Abstract
Here, we test whether the increase in tooth height in insular endemics results from the expansion of the dietary niche under resource limitation, as widely considered, or whether it represents an investment in dental durability in response to the selection for extended longevity under low levels of extrinsic mortality. We tested these hypotheses in the extremely hypsodont fossil bovid Myotragus balearicus from the Balearic Islands, an ideal model to study the evolutionary trends on islands. Dental abrasion was significantly lower in the insular bovid than in highly hypsodont continental artiodactyls, suggesting that feeding habits are not the sole driving force behind increased crown height. However, the estimated longevity for M. balearicus based on dental durability was two times that predicted from body mass. Survivorship curves confirm that an extraordinarily large number of individuals approached the longevity of the species. Our results, hence, provide evidence that hypsodonty in insular endemics is the outcome of selection for increased durability of the permanent dentition in association with an extended lifespan. In the context of insularity, our results lend additional support to the disposable soma theory of ageing confirming the dependency of somatic maintenance and repair on lifespan, and its control by resource availability and extrinsic mortality.
Keywords: ageing, lifespan, life-history theory, disposable soma theory, tooth height, dental abrasion
1. Introduction
Understanding differences in longevity between species is a major question in evolutionary biology, because lifespan is one of the most influential life-history traits related to individual fitness [1–3]. The evolutionary theory of ageing predicts that the level of extrinsic mortality exerts a direct influence on the rate of senescence (a decline in fitness with advancing age) and on the longevity (maximum lifespan) of organisms [4–6]. On the basis of the weakening of natural selection with increasing age [1,7], this theory predicts that high levels of extrinsic mortality select for a faster rate of ageing and a shorter reproductive lifespan. Conversely, the selection for postponed senescence (slower ageing) is predicted for populations with low extrinsic mortality, which in turn should increase the species’ longevity over evolutionary time [8–10].
Insular populations provide an excellent natural framework to look for the long-term evolution of selective traits [11,12]. Because insular faunas evolved under special selection pressures (low predation pressure, high intraspecific competition and resources limitation), they are predicted to share a series of unique traits irrespective of phylogeny (often called ‘island syndrome’) [13,14]. One of these traits is an increase in tooth height, typically molars, relative to their mainland ancestors, leading to accentuated hypsodonty (high-crowned teeth) in herbivores [15–17]. Hypsodonty in mammalian herbivores is generally found to be related to increased dental wear rate because of feeding habits [18–23]. Accordingly, the parallel evolution of hypsodonty in phylogenetically unrelated insular mammals is widely considered to result from the expansion of the dietary niche under resource limitation [16,24]. However, some recent work on herbivores suggests that increased tooth height may also evolve in response to shifts in life-history traits, specifically to an increase in reproductive lifespan/longevity [25–27], because tooth wear is the main proximal cause of senescence in mammalian herbivores [28,29]. This latter hypothesis is in agreement with the evolutionary theory of ageing, specifically the disposable soma theory [30], that predicts a relative increase in investment of metabolic resources in somatic maintenance and repair at the expense of investment in reproduction under low extrinsic mortality, and suggests a planned durability of somatic permanent structures that extend the length of the reproductive lifespan [1].
Here, we test whether the overall trend towards the increase in molar crown height in insular endemic mammals represents an investment in the durability of somatic structures (feeding apparatus) in response to the selection for a longer reproductive lifespan/longevity under low levels of extrinsic mortality that characterize insular populations [8,31], or whether crown height increase is related only to shifts in feeding habits as upheld by the prevailing hypothesis. We used the extremely hypsodont insular bovid Myotragus balearicus (Majorca, Balearic Islands, Spain) as a test species. We analysed dental mesowear and calculated molar wear rate as proxies for the relative degree of dental abrasion in this species, which was compared with the degree of hypsodonty to determine whether they give compatible signals. Moreover, we estimated molar durability to determine the potential lifespan (longevity) and analysed the level of extrinsic mortality through a comparative analysis of mortality patterns.
