Abstract
Chemical contamination and disease outbreaks have increased in many ecosystems. However, connecting pollution to disease spread remains difficult, in part, because contaminants can simultaneously exert direct and multi-generational effects on several host and parasite traits. To address these challenges, we parametrized a model using a zooplankton–fungus–copper system. In individual-level assays, we considered three sublethal contamination scenarios: no contamination, single-generation contamination (hosts and parasites exposed only during the assays) and multi-generational contamination (hosts and parasites exposed for several generations prior to and during the assays). Contamination boosted transmission by increasing contact of hosts with parasites. However, it diminished parasite reproduction by reducing the size and lifespan of infected hosts. Multi-generational contamination further reduced parasite reproduction. The parametrized model predicted that a single generation of contamination would enhance disease spread (via enhanced transmission), whereas multi-generational contamination would inhibit epidemics relative to unpolluted conditions (through greatly depressed parasite reproduction). In a population-level experiment, multi-generational contamination reduced the size of experimental epidemics but did not affect Daphnia populations without disease. This result highlights the importance of multi-generational effects for disease dynamics. Such integration of models with experiments can provide predictive power for disease problems in contaminated environments.
Keywords: contamination, disease, multi-generational effects, Daphnia, transmission, parasite reproduction
1. Introduction
Disease outbreaks and chemical contamination are increasing in many ecosystems [1–3]. In some cases, contamination is associated with elevated disease prevalence and intensity [4,5]. Does pollution exacerbate wildlife disease? If so, do contaminants and parasitism synergistically depress host populations? There is mixed evidence for this concerning scenario: sometimes disease is more prevalent in contaminated habitats, whereas in other cases parasites may be rare or absent [6,7]. Thus, pollution does not always exacerbate disease, but the mechanisms underlying these highly variable outcomes remain unresolved. Without better understanding of the combined effects of contamination and disease, ecologists and managers have limited power to confront these two rapidly growing ecological threats.
Contaminants can alter disease spread by changing the traits of hosts and parasites. For example, pollutants can increase the virulence of pathogens [8], raise the susceptibility of hosts [4] and increase the mortality of the free-living parasite stages [9]. Still, it remains difficult to concretely link these effects to disease spread in populations. One fundamental challenge is that contaminants can affect several disease-related traits simultaneously. For example, large pesticide doses can increase not only host susceptibility, but also decrease free-living parasite survival in a frog–trematode system [10]. When contaminants simultaneously perturb many traits, it becomes critical to determine their ‘net effects’ on disease spread [10]. We can evaluate these net effects by synthesizing contaminant-dependent disease traits with epidemiological models. Models of disease yield criteria for parasite invasion, persistence of hosts during epidemics and extinction of host or parasite. These factors critically shape the size of epidemics. Thus, by linking the individual and population levels, models can delineate the joint effects of these two ecological stressors on host populations.
A predictive framework linking contamination and disease must also confront temporal scale. Contamination can occur with high temporal variability. For example, pesticide use may cause large, short-lived spikes of contamination. Conversely, chronic pollution may persistently elevate toxicant levels, exposing organisms for many generations. Generally, for an individual, toxic effects increase with exposure time owing to bioaccumulation. Exposed organisms may produce lower quality offspring that can be more vulnerable to contamination or additional stressors [11]. These multi-generational effects of contamination could strongly influence disease spread in chronically polluted environments.
Here, we link individual-, multi-generational and population effects of contamination on disease using a case study. We focus on a common freshwater pollutant (copper), a zooplankton host (Daphnia dentifera) and a fungal pathogen (Metschnikowia bicuspidata). Daphnia dentifera is a non-selective grazer in small, thermally stratified lakes. Hosts become infected after consuming free-living fungal spores [12]. Infection reduces host reproduction and survival [13]. Parasites reproduce in the host's haemolymph and body cavity. Spores are released into the environment only after host death [12]. Hosts and parasites may experience temporally diverse contamination events. For example, use of copper sulphate (an algicide) elevates concentrations in the water for days or weeks [14], whereas pollution from mining can raise metal levels for years [15].
Copper contamination should exert opposing influences on several traits central to disease in this system. Daphnia can increase their feeding rate when exposed to sublethal levels of copper [16]. Because infection requires consumption of parasites, copper should increase transmission rate, thereby boosting disease spread. Copper may also impair immune response to consumed spores, further enhancing transmission. On the other hand, copper should reduce parasite production within infected hosts, inhibiting disease spread. Two mechanisms should cause this reduction. First, copper could decrease the lifespan of infected hosts, a common effect of contaminants [6]. This reduces the time during which parasites could reproduce in hosts. Second, contamination increases metabolic costs for hosts. These costs reduce growth rate and size of infected hosts, limiting the production of parasites owing to metabolic connections among size, growth rate and spore yield from hosts [13,17]. Multi-generational exposure of hosts to contamination should exacerbate these effects in chronically contaminated environments [18].
To study the net outcome of chronic copper contamination on disease, we started with a general epidemiological model. From the model, we determined R0, the parasite's basic reproductive ratio, which is an indicator of the potential for disease spread. Using laboratory experiments, we estimated parameters for this model and calculated R0 under contamination scenarios of different duration. We used a single host genotype and parasite isolate to examine the direct and transgenerational effects of contamination while controlling for potential evolutionary responses during epidemics. The R0 criterion indicated that the time scale of contamination determines the effect on disease spread, and chronic contamination should decrease epidemic size. We tested this prediction with a population-level experiment.
