/S0031182000077003) [DOI] [PubMed] [Google Scholar]
- 67.Lambrechts L., Paaijmans K. P., Fansiri T., Carrington L. B., Kramer L. D., Thomas M. B., Scott T. W. 2011. Impact of daily temperature fluctuations on dengue virus transmission by Aedes aegypti. Proc. Natl Acad. Sci. USA 108, 7460–7465 10.1073/pnas.1101377108 (doi:10.1073/pnas.1101377108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Paaijmans K. P., Blanford S., Chan B. H. K., Thomas M. B. In press Warmer temperatures reduce the vectorial capacity of malaria mosquitoes. Biol. Lett. 8 10.1098/rsbl.2011.1075 (doi:10.1098/rsbl.2011.1075) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Muirhead-Thomson R. C. 1951. Mosquito behaviour. London, UK: Edward Arnold and Company [Google Scholar]
- 70.Lim J. H., Gowda D. C., Krishnegowda G., Luckhart S. 2005. Induction of nitric oxide synthase in Anopheles stephensi by Plasmodium falciparum: mechanism of signaling and the role of parasite glycosylphosphatidylinositols. Infect Immun. 73, 2778–2789 10.1128/iai.73.5.2778-2789.2005 (doi:10.1128/iai.73.5.2778-2789.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ikemoto T. 2008. Tropical malaria does not mean hot environments. J. Med. Entomol. 45, 963–969 10.1603/0022-2585(2008)45[963:TMDNMH]2.0.CO;2 (doi:10.1603/0022-2585(2008)45[963:TMDNMH]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 72.Paaijmans K. P., Read A. F., Thomas M. B. 2009. Understanding the link between malaria risk and climate. Proc. Natl Acad. Sci. USA 106, 13 844–13 849 10.1073/pnas.0903423106 (doi:10.1073/pnas.0903423106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Suwanchaichinda C., Paskewitz S. M. 1998. Effects of larval nutrition, adult body size, and adult temperature on the ability of Anopheles gambiae (Diptera: Culicidae) to melanize Sephadex beads. J. Med. Entomol. 35, 157–161 [DOI] [PubMed] [Google Scholar]
- 74.Hillyer J. F., Christensen B. M. 2005. Mosquito phenoloxidase and defensin colocalize in melanization innate immune responses. J. Histochem. Cytochem. 53, 689–698 10.1369/jhc.4A6564.2005 (doi:10.1369/jhc.4A6564.2005) [DOI] [PubMed] [Google Scholar]
- 75.Chown S. L., Nicolson S. W. 2004. Insect physiological ecology: mechanisms and patterns. New York, NY: Oxford University Press [Google Scholar]
- 76.Collazos M. E., Ortega E., Barriga C. 1994. Effect of temperature on the immune system of a cyprinid fish (Tinca tinca, L): blood phagocyte function at low temperature. Fish Shellfish Immunol. 4, 231–238 10.1006/fsim.1994.1021 (doi:10.1006/fsim.1994.1021) [DOI] [Google Scholar]
- 77.Le Morvan C., Clerton P., Deschaux P., Troutaud D. 1997. Effects of environmental temperature on macrophage activities in carp. Fish Shellfish Immunol. 7, 209–212 10.1006/fsim.1996.0075 (doi:10.1006/fsim.1996.0075) [DOI] [Google Scholar]
- 78.Hardie L. J., Fletcher T. C., Secombes C. J. 1994. Effect of temperature on macrophage activation and the production of macrophage activating factor by rainbow trout (Oncorhyncus mykiss) leukocytes. Dev. Comp. Immunol. 18, 57–66 10.1016/0145-305X(94)90252-6 (doi:10.1016/0145-305X(94)90252-6) [DOI] [PubMed] [Google Scholar]
- 79.Mitri C., et al. 2009. Fine pathogen discrimination within the APL1 gene family protects Anopheles gambiae against human and rodent malaria species. PLoS Pathog. 5, 1–10 10.1371/journal.ppat.1000576 (doi:10.1371/journal.ppat.1000576) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rivero A., Vezilier J., Weill M., Read A. F., Gandon S. 2010. Insecticide control of vector-borne diseases: when is insecticide resistance a problem? PLoS Pathog. 6, 1–9 10.1371/journal.ppat.1001000 (doi:10.1371/journal.ppat.1001000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Patil N. S., Lole K. S., Deobagkar D. N. 1996. Adaptive larval thermotolerance and induced cross-tolerance to propoxur insecticide in mosquitoes Anopheles stephensi and Aedes aegypti. Med. Vet. Entomol. 10, 277–282 10.1111/j.1365-2915.1996.tb00743.x (doi:10.1111/j.1365-2915.1996.tb00743.x) [DOI] [PubMed] [Google Scholar]
- 82.Blanford S., Shi W. P., Christian R., Marden J. H., Koekemoer L. L., Brooke B. D., Coetzee M., Read A. F., Thomas M. B. 2011. Lethal and pre-lethal effects of a fungal biopesticide contribute to substantial and rapid control of malaria vectors. PLoS ONE 6, e23591. 10.1371/journal.pone.0023591 (doi:10.1371/journal.pone.0023591) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kikankie C. K., Brooke B. D., Knols B. G. J., Koekemoer L. L., Farenhorst M., Hunt R. H., Thomas M. B., Coetzee M. 2010. The infectivity of the entomopathogenic fungus Beauveria bassiana to insecticide-resistant and susceptible Anopheles arabiensis mosquitoes at two different temperatures. Malar. J. 9, 9–71 10.1186/1475-2875-9-71 (doi:10.1186/1475-2875-9-71) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mittal P. K., Adak T., Sharma V. P. 1993. Effect of temperature on toxicity of two bioinsecticides spherix (Bacillus sphaericus) and bactoculicide (Bacillus thuringiensis) against larvae of four vector mosquitoes. Indian J. Malariol. 30, 37–41 [PubMed] [Google Scholar]
- 85.Ren X., Hoiczyk E., Rasgon J. L. 2008. Viral paratransgenesis in the malaria vector Anopheles gambiae. PLoS Pathog. 4, 1–8 10.1371/journal.ppat.1000135 (doi:10.1371/journal.ppat.1000135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hoffmann A. A., et al. 2011. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 476, 454–457 10.1038/nature10356 (doi:10.1038/nature10356) [DOI] [PubMed] [Google Scholar]