The fossil genus Myotragus [32] (Bovidae, Caprinae) is an ideal model to study evolutionary trends on islands [14,33,34]. Myotragus balearicus is the terminal species of this endemic genus that survived under complete geographical isolation for over 5 million years, from the Pliocene to the Holocene, until its extinction more than 3000 years ago [14]. During its evolution, the genus Myotragus underwent significant changes in body design that essentially affected the locomotor system and body size (dwarfism) [35], as well as changes in the nervous system (brain size and sense organs reduction) [14] and in the feeding apparatus [36]. Changes in dentition are characterized by the progressive reduction of the number of incisors and premolars, the acquisition of evergrowing incisors (hypselodonty) and the increase in the degree of hypsondonty in all teeth [37]. Myotragus balearicus presents an extremely modified lower jaw dentition that is not shared by any other known extant Caprinae. Adults of this species display a single rodent-like hypselodont incisor, a single premolar and three highly hypsodont molars per jaw. This type of dentition has traditionally been interpreted as a functional adaptation to increase feeding efficiency on an abrasive diet [37,38]. While hypselodonty is a trait essentially restricted to Myotragus (as well as Maremmia lorenzi, a Late Miocene antelope of Tuscany), increase in molar crown height is a common morphological trend in insular faunas [16]. That the extreme hypsodonty of M. balearicus is not due to a phylogenetic effect is evidenced by the crown height of the earliest species of the genus that is similar to the mainland relatives (see the electronic supplementary material, figure S1).
2. Material and methods
(a). Dental mesowear
We analysed dental mesowear in M. balearicus from material housed at Institut Català de Paleontologia Miquel Crusafont and belonging to several sites of Majorca (Cova Moleta, Cova de Llenaire, Es Bufador, Son Bouzà, Son Maiol and Cova Estreta de Gabellí). Dental mesowear [39] was used as a method to provide insights into the abrasiveness of the items eaten by M. balearicus. Also, its dietary trait was investigated based on the assumption that, essentially, low-abrasion signatures are typical of browsers, abrasion-dominated signatures are associated with grazing and intermediate signatures are commonly recognized in mixed feeders [39–42]. Whenever possible, occlusal relief and cusp shape were examined in all available upper third molars because of the considerable degree of wear of the upper M1s and M2s. Thus, our sample comprises 17 specimens after excluding unworn teeth and specimens with broken cusp apices. A comparative database composed by 54 well-known extant ungulates was used as a reference, and conservative and radical dietary classifications were employed according to Fortelius & Solounias [39]. Subsequently, statistical analyses were performed using SPSS v. 11.5 following DeMiguel et al. [42,43].
(b). Dental wear rate and durability
Dental wear rate and dental durability in lower first and third molars were estimated by using regression of molar crown height (the height of the enamel on the buccal side of the tooth) on age estimated from counts of cementum annuli of the same tooth [20]. It is widely established that the counting of growth layers in dental cementum provides reliable estimates for age at death in mammals [44]. Cementum layers were quantified in longitudinal thin sections of isolated lower molars. The best area of the section for counting the layers was the cervical region where the layers begin to spread. These analyses were performed on both M1s and M3s. First molars are widely used to estimate tooth wear, while the durability of the third molar is known to be tightly correlated with lifespan and animal's performance [20,27]. A total of 22 teeth were sectioned following the standard procedures in our laboratory of thin/ground sections [34]. Although the cementum lines were well defined in some of the teeth (see the electronic supplementary material, figure S2), in others they were too weak to be useful for our study. Therefore, the final sample size was 13 molars (electronic supplementary material, table S1). The material used for this analysis comes from two sites in Majorca: Cova Moleta, housed at Museu Balear de Ciències Naturals and Cova de Llenaire, housed at Institut Català de Paleontologia Miquel Crusafont.
Wear rate was obtained by the slope of the linear regression between crown height and age. The x-intercept of the regression line gives an estimate of dental durability (the time expected for a molar to wear down completely) [20]. Previous studies have shown that molar wear rates and durability differ between sexes in extant herbivores [25,29]; however, our sample could not be sexed because of obvious reasons (isolated teeth). On the basis of the fact that growth of the cementum layers begins with dental eruption [44], longevity was determined by adding the molar eruption times [34] to the molar durability. The scaling relationship between longevity and body size was represented for the family of bovidae using 115 species, including M. balearicus [45]. A mean body mass of 26 kg in M. balearicus was calculated by multiple regressions of six articular dimensions from material of different sites [14].
(c). Mortality pattern
For the analysis of mortality pattern, we gathered data of molar stage of eruption and wear [46] in mandibles from two sites in Majorca (Cova Estreta, n = 81 and Cova des Moro, n = 131) with natural accumulations of M. balearicus material. These caves may have served as a refuge for these animals, which is a habitual behaviour in certain ruminants [16,47], leading to the accumulation of abundant fossil material inside the caves. The material is housed at Institut Mediterrani d'Estudis Avançats. Absolute age at death of young specimens was determined by the stage of molar eruption based on Jordana & Köhler [34]. For age at death estimation of adult specimens (third molar erupted), third lower molar wear stages [46] were regressed against age estimated by cementum layers on isolated teeth. We obtained a good linear fit (r2 = 0.83) that allowed the establishment of five year age intervals for the wear stages. This method was applied to the adult mandible samples in order to estimate absolute age at death. Survivorship curves of M. balearicus were calculated from the age distribution in the two sites, following the study of Pianka [48]. For comparative purposes, survivorship curves of extant bovid populations were also calculated, including Ovis dalli [49], Bos gaurus [50], Syncerus caffer [51] and Hemitragus jemlahicus [52].