2. Methods
(a). Epidemiological model
We connected copper to disease spread with a model that captures key elements of the Daphnia–fungus epidemiology [17]. It tracks the changes in density of susceptible hosts (S), infected hosts (I) and fungal spores (Z) of the single clone–single isolate system through time (t):
| 2.1 |
| 2.2 |
| 2.3 |
Susceptible hosts increase with births, at a maximum rate for susceptible (b) and infected hosts (ρb; usually ρ < 1), modulated by density dependence (c). Hosts die at background rate d but also decrease after contacting free-living spores. Infected hosts arise through infection at rate β but die at an elevated rate owing to virulence of the parasite, d + v (equation (2.2)). Free-living spores are released after host death, at per capita yield σ, but are lost at rate m (equation (2.3)).
Using this model, we determined R0, a key indicator of the parasite's success:
| 2.4 |
This quantity estimates the average number of infections caused by a single infected individual introduced into a disease-free population of susceptible hosts at equilibrium. Higher R0 generally corresponds to larger epidemics and more severe effects on host density. Therefore, it predicts the potential for disease [19].
The R0 criterion focuses our experiments below on traits that govern disease spread. The parasite's success is determined by equilibrial population density of hosts in the absence of disease, the first term in parentheses, (b–d)/(bc). Therefore, contaminants that reduce the birth rate (b) or increase the death rate (d) of hosts depress R0. It also depends on three disease-related traits, (σβ/m): R0 increases with host susceptibility (β) or spore yield (σ) but decreases with higher loss rates of spores (m). We focus on sublethal copper exposures, because lethal concentrations would threaten host populations independent of disease. If contamination enhances σ or β, larger epidemics could ensue. Alternatively, copper may inhibit epidemics by reducing these traits.
(b). Parameter estimation
We described the experimental and statistical methods used to estimate parameters in more detail in the electronic supplementary material, appendix. Therefore, we briefly outline our methods here.
(i). Hosts and parasites
We used a single host clone and fungal isolate collected from Baker Lake, an uncontaminated lake in Michigan, USA. Compared with other Daphnia genotypes collected from this area, this genotype is moderately susceptible to fungal infection [20], and all genotypes were similarly sensitive to copper contamination (D. J. Civitello 2011, unpublished data). Daphnia genotypes vary within and among lakes in susceptibility to infection [20,21]. However, unlike in other Daphnia-parasite systems (e.g. with the bacterium Pasteuria; [22,23]), there is very little variation among fungal isolates. Metschnikowia isolates collected from different lakes in this region exhibited no variation in overall infectivity nor evidence of host genotype–parasite isolate interactions [21].
(ii). Culturing conditions
We reared hosts and parasites in high hardness COMBO [24] with 5 μg l−1 phosphorus at 20°C and 16 L : 8 D cycle in acid-washed glassware. We added copper as copper sulphate pentahydrate (CuSO4·5H2O) and provided ample algal food (1.0 mg l−1 dry weight of Scenedesmus acutus reared in P-replete media). In a preliminary life table assay, daily exposure of uninfected Daphnia to less than or equal to 200 μg Cu l−1 for 21 days did not affect survival or reproduction. However, Daphnia rapidly died at 400 μg Cu l−1 (see electronic supplementary material, appendix and figure A1). Thus, we chose sublethal exposures, less than or equal to 200 μg Cu l−1, for our experimental manipulations. These concentrations are higher than those often measured in chronically contaminated lakes (see electronic supplementary material, appendix). However, Daphnia tolerate five- to 10-fold higher copper concentrations in COMBO than in lake water (see electronic supplementary material, appendix). Despite this difference, we use COMBO because it is highly reproducible. Furthermore, among defined media, it uniquely enables long-term culturing required for population-level experiments [24]. Here, 200 μg Cu l−1 provides the sublethal dose desired for the experiments, calibrated for the chemistry of the COMBO media. Comparable sublethal exposures can occur in lakes chronically contaminated with copper (see electronic supplementary material, appendix). We reared hosts for at least 10 generations at ambient copper (1 μg Cu l−1, ‘naive’ lineages) for uncontaminated and single-generation exposure treatments or elevated copper (200 μg Cu l−1, ‘exposed’ lineages) for multi-generational exposure treatments. (‘Multi-generational exposure’ always refers to exposure of hosts (or parasites) to copper, not of hosts to parasites.) Separately, we raised parasites in vivo at ambient or elevated copper for at least six infection cycles.
(iii). Transmission (β)
We estimated the copper-dependent transmission rate (β) with an infection assay. We placed groups of six 6-day-old naive Daphnia into beakers containing 100 ml of media with added copper (0, 50, 100, 200 μg Cu l−1). To each beaker, we added naive fungal spores (25, 50 or 75 spores ml−1). To test for a multi-generational effect of copper exposure on transmission, we replicated the 200 μg Cu l−1 treatment with hosts and parasites from exposed lineages (i.e. multi-generational exposure of both hosts and parasites, identified as the ‘200-multi-’ treatment in figure 1). After 24 h, all animals were transferred to new media and reared until visual diagnosis [12]. In the highest spore density treatment, we estimated the density of spores remaining in the beakers following exposure. With these data, we estimated the per parasite consumption rate of Daphnia [25] (see electronic supplementary material, appendix). This per parasite consumption rate represents the volume of environment that is depleted of parasites per unit time (i.e. foraging or filtration rate). It does not depend on parasite density (D. J. Civitello, S. Pearsall and S. R. Hall 2011, unpublished data). However, the total parasite consumption rate, the product of the per parasite consumption rate and parasite density, increases with parasite density. Therefore, rates estimated at one parasite density provide an unbiased estimate of the host–parasite contact rate.