3. Results
Regarding mesowear, teeth from M. balearicus are characterized by a predominance of sharp cusps (67.74%) and high relief (75%), which presumably excludes an abundance of highly abrasive material in their diet such as phytolith-rich grasses. Teeth also exhibit significant proportions of rounded cusps (29.03%). Finally, the incidence of low occlusal relief (25%) and blunt apices (3.23%) suggest that M. balearicus fed on a larger amount of abrasive material than typical browsers.
Cluster analyses were applied as an explorative technique for identifying species groups and emphasizing any inherent structure based on similarities in mesowear signature (see the electronic supplementary material figure S3 for further explanation). In a first explorative analysis (see the electronic supplementary material, figure S3a), M. balearicus is grouped in a cluster containing all traditional mixed feeders from the comparative database, thus indicating moderate levels of abrasives in their diet. In a more rigorous analysis (see the electronic supplementary material, figure S3b), M. balearicus is grouped with traditional mixed feeders and undisputed browsers.
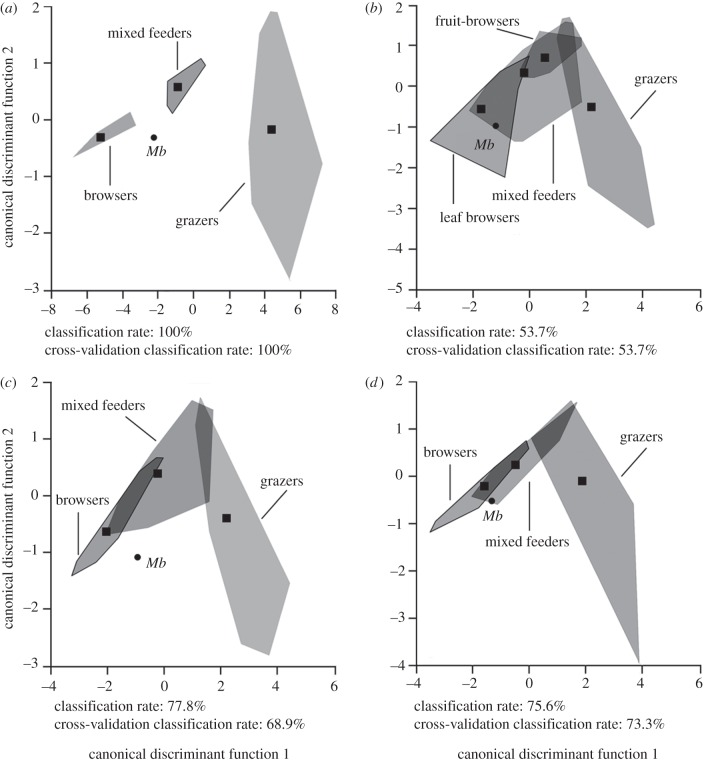
Results from the discriminant analyses show a very high discrimination among the 27 ‘typical’ extant species, thus displaying a correct classification rate of 100 per cent. Myotragus balearicus was placed in the category of mixed feeders (figure 1a). When its wear signature is compared with the full dataset, the mean percentage correctly classified was only 53.7 per cent (29 of 54 species), with M. balearicus clearly not fitting the range of mixed feeders, but that of leaf browsers (figure 1b). Removing the ‘MABRA’ group (for ‘minute abraded brachydonts’; see Fortelius & Solounias [39] for further explanation) notably contributed to increasing the correct classification rate for both the conservative (77.8%; figure 1c) and the radical (75.6%; figure 1d) dietary classifications. The most probable dietary assignment for M. balearicus corresponds to a browser according to both classifications.
Figure 1.
Bivariate diagrams based on discriminant analyses. Grey areas indicating the morphospace defined by the dental mesowear signatures of extant ungulates, and squares representing centroids. Distribution of Myotragus balearicus (Mb) based on mesowear signatures with (a) a set of ‘typical’ species, and with (b) a larger dataset with ‘MABRA’ and without ‘MABRA’ using (c) conservative and (d) radical classifications. Outlined morphospaces indicate the dietary assignation obtained for M. balearicus in each analysis.
The decrease of molar crown height over time during ontogeny is shown in figure 2. Because of the very good fits (r2 > 0.9), we used a linear model, although this relationship has been reported to be nonlinear in other ungulates [20,25,29]. The slopes of the linear regressions give an estimate of the wear rate. First molar wear rate in M. balearicus (2.32 mm yr−1) is higher than the average wear rate in browsers (0.33 mm yr−1) [20] and in mixed feeders (1.22 mm yr−1) [20]. Nevertheless, it is lower than wear rates in extant highly hypsodont artiodactyls, such as grazers averaged (2.93 mm yr−1) [20] or Antilocapra americana (4.19 mm yr−1) [52], which is a mixed feeder that consumes a large amount of soil just like grazers [21].
Figure 2.
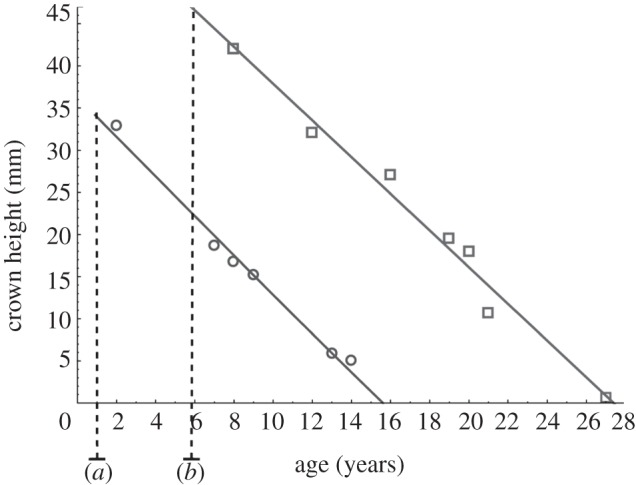
Regression of molar crown height on age estimated from counts of cementum annuli. M1: r2 = 0.99, slope = 2.32; M3: r2 = 0.98, slope = 2.17. For age estimations, we added molar eruption times [34] to the number of cementum annuli: (a) age of M1 eruption, (b) age of M3 eruption. The slopes of the linear regression are estimates of molar wear rate, while the x-intercepts give an estimation of dental durability. Circles with solid line, M1; squares with solid line, M3.
The durability of third molars in M. balearicus (21 years), which are also the highest crowned teeth, was higher than that of the first ones (15 years). When the data of molar eruption times is taken into account, the results show that the third molar retains high functionality (11 years) long after the first molar has been completely worn, which occurs at the age of 16 years. Depletion of the third molar crown occurs after the age of 27 years. The tight matching between the height of the molars in a mandible measured on X-ray images and the height obtained using the linear regressions for a given age corroborates the results (see the electronic supplementary material, figure S4).
The potential lifespan (longevity) in M. balearicus estimated by dental durability (27 years) coincides with the minimum age of 27 years determined by Marín-Moratalla et al. [54] using bone skeletochronology. This exceptional longevity is about two times that predicted from average body mass (26 kg) [14], fitting the upper limit of the family of bovidae (figure 3).
Figure 3.
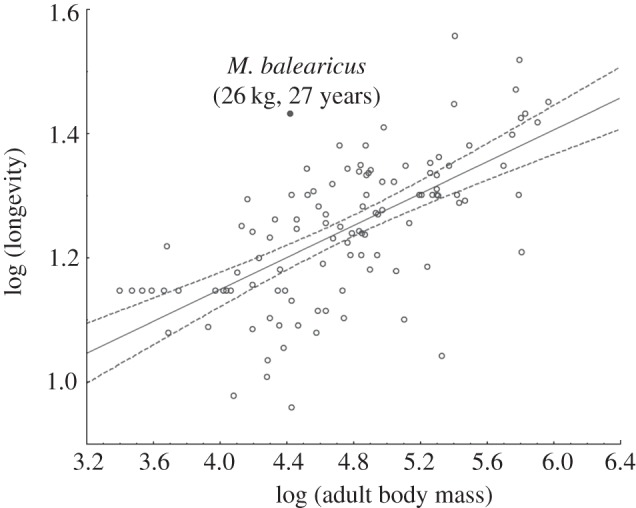
Longevity plotted against body mass for 115 species of bovidae with individual body masses ranging from 2.5 (Madoqua piacentinii) to 929.5 kg (Bubalus bubalis). Slope = 0.13, r2 = 0.41.