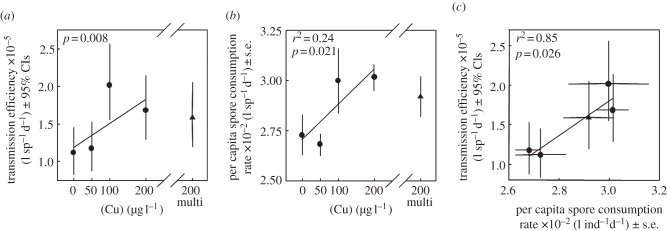
Figure 1.
Results from the infection assay. (a) Copper contamination increased host susceptibility to infection as indexed by the density-dependent transmission rate, β (0–200 μg Cu l−1, circles). However, maternal exposure did not further elevate host susceptibility (compare 95% CIs of 200 μg Cu l−1 and 200-multi-generational treatments, triangles). (b) Daphnia exposed to copper during the assay consumed fungal spores at a higher rate. (c) Spore consumption by Daphnia was highly correlated with transmission rate.
We fit a simplified model to estimate the transmission rate parameter, β, assuming binomially distributed infections (see electronic supplementary material, appendix). Using a likelihood ratio test [26], we competed a null model in which copper does not influence β (β = β0), with an alternative model with β as a linear function of copper concentration (β = β0 + β1 × [Cu]). We also fit three nonlinear functions relating transmission rate to copper: saturating, sigmoid and quadratic (see electronic supplementary material, appendix). In order to facilitate comparisons with parameters estimated in the life table assay, we also tested the hypotheses that a single generation of exposure to 200 μg Cu l−1 altered transmission rate (HA: β0 ≠ β200) and that multi-generational copper exposure further influenced transmission (HA: β200 ≠ β200 − multi-generation) using likelihood ratio tests [26]. For each test, we compared a null model that fit the same β with both treatments versus a model that estimated different β from each treatment. We assessed relationships between contamination and spore consumption rate, and then spore consumption and transmission rate with linear regression.
(iv). Fecundity (b), mortality (d), virulence and spore production (σ)
We estimated other key parameters influencing disease spread with a life table assay (n = 124). We considered three scenarios involving exposure to 200 μg Cu l−1: no contamination, single-generation contamination and multi-generational contamination. We crossed these host contamination scenarios with three infection treatments (no infection, infection with Cu naive parasites or infection with Cu-exposed parasites). See electronic supplementary material, appendix for pre-infection culturing details. We exposed 7-day-old Daphnia in the infected treatments to a high spore dose (1200 spores ml−1, to ensure infection) for 24 h. We then transferred each animal individually into 100 ml fresh media daily, recording survival and reproduction. We estimated population growth rate (r) for each uninfected host treatment with the Euler–Lotka equation [27]. We estimated the background death rate, d, by fitting an exponential survivorship curve for the uninfected hosts [27]. We calculated instantaneous birth rates (b) as the sum of r and d (i.e. b = r + d; [27]). After infected hosts died, we measured eye-to-tail length and counted spores (σ) using a haemocytometer. We analysed infected host lifespan, length at death and spore yield using two-way ANOVAs (contamination scenario × infection treatment; parasites exposed or naive) with type III SS (PROC GLM; SAS Institute Inc. 2004). Using likelihood ratio tests, we found that parasite exposure history proved insignificant in all analyses. Therefore, we tested the hypotheses that a single generation of copper exposure reduced spore yield, lifespan and size for naive animals (HA: µsingle-generation exposure < µno exposure) and that multi-generational copper exposure further reduced relative to single-generation exposure (HA: µmulti-generational exposure < µsingle-generation exposure) using one-tailed planned contrasts [28]. We used partial regression to assess the contribution of lifespan and length at death on spore yield of individual infected hosts [29] (see electronic supplementary material, appendix).
(c). Population-level predictions
We assessed the potential for disease spread under each scenario by estimating R0 (equation (2.4)) with data from the infection and life table assays. We estimated four of the six parameters in the R0 expression (equation (2.4)): birth rate of uninfected hosts (b), background death rate (d), spore yield (σ) and transmission rate (β). Without knowing the sensitivity of the two remaining parameters to copper, we calculated R0 at different plausible (but Cu-independent) values of density dependence (c) and loss rate of spores (m). Because the relative differences in R0 were not sensitive to c and m, we present estimates with single values for these two parameters.
(d). Population-level experiment
We tested the prediction that multi-generational contamination would inhibit epidemics with a population-level experiment manipulating contamination and parasite introduction. We established populations of hosts (initial density: approx. 30 Daphnia per litre) and algae (initial density: 1 mg dry weight l−1) in aquaria filled with 35 l of COMBO with 50 μg P l−1 maintained at 20°C with a 16 L : 8 D cycle (n = 12 aquaria). Each day, we stirred the tanks, and supplied 5 per cent of the initial amount of N and P as NaNO3 and K2HPO4 to maintain algal productivity. Starting on day six, we sampled the tanks every 4 to 5 days by stirring the tanks, removing a 1 l sample, and replacing it with fresh media. We estimated host density and infection prevalence with these samples. Half of the tanks received copper (4 μg Cu l−1 per day), initiated on day six; this provided 23 days (two to three generations) to install multi-generational effects of copper exposure on hosts before parasite introduction (see electronic supplementary material, appendix for additional details and copper dynamics). On day 29, we inoculated half of the tanks with fungal spores (104 spores l−1) and then maintained the experiment for 87 more days. We quantified epidemic size by estimating the area under the prevalence versus time curve (i.e. integrated prevalence). Similarly, we estimated the area under the host density versus time curve (integrated host density) from day 29 to 116. Decreases in integrated density of hosts reflect the parasite's ability to reduce the host population. We accidentally supplied a large dose of copper to one copper/no parasite replicate around day 50, causing a population crash. We omitted this replicate from the analysis. We tested the hypotheses that copper contamination would reduce epidemic size and therefore yield higher host density with parasites with one-tailed planned contrasts. We also tested for toxic effects of copper on integrated host density without parasites with a one-tailed planned contrast.
3. Results
(a). Individual-level assays
(i). Transmission (β)
Copper significantly increased transmission rate (likelihood ratio test: n = 80, d.f. = 1, p = 0.008; figure 1a). Further, the nonlinear models did not significantly outperform the linear model (ΔAICc quadratic: 0, linear: 0.1, sigmoid: 0.5, saturating: 1.2; see electronic supplementary material, appendix). Single-generation exposure of Daphnia to 200 μg Cu l−1 increased transmission (likelihood ratio test, n = 40, d.f. = 1, p = 0.031), whereas multi-generational exposure to copper did not further affect transmission (likelihood ratio test, n = 40, d.f. = 1, p = 0.73). Spore consumption increased with copper addition (linear regression: n = 22, r2 = 0.24, p = 0.021; figure 1b). Transmission rate increased with per capita spore consumption rate for each treatment (linear regression: n = 5, r2 = 0.85, p = 0.026; figure 1c).
(ii). Fecundity (b), mortality (d), virulence and spore production (σ)
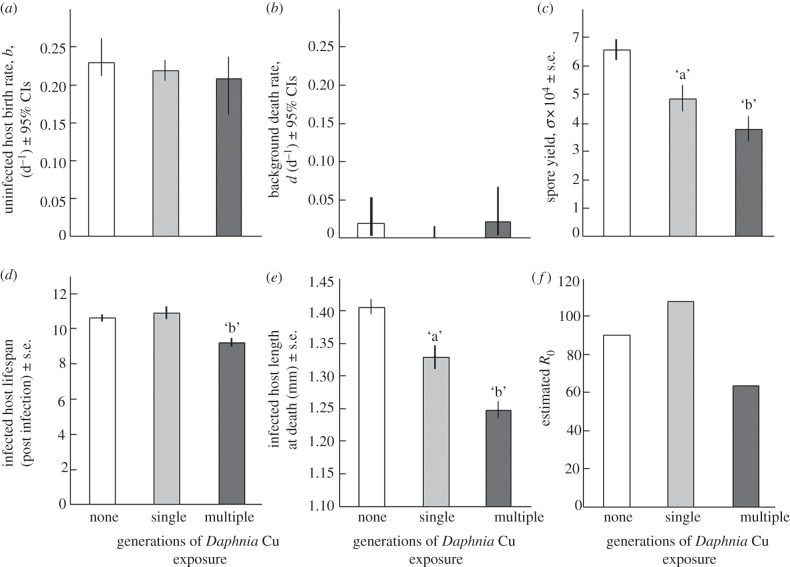
Birth and death rates of uninfected hosts did not differ among the three contamination scenarios (figure 2a,b). However, copper contamination had profound effects on infected individuals. Single-generation exposure reduced spore yield in infected hosts (planned contrast: p = 0.002; figure 2c). Multi-generational exposure further depressed spore yield relative to the single-generation treatment (planned contrast: p = 0.045). Exposure to copper during the assay did not decrease lifespan of naive infected hosts (planned contrast: p = 1; figure 2d), but hosts exposed to multi-generational contamination died earlier than those experiencing only single-generation exposure (planned contrast: p < 10−5). Single-generation contamination reduced the size at death of infected naive hosts (planned contrast: p < 10−4; figure 2e). Multi-generational exposure exacerbated this effect (planned contrast: p = 10−4). Spore yield significantly increased with lifespan (multiple linear regression: n = 86, p < 10−10) and length at death (n = 86, p < 10−6; electronic supplementary material, figure A2). Together, both factors explained 52.4 per cent of the variation in spore yield; 24.7 per cent of the variation is attributable to lifespan alone when controlling for length, 11.5 per cent was explained by length alone when controlling for lifespan, and the remaining 16.1 per cent could be explained by either factor.
Figure 2.
Parameter estimates derived for three contamination scenarios. Naive lineages of Daphnia were not exposed (‘none’, white bars) or were exposed (‘single’, grey bars) to copper during the life table experiment; meanwhile, Daphnia from exposed lineages also received copper during the experiment (‘multiple’, dark grey). (a) Cu treatments did not affect the birth rate or (b) death rate of uninfected Daphnia. (c–e) However, contamination significantly affected three traits of infected hosts (letters ‘a’ and ‘b’ denote significant differences between no exposure–single generation and single-generation–multi-generational exposure comparisons): (c) spore yield, (d) lifespan and (e) length at death. (f) Compared with the no contamination scenario, single-generation contamination increased the parasite's R0, whereas multi-generational contamination decreased it. R0 calculated with transmission rate (β) from figure 1a; also c = 0.01 and m = 0.75.