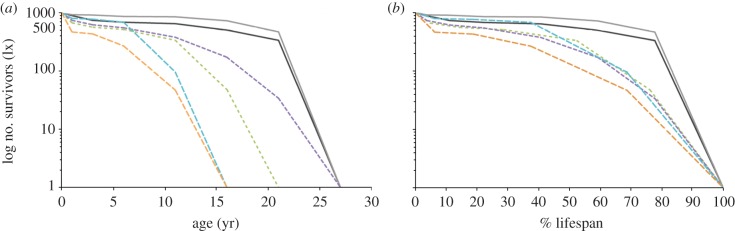
The age profiles from two sites (caves) with natural accumulation of M. balearicus material (Cova Estreta and Cova des Moro) are clearly U-shaped (figure 4), which is typical of ‘attritional’ mortality, as opposed to ‘catastrophic’ mortality, validating the data for demographic analyses. Survivorship curves (figure 5) in M. balearicus populations belong to type I that is low mortality up to a certain age, usually an adult stage, followed by a fairly steep increase in mortality [48]. Our M. balearicus populations show higher juvenile and adult survival rates than extant bovid populations, with a large proportion of the populations surviving to old ages, indicating a slower rate of senescence than in mainland bovids. The survivorship curves provide evidence that both fossil insular populations were approaching their potential lifespan. These results are consistent with evolutionary theory in that senescence is delayed in populations that suffer relatively low extrinsic mortality [6].
Figure 4.
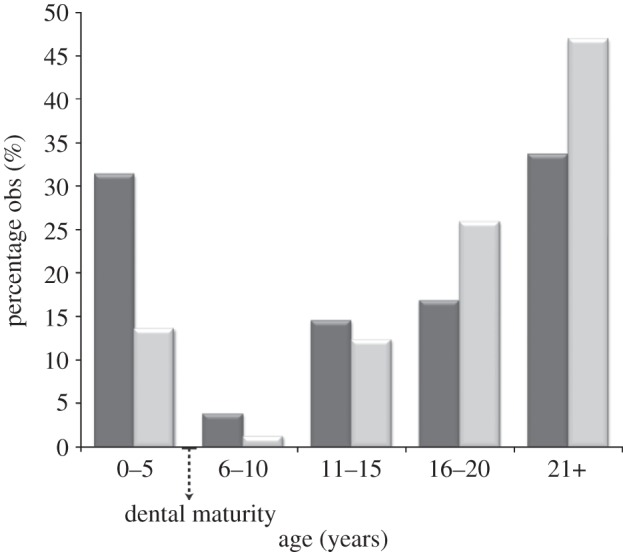
Age distribution of M. balearicus from right mandibles in two sites of Majorca. Dental maturity [34] indicates the age at somatic maturity. Dark grey bars, Cova des Moro (n = 131); light grey bars, Cova Estreta (n = 81).
Figure 5.
Semilogarithmic plots of survivorship curves of M. balearicus and extant bovids populations, the African buffalo (Syncerus caffer), the Gaur (Bos gaurus), the Dall sheep (Ovis dalli) and the Himalayan Tahr (Hemitragus jemlahicus). (a) Absolute age at death, (b) percentage of lifespan. Solid black line, M. balearicus (Cova des Moro); grey line, M. balearicus (Cova Estreta); green dashed line, Syncerus caffer; purple dashed line, Bos gaurus; blue dashed line, Ovis dalli; orange dashed line, Hemitragus jemlahicus.
4. Discussion
In this study, we tested whether the parallel evolution of hypsodonty in insular mammals is associated with an increase in longevity as predicted for insular populations because of their lower extrinsic mortality (the evolutionary theory of ageing), or whether it is related to expansion of the dietary niche under resource limitation (the prevailing hypothesis). We used the endemic goat-like bovid M. balearicus, a fossil species that survived for a long geological time span under the characteristic ecological conditions of islands [12,13]. The pristine laboratory-like environmental conditions unaltered by anthropogenic interventions make the genus Myotragus an ideal model to study evolutionary trends in life-history traits on islands [33,34,54].
The two hypotheses, not mutually exclusive, attempt to explain the increase in relative molar crown height (hypsodonty) in mammalian herbivores as follows: (i) the food habits hypothesis suggests increase in molar height to be an adaptation to resist high rates of tooth wear during food processing, whatever the reason for the abrasive nature [19,20,23]; and (ii) the life-history hypothesis predicts molar height to be related to expected longevity and to be independent of dental wear rate variation [25–27].
The food habits hypothesis is based on three major causal agents (see a review in Damuth & Janis [21]): (i) type of diet, grass or browse (grass is more rich in phytoliths than browse, thus is more abrasive), (ii) presence of soil particles when feeding at ground level in open habitats, and (iii) the amount of foot eaten per day. Generally, hypsodonty appears to be associated with the occurrence of open and dry environments with extensive grasslands [19,21]. Despite the lack of specific palaeoenvironmental studies of the Plio-Pleistocene of Majorca, analyses on coprolites [38], cranial and dental morphology [38,55] and stable isotopes [56] in M. balearicus pointed towards essentially browsing habits, however, with an abrasive component in the diet [38]. These analyses are in agreement with our results on dental mesowear and wear rate, which indicate that M. balearicus fed on a larger amount of abrasive material than typical browsers. Results show that it could either be referred to as a browser that could well have consumed considerable levels of abrasives (more than expected for a typical browser) or a mixed feeder shifted towards the browse-dominated end of the continuum.