(b). Population-level predictions
Contamination for a single generation increased R0 by 19.8 per cent relative to uncontaminated conditions (figure 2f) largely because β increased 51.3 per cent (figure 1a; compare 0 and 200 Cu treatments), but σ decreased only 25.5 per cent (figure 2c). However, multi-generational contamination increased β by 42.0 per cent (figure 1a; compare 0 Cu and 200 multi-treatments), but reduced σ by 42.3 per cent (figure 2c). In this case, R0 decreased 20.0 per cent relative to uncontaminated conditions (figure 2f).
(c). Population-level experiment
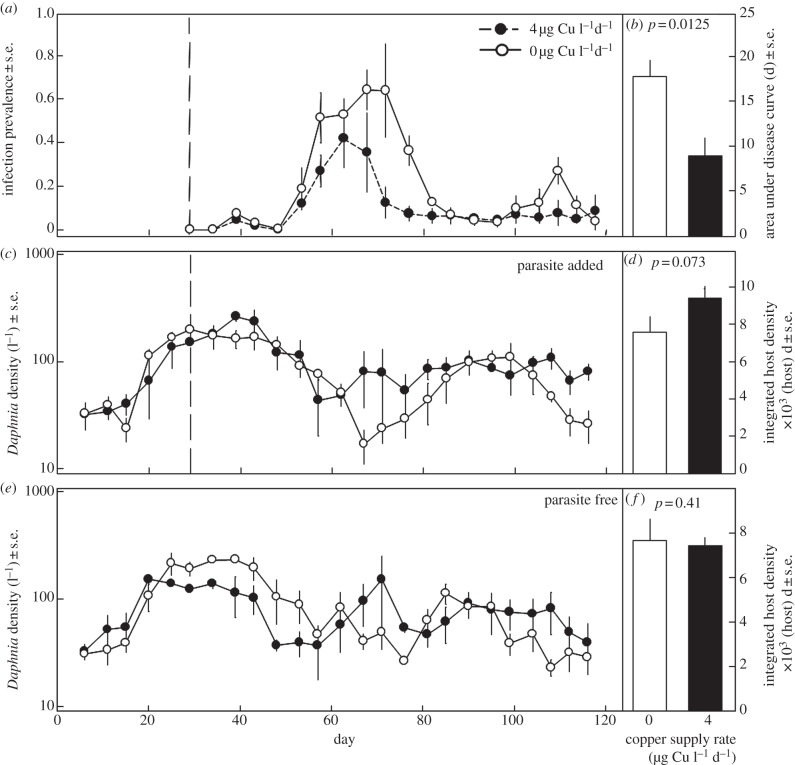
Infection prevalence peaked roughly 30–40 days following parasite addition to maxima that ranged from 20 to 91 per cent (figure 3a). Daphnia population densities varied from approximately 10–300 l−1 throughout the experiment (figure 3c,e). Dissolved copper concentrations remained less than or equal to 200 μg l−1 throughout the experiment (see electronic supplementary material, appendix and figure A3). Thus, Daphnia in these populations were exposed to sublethal levels of contamination throughout the experiment. Contamination reduced the size of fungal epidemics (one-tailed p = 0.0125; figure 3b). Following parasite addition, contaminated aquaria—which had smaller epidemics—tended to have higher integrated densities of hosts (one-tailed p = 0.073; figure 3c,d). By contrast, in aquaria without the parasite, copper contamination did not significantly reduce integrated density of hosts (one-tailed p = 0.42; figure 3e,f).
Figure 3.
Results of the population-level experiment. (a) Infection prevalence through time. (b) Copper contamination reduced the total size of epidemics in Daphnia populations. Host density through time for (c) the parasites-added and (e) parasite-free treatments. (d) Contamination caused a marginally significant increase in the integrated density of hosts in aquaria with the parasite. However, (f) contamination alone did not affect the integrated density of hosts with the parasite. The dashed line in (a) and (c) indicates the date of parasite introduction.
4. Discussion
Copper exerted strong but opposing effects on traits predicted to govern disease spread. Contamination increased disease transmission, which should exacerbate epidemics. This occurred because contamination stimulated consumption of parasites by hosts. Such contaminant-mediated exposure to parasites may apply broadly because trophic acquisition of infection by animal hosts arises commonly [30,31]. Other contaminants, e.g. cadmium, often reduce animal foraging rates [32]. These pollutants should decrease transmission rate (all else equal). Although we have strong evidence for the foraging-transmission mechanism here, we cannot determine whether copper also impaired host immunity. Many contaminants suppress immunity [33]; if suppressed immunity operated simultaneously with the foraging rate mechanism, then it could further increase infection prevalence. However, our infection experiment could not assess immunotoxicity. Finally, while short-term contamination increased transmission, multi-generational contamination did not elevate it further.
Although copper increased transmission, it reduced parasite reproduction in infected hosts, potentially inhibiting disease spread. Two mechanisms caused this effect. First, infected hosts died earlier when confronted with otherwise non-lethal copper doses. Parasites had less time to reproduce within these hosts. Because many contaminants reduce the lifespan of infected hosts [8,34], contaminants should inhibit disease spread by limiting transmission potential [7]. Second, infected hosts that were exposed to copper reached smaller sizes. Small host size slows parasite production owing to links among size, feeding rate and the availability of energy or nutrients for the parasite [13]. Multi-generational exposure of Daphnia to copper exacerbated these effects—it produced even smaller infected hosts yielding even less spores—but exposure history of parasites to contamination did not affect these traits.