Attempts to explain the abrasiveness of the diet of this fossil bovid evoked adaptations to significantly enlarge the range of consumable plants and parts of plants such as roots, thus ingesting large amounts of soil, in response to resource limitation [16,37,38]. Actually, the evergrowing incisor in M. balearicus is usually related to increased grinding force [16]. Resource limitation in Majorca is evidenced by the low species diversity and the absence of predators. Only three mammalian species (the bovid Myotragus, an insectivore of the genus Nesiotites and a glirid of the genus Hypnomys), some birds and a series of reptiles and amphibians survived under the low levels of food supply imposed by the small geographical area of the island [35,36]. Hypsodonty in M. balearicus, therefore, is widely considered as an adaptation to cope with increasingly abrasive component in the diet.
Nevertheless, though high tooth wear could be among the causes underlying an increase in molar crown height, this alone is not enough to explain the outstanding hypsodonty in M. balearicus. Our results show that the wear signature in the diet of M. balearicus is significantly lower than in extant continental artiodactyls with a similar, or even lower, degree of hypsodonty than M. balearicus, such as the African mixed-feeders impala (Aepyceros melampus) and oribi (Ourebia ourebi) or the grazer American bison (Bison bison). The hypsodonty of lower third molars in two M. balearicus populations (5.15 ± 0.99 in Cova des Moro and 6.11 ± 0.63 in Cova Estreta) [55] falls within the category of ‘highly hypsodont’ according to Janis [19], typical only of grasslands grazers [21]. Actually, if hypsodonty is essentially a reflection of overall wear rate as Fortelius & Solounias [39] suggested, the degree of hypsodonty in the insular fossil bovid is higher than expected for its tooth wear signature. Hence, the feeding habits hypothesis alone cannot explain the evolution of hypsodonty in M. balearicus.
The life-history hypothesis on hypsodonty, which predicts a correlated evolution of tooth height and longevity [25,26], relies on the deleterious effects of tooth wear (dental senescence) on life-history parameters and the animal's performance, specifically on reproductive success and survival [27–29]. According to this hypothesis, an increase in crown height allows an increase in tooth durability independent of wear rate variation. While the extreme hypsodonty of M. balearicus cannot be explained alone by feeding habits because of the relative degree of dental abrasion, it makes sense in association with the estimated exceptional longevity, suggesting that selection favoured an increase in hypsodonty to extend the durability of the feeding apparatus over a longer lifespan. Our analysis of mortality patterns in two populations of M. balearicus shows that they were approaching their potential lifespan estimated by dental durability, providing support to the life-history hypothesis predicting hypsodonty to be related to expected longevity.
This confirms the result of a previous study on the enamel microstructure of the lower molars in M. balearicus [34]. In this work, we provided evidence of a relationship between the extreme molar crown height and the delay in timing and rates in crown formation and tooth eruption, which doubles that of extant bovids of similar body size. This slow pace of dental development in M. balearicus was coupled to a general delay in the growth period and, hence, in maturity [33]. This evolution towards a slow life history [57] has been suggested to result from changes in energy allocation from reproduction to growth and maintenance that facilitated survival in a resource-limited environment without predators [36].
Our results, hence, provide empirical evidence for a positive correlation between delayed maturity and extended reproductive lifespan/longevity under low levels of extrinsic mortality as predicted in the life-history theory [57] and are in agreement with the fundamental trade-off between reproduction and survival [6,9,58]. Some authors, however, suggested that in populations with low extrinsic mortality, the shift of resources from reproduction to survival only occurs under conditions of food stress during the breeding seasons [10,57]. These special ecological conditions converge on the islands [13,59].
Our results lend strong support to the disposable soma theory of ageing [1,30], which is based on optimal allocation of metabolic resources between somatic maintenance and reproduction. This theory predicts that species with low extrinsic mortality should invest relatively more in maintenance and repair at the expense of reproduction than species with high extrinsic mortality, resulting in slower ageing [1]. Our findings are in agreement with those of Carranza et al. [25] on a mainland ungulate.