These contaminant-modulated traits should exert opposing influence on disease spread, so we synthesized their net effect with an epidemiological model. Epidemics should be largest under contamination scenarios that maximize R0, the indicator of parasite success. Among our three contamination scenarios (none, single generation and multiple generation), R0 was maximized under single-generation contamination. In this case, elevated transmission sufficiently offsets the decrease in spore yield from infected hosts. However, multi-generational contamination caused a much larger reduction in spore yield. This reduction overwhelmed the increase in transmission owing to contamination; therefore, it reduced R0 relative to uncontaminated conditions. This strong multi-generational effect on spore production, then, drove distinct predictions for disease in chronically contaminated environments.
We tested the prediction that chronic contamination would inhibit epidemics in Daphnia populations with a population-level experiment. Contamination reduced the total size of epidemics in Daphnia populations by 50 per cent relative to epidemics occurring in uncontaminated conditions. This reduction in epidemic size matters because larger epidemics typically reduce densities of Daphnia more severely [35]. Indeed, Daphnia populations experienced a smaller parasite-induced decline in densities in copper contaminated tanks with smaller epidemics. Overall, these results supported our predictions and illustrated the importance of multi-generational effects for disease spread. Transgenerational effects on traits such as size/quality, immunity and abiotic stress resistance are widespread [11,36,37]. Thus, multi-generational effects might shape disease dynamics in variety of contaminated systems.
The parametrized model demonstrates the potential for theory to predict the consequences of pollution for disease. Still, several modifications could extend our understanding of contamination and disease in natural populations. First, we took a ‘classic ecological’ approach and determined the average traits of a single host genotype and parasite isolate. This strategy enhanced our general but mechanistic focus on transgenerational and physiological effects of contaminants on disease spread. Using our design, the relevance of the multi-generational effects could more clearly emerge in the population-level experiment. In a next step, we could extend this contamination framework to encompass genetic variation of hosts and parasites. This expansion might prove important because genetic variation among hosts or parasite genotypes in toxicological traits [38,39] could lead to diverse responses to contamination within and among populations. Furthermore, rapid evolution of hosts during epidemics [21] and/or to contamination [38,39] might change the predictions offered here—especially if tradeoffs exist between toxicological and epidemiological traits among host genotypes [38,39]. Second, we viewed contamination scenarios as distinct, yet constant, environments, which provided a useful qualitative prediction here. However, contamination can be dynamic and multi-generational effects accumulate. Therefore, future models could incorporate toxicokinetics [40] and track parental effects during temporally variable contamination scenarios [41]. Third, the model could include the community ecology of disease. Other species, such as predators or competitors of hosts and parasites, can influence disease spread [42]. These models could link disease prevalence to the toxic effects of contaminants on the traits or densities of other interacting species [43].
Concern grows over possible links between toxic chemicals and disease in many systems [1–3]. However, it remains challenging to predict the net effects of pollutants on disease spread. Here, we relied on an epidemiological model to synthesize the contrasting effects of copper contamination on key epidemiological traits (transmission versus parasite production). The model predicted that chronic copper contamination can actually inhibit epidemics, largely owing to multi-generational effects of contamination on parasite reproduction. Overall, the data–model combination illustrated here provides a more mechanistic framework for understanding disease in contaminated environments. Such frameworks can facilitate the assessment and mitigation of harmful effects of these two stressors in natural populations.
Acknowledgements
We thank K. Boatman, Z. Brown, C. Howland and J. Lawitschka for assistance and C. Cáceres for comments on the manuscript. This research was supported by the National Science Foundation (grant no. DEB 0614316, 0614361, 1010929) and a STAR fellowship from the US EPA (D.J.C.). We appreciate cooperation from S. Siscoe at the Indiana DNR's Division of Forestry.
References
- 1.Lafferty K. D., Porter J. W., Ford S. E. 2004. Are diseases increasing in the ocean? Ann. Rev. Ecol. Evol. Syst. 35, 31–54 10.1146/annurev.ecolsys.35.021103.105704 (doi:10.1146/annurev.ecolsys.35.021103.105704) [DOI] [Google Scholar]
- 2.Schwarzenbach R. P., Escher B. I., Fenner K., Hofstetter T. B., Johnson C. A., Von Gunten U., Wehrli B. 2006. The challenge of micropollutants in aquatic systems. Science 313, 1072. 10.1126/science.1127291 (doi:10.1126/science.1127291) [DOI] [PubMed] [Google Scholar]
- 3.Van Bressem M.-F., et al. 2009. Emerging infectious diseases in cetaceans worldwide and the possible role of environmental stressors. Dis. Aquat. Organ. 86, 143–157 10.3354/dao02101 (doi:10.3354/dao02101) [DOI] [PubMed] [Google Scholar]
- 4.Khan R. A. 1990. Parasitism in marine fish after chronic exposure to petroleum hydrocarbons in the laboratory and to the Exxon Valdez oil spill. Bull. Environ. Contam. Toxicol. 44, 759–763 10.1007/BF01701799 (doi:10.1007/BF01701799) [DOI] [PubMed] [Google Scholar]
- 5.Rohr J. R., et al. 2008. Agrochemicals increase trematode infections in a declining amphibian species. Nature 455, 1235–1239 10.1038/nature07281 (doi:10.1038/nature07281) [DOI] [PubMed] [Google Scholar]
- 6.Lafferty K. D., Kuris A. M. 1999. How environmental stress affects the impacts of parasites? Limnol. Oceanogr. 44, 925–931 10.4319/lo.1999.44.3_part_2.0925 (doi:10.4319/lo.1999.44.3_part_2.0925) [DOI] [Google Scholar]
- 7.Lafferty K. D., Holt R. D. 2003. How should environmental stress affect the population dynamics of disease? Ecol. Lett 6, 654–664 10.1046/j.1461-0248.2003.00480.x (doi:10.1046/j.1461-0248.2003.00480.x) [DOI] [Google Scholar]
- 8.Chou H. Y., Peng T. Y., Chang S. J., Hsu Y. L., Wu J. L. 1999. Effect of heavy metal stressors and salinity shock on the susceptibility of grouper (Epinephelus sp.) to infectious pancreatic necrosis virus. Virus Res. 63, 121–129 10.1016/S0168-1702(99)00065-9 (doi:10.1016/S0168-1702(99)00065-9) [DOI] [PubMed] [Google Scholar]
- 9.Griggs J., Belden L. 2008. Effects of Atrazine and metolachlor on the survivorship and infectivity of Echinostoma trivolvis; Trematode Cercariae. Arch. Environ. Contam. Toxicol. 54, 195–202 10.1007/s00244-007-9029-x (doi:10.1007/s00244-007-9029-x) [DOI] [PubMed] [Google Scholar]
- 10.Rohr J. R., Raffel T. R., Sessions S. K., Hudson P. J. 2008. Understanding the net effects of pesticides on amphibian trematode infections. Ecol. Appl. 18, 1743–1753 10.1890/07-1429.1 (doi:10.1890/07-1429.1) [DOI] [PubMed] [Google Scholar]
- 11.Marshall D. J. 2008. Transgenerational plasticity in the sea: context-dependent maternal effects across the life history. Ecology 89, 418–427 10.1890/07-0449.1 (doi:10.1890/07-0449.1) [DOI] [PubMed] [Google Scholar]
- 12.Ebert D. 2005. Ecology, epidemiology, and evolution of parasitism in Daphnia. Bethesda, MD: National Library of Medicine (US), National Center for Biotechnology Information [Google Scholar]
- 13.Hall S. R., Simonis J. L., Nisbet R. M., Tessier A. J., Caceres C. E. 2009. Resource ecology of virulence in a planktonic host-parasite system: an explanation using dynamic energy budgets. Am. Nat. 174, 149–162 10.1086/600086 (doi:10.1086/600086) [DOI] [PubMed] [Google Scholar]
- 14.van Hullebusch E., Chatenet P., Deluchat V., Chazal P. M., Froissard D., Botineau M., Ghestem A., Baudu M. 2003. Copper accumulation in a reservoir ecosystem following copper sulfate treatment (St Germain Les Belles, France). Water, Air Soil Pollut. 150, 3–22 10.1023/A:1026148914108 (doi:10.1023/A:1026148914108) [DOI] [Google Scholar]
- 15.Hutchinson T. C., Havas M. 1986. Recovery of previously acidified lakes near Coniston, Canada following reductions in atmospheric sulphur and metal emissions. Water, Air, Soil Pollut. 28, 319–333 10.1007/BF00583498 (doi:10.1007/BF00583498) [DOI] [Google Scholar]
- 16.Bossuyt B. T. A., Janssen C. R. 2004. Influence of multigeneration acclimation to copper on tolerance, energy reserves, and homeostasis of Daphnia magna straus. Environ. Toxicol. Chem. 23, 2029–2037 10.1897/03-377 (doi:10.1897/03-377) [DOI] [PubMed] [Google Scholar]
- 17.Hall S. R., Knight C. J., Becker C. R., Duffy M. A., Tessier A. J., Caceres C. E. 2009. Quality matters: resource quality for hosts and the timing of epidemics. Ecol. Lett. 12, 118–128 10.1111/j.1461-0248.2008.01264.x (doi:10.1111/j.1461-0248.2008.01264.x) [DOI] [PubMed] [Google Scholar]
- 18.Fernández-González M. A., González-Barrientos J., Carter M.J., Ramos-Jiliberto J. 2011. Parent-to-offspring transfer of sublethal effects of copper exposure: metabolic rate and life-history traits of Daphnia. Rev. Chilena Hist. Nat. 84, 195–201 10.4067/S0716-078X2011000200005 (doi:10.4067/S0716-078X2011000200005) [DOI] [Google Scholar]
- 19.Anderson R. M., May R. M. 1991. Infectious diseases of humans. Oxford, UK: Oxford University Press [Google Scholar]
- 20.Hall S. R., Becker C. R., Duffy M. A., Cáceres C. E. 2010. Variation in resource acquisition and use among host clones creates key epidemiological trade-offs. Am. Nat. 176, 557–565 10.1086/656523 (doi:10.1086/656523) [DOI] [PubMed] [Google Scholar]