In summary, we provide empirical evidence of increased tooth height in an endemic insular mammal as a result of delayed senescence and exceptional longevity. Our estimates on the level of dental abrasion in a highly hypsodont fossil insular bovid exclude increased tooth height as a mere adaptation to an enlarged range of the dietary niche as hitherto suggested. Our demographic analysis confirms that this endemic fossil bovid aged at a lower rate than mainland relatives, providing support to the hypothesis of a correlated evolution of hypsodonty and longevity. The acquisition of a more durable permanent dentition through selection on increased hypsodonty allowed Myotragus to delay senescence and to extend their reproductive lifespan, thus increasing fitness under the chronic resource limitation and the low extrinsic mortality that characterizes insular ecosystems. Myotragus further provides evidence of a trade-off between an extended reproductive span and a delayed maturity. This is consistent with the prediction of the disposable soma theory that under the ecological conditions of low extrinsic mortality combined with low-resource availability, allocation of metabolic resources is conveyed from reproduction to growth and maintenance. Finally, our results emphasize the power of selection on life-history traits in shaping dental adaptations, which are usually interpreted as a response to abiotic changes (climate), and the importance of life-history theory for interpreting palaeoenvironments.
Acknowledgments
We thank C. Constantino for access to the collections of the Museu Balear de Ciències Naturals (MBCN), Salvador Moyà for providing the hypsodonty index of Myotragaus species as well as for access to his Myotragus collections at the ICP, A. Alcover for access to the collections of the Institut Mediterrani d'Estudis Avançats (IMEDEA) and R. García for technical help. We are grateful to two anonymous reviewers for their useful comments and suggestions on the manuscript. This work was supported by the Spanish Ministry of Science and Innovation MICINN (M.K. CGL2008–06204/BTE, N.M.-M. BES-2009-02641, X.J. JCI-2010-08157 and D.DeM. JCI-2011-11697).
References
- 1.Kirkwood T. B. L., Austad S. N. 2000. Why do we age? Nature 408, 233–238 10.1038/35041682 (doi:10.1038/35041682) [DOI] [PubMed] [Google Scholar]
- 2.Gaillard J.-M., Loison A., Festa-Bianchet M., Yoccoz N. G., Solberg E. 2003. Ecological correlates of life span in populations of large herbivorous mammals. In Life span: evolutionary, ecological, and demographic perspectives (eds Carey J. R., Tuljapurkar S.), pp. 39–56 New York, NY: Population and Development Review [Google Scholar]
- 3.Ricklefs R. E. 2010. Insights from comparative analyses of aging in birds and mammals. Aging Cell 9, 273–284 10.1111/j.1474-9726.2009.00542.x (doi:10.1111/j.1474-9726.2009.00542.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ricklefs R. E. 1998. Evolutionary theories of aging: confirmation of a fundamental prediction, with implications for the genetic basis and evolution of life span. Am. Nat. 152, 24–44 10.1086/286147 (doi:10.1086/286147) [DOI] [PubMed] [Google Scholar]
- 5.Reznick D. N., Bryant M. J., Roff D., Ghalambor C. K., Ghalambor D. E. 2004. Effect of extrinsic mortality on the evolution of senescence in guppies. Nature 431, 1095–1099 10.1038/nature02936 (doi:10.1038/nature02936) [DOI] [PubMed] [Google Scholar]
- 6.Ricklefs R. E. 2008. The evolution of senescence from a comparative perspective. Funct. Ecol. 22, 379–392 10.1111/j.1365-2435.2008.01420.x (doi:10.1111/j.1365-2435.2008.01420.x) [DOI] [Google Scholar]
- 7.Charlesworth B. 1994. Evolution in age-structured populations. Cambridge, UK: Cambridge University Press [Google Scholar]
- 8.Austad S. N. 1997. Comparative aging and life histories in mammals. Exp. Gerontol. 32, 23–38 10.1016/S0531-5565(96)00059-9 (doi:10.1016/S0531-5565(96)00059-9) [DOI] [PubMed] [Google Scholar]
- 9.Wilkinson G. S., South J. M. 2002. Life history, ecology and longevity in bats. Aging Cell 1, 124–131 10.1046/j.1474-9728.2002.00020.x (doi:10.1046/j.1474-9728.2002.00020.x) [DOI] [PubMed] [Google Scholar]
- 10.Speakman J. R., Król E. 2010. The heat dissipation limit theory and evolution of life histories in Endotherms: time to dispose of the disposable soma theory? Integr. Comp. Biol. 50, 793–807 10.1093/icb/icq049 (doi:10.1093/icb/icq049) [DOI] [PubMed] [Google Scholar]