- 21.Duffy M. A., Sivars-Becker L. 2007. Rapid evolution and ecological host–parasite dynamics. Ecol. Lett. 10, 44–53 10.1111/j.1461-0248.2006.00995.x (doi:10.1111/j.1461-0248.2006.00995.x) [DOI] [PubMed] [Google Scholar]
- 22.Carius H. J., Little T. J., Ebert D. 2001. Genetic variation in host–parasite association: potential for coevolution and frequency-dependent selection. Evolution 55, 1136–1145 [DOI] [PubMed] [Google Scholar]
- 23.Luijckx P., Ben-Ami F., Mouton L., Du Pasquier L., Ebert D. 2011. Cloning of the unculturable parasite Pasteuria ramosa and its Daphnia host reveals extreme genotype-genotype interactions. Ecol. Lett. 14, 125–131 10.1111/j.1461-0248.2010.01561.x (doi:10.1111/j.1461-0248.2010.01561.x) [DOI] [PubMed] [Google Scholar]
- 24.Baer K. N., Goulden C. E. 1998. Evaluation of a high-hardness COMBO medium and frozen algae for Daphnia magna. Ecotoxicol. Environ. Saf. 39, 201–206 10.1006/eesa.1997.1627 (doi:10.1006/eesa.1997.1627) [DOI] [PubMed] [Google Scholar]
- 25.Sarnelle O., Wilson A. E. 2008. Type III functional response in Daphnia. Ecology 89, 1723–1732 10.1890/07-0935.1 (doi:10.1890/07-0935.1) [DOI] [PubMed] [Google Scholar]
- 26.Hilborn R., Mangel M. 1997. The ecological detective: confronting models with data. Princeton, NJ: Princeton University Press [Google Scholar]
- 27.McCallum H. 2000. Population parameters: estimation for ecological models. Oxford, UK: Wiley-Blackwell [Google Scholar]
- 28.Sokal R. R., Rohlf F. J. 1995. Biometry, 3rd edn New York, NY: WH Freeman [Google Scholar]
- 29.Legendre P., Legendre L. 1998. Numerical ecology. Amsterdam, The Netherlands: Elsevier Science [Google Scholar]
- 30.Arneberg P., Skorping A., Grenfell B., Read A. F. 1998. Host densities as determinants of abundance in parasite communities. Proc. R. Soc. Lond. B 265, 1283–1289 10.1098/rspb.1998.0431 (doi:10.1098/rspb.1998.0431) [DOI] [Google Scholar]
- 31.Parker B. J., Elderd B. D., Dwyer G. 2010. Host behaviour and exposure risk in an insect-pathogen interaction. J. Anim. Ecol. 79, 863–870 10.1111/j.1365-2656.2010.01690x (doi:10.1111/j.1365-2656.2010.01690x) [DOI] [PubMed] [Google Scholar]
- 32.Kooijman S. 2009. Dynamic energy and mass budgets in biological systems. Cambridge, UK: Cambridge University Press [Google Scholar]
- 33.Galloway T., Handy R. 2003. Immunotoxicity of organophosphorous pesticides. Ecotoxicology 12, 345–363 10.1023/A:1022579416322 (doi:10.1023/A:1022579416322) [DOI] [PubMed] [Google Scholar]
- 34.Coors A., De Meester L. 2011. Fitness and virulence of a bacterial endoparasite in an environmentally stressed crustacean host. Parasitology 138, 122–131 10.1017/S0031182010000995 (doi:10.1017/S0031182010000995) [DOI] [PubMed] [Google Scholar]
- 35.Hall S. R., Becker C., Duffy M. A., Cáceres C. 2011. Epidemic size determines population-level effects of fungal parasites on Daphnia hosts. Oecologia 166, 833–842 10.1007/s00442-011-1905-4 (doi:10.1007/s00442-011-1905-4) [DOI] [PubMed] [Google Scholar]
- 36.Grindstaff J. L., Brodie E. D., Ketterson E. D. 2003. Immune function across generations: integrating mechanism and evolutionary process in maternal antibody transmission. Proc R. Soc. Lond. B 270, 2309–2319 10.1098/rspb.2003.2485 (doi:10.1098/rspb.2003.2485) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ben-Ami F., Ebert D., Regroes R. 2010. Pathogen dose infectivity curves as a method to analyze the distribution of host susceptibility: a quantitative assessment of maternal effects after food stress and pathogen exposure. Am. Nat. 175, 106–115 10.1086/648672 (doi:10.1086/648672) [DOI] [PubMed] [Google Scholar]
- 38.Jansen M., Coors A., Stoks R., De Meester L. 2011. Evolutionary ecotoxicology of pesticide resistance: a case study. Ecotoxicology 20, 543–551 10.1007/s10646-011-0627-z (doi:10.1007/s10646-011-0627-z) [DOI] [PubMed] [Google Scholar]
- 39.Jansen M., Stoks R., Coors A., van Doorslaer W., De Meester L. 2011. Collateral damage: rapid exposure-induced evolution of pesticide resistance leads to increased susceptibility to parasites. Evolution 65, 2681–2691 10.1111/j.1558-5646.2011.01331.x (doi:10.1111/j.1558-5646.2011.01331.x) [DOI] [PubMed] [Google Scholar]
- 40.Kooi B. W., Bontje D., van Voorn G. A. K., Kooijman S. A. L. M. 2008. Sublethal toxic effects in a simple aquatic food chain. Ecol. Model. 212, 304–318 10.1016/j.ecolmodel.2007.10.042 (doi:10.1016/j.ecolmodel.2007.10.042) [DOI] [Google Scholar]
- 41.Ginzburg L. R. 1998. Inertial growth: population dynamics based on maternal effects. In Maternal effects as adaptations (eds Mousseau T., Fox C.), pp. 42–53 Oxford, UK: Oxford University Press [Google Scholar]
- 42.Keesing F., Holt R. D., Ostfeld R. S. 2006. Effects of species diversity on disease risk. Ecol. Lett. 9, 485–498 10.1111/j.1461-0248.2006.00885.x (doi:10.1111/j.1461-0248.2006.00885.x) [DOI] [PubMed] [Google Scholar]
- 43.Relyea R., Hoverman J. 2006. Assessing the ecology in ecotoxicology: a review and synthesis in freshwater systems. Ecol. Lett. 9, 1157–1171 10.1111/j.1461-0248.2006.00966.x (doi:10.1111/j.1461-0248.2006.00966.x) [DOI] [PubMed] [Google Scholar]