- 11.Foster J. B. 1964. Evolution of mammals on islands. Nature 202, 234–235 10.1038/202234a0 (doi:10.1038/202234a0) [DOI] [Google Scholar]
- 12.MacArthur R. H., Wilson E. O. 1967. The theory of island biogeography. Princeton, NJ: Princeton University Press [Google Scholar]
- 13.Grant P. 1998. Patterns on islands and microevolution. In Evolution on islands (ed. Grant P. R.), pp. 1–17 Oxford, UK: Oxford University Press [Google Scholar]
- 14.Köhler M., Moyà-Solà S. 2004. Reduction of brain and sense organs in the fossil insular bovid Myotragus. Brain Behav. Evol. 63, 125–140 10.1159/000076239 (doi:10.1159/000076239) [DOI] [PubMed] [Google Scholar]
- 15.Köhler M., Moyà-Solà S. 1997. Ape-like or hominid-like? The positional behavior of Oreopithecus bambolii reconsidered. Proc. Natl Acad. Sci. USA 94, 11 747–11 750 10.1073/pnas.94.21.11747 (doi:10.1073/pnas.94.21.11747) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van der Geer A., Lyras G., de Vos J., Dermitzakis M. 2010. Evolution of island mammals: adaptation and extinction of palcental mammals on islands. Oxford, UK: Wiley-Blackwell [Google Scholar]
- 17.Casanovas-Vilar I., van Dam J. A., Moyà-Solà S., Rook L. 2011. Late Miocene insular mice from the Tusco-Sardinian palaeobioprovince provide new insights on the palaeoecology of the Oreopithecus faunas . J. Hum. Evol. 61, 42–49 10.1016/j.jhevol.2011.01.003 (doi:10.1016/j.jhevol.2011.01.003) [DOI] [PubMed] [Google Scholar]
- 18.Fortelius M. 1985. Ungulate cheek teeth: developmental, functional, and evolutionary interrelations. Acta Zool. Fenn. 180, 1–76 [Google Scholar]
- 19.Janis C. M. 1988. An estimation of tooth volume and hypsodonty indices in ungulate mammals. In Teeth revisited: Proc. 7th Int. Symp. on Dental Morphology, 20–24 May, 1986, Paris (eds Russell D. E., Santoro J. P., Sigogneau-Russel D.), pp. 367–387 Paris, France: Museum National d'histoire Naturelle [Google Scholar]
- 20.Solounias N., Fortelius M., Freeman P. 1994. Molar wear rates in ruminants: a new approach. Ann. Zool. Fenn. 31, 219–227 [Google Scholar]
- 21.Damuth J., Janis C. M. 2011. On the relationship between hypsodonty and feeding ecology in ungulate mammals, and its utility in palaeoecology. Biol. Rev. 86, 733–758 10.1111/j.1469-185X.2011.00176.x (doi:10.1111/j.1469-185X.2011.00176.x) [DOI] [PubMed] [Google Scholar]
- 22.Mihlbachler M. C., Rivals F., Solounias N., Semprebon G. M. 2011. Dietary change and evolution of horses in North America. Science 331, 1178–1181 10.1126/science.1196166 (doi:10.1126/science.1196166) [DOI] [PubMed] [Google Scholar]
- 23.Hummel J., Findeisen E., Südekum K.-H., Ruf I., Kaiser T. M., Bucher M., Clauss M., Codron D. 2011. Another one bites the dust: faecal silica levels in large herbivores correlate with high-crowned teeth. Proc. R. Soc. B 278, 1742–1747 10.1098/rspb.2010.1939 (doi:10.1098/rspb.2010.1939) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Geer A. E. 2005. Island ruminants and parallel evolution of functional structures. Quaternaire 2, 231–240 [Google Scholar]
- 25.Carranza J., Alarcos S., Sánchez-Prieto C. B., Valencia J., Mateos C. 2004. Disposable-soma senescence mediated by sexual selection in an ungulate. Nature 432, 215–218 10.1038/nature03004 (doi:10.1038/nature03004) [DOI] [PubMed] [Google Scholar]
- 26.Veiberg V., Mysterud A., Gaillard J.-M., Delorme D., van Laere G., Klein F. 2007. Bigger teeth for longer life? Longevity and molar height in two roe deer populations. Biol. Lett. 3, 268–270 10.1098/rsbl.2006.0610 (doi:10.1098/rsbl.2006.0610) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ozaki M., et al. 2010. The relationship between food habits, molar wear and life expectancy in wild sika deer populations. J. Zool. 280, 202–212 10.1111/j.1469-7998.2009.00653.x (doi:10.1111/j.1469-7998.2009.00653.x) [DOI] [Google Scholar]
- 28.Gaillard J.-M., Delorme D., Boutin J. M., van Laere G., Boisaubert B., Pradel R. 1993. Roe deer survival patterns: a comparative-analysis of contrasting populations. J. Anim. Ecol. 62, 778–791 10.2307/5396 (doi:10.2307/5396) [DOI] [Google Scholar]
- 29.Loe L. E., Mysterud A., Langvatn R., Stenseth N. C. 2003. Decelerating and sex-dependent toothwear in Norwegian red deer. Oecologia 135, 346–353 10.1007/s00442-003-1192-9 (doi:10.1007/s00442-003-1192-9) [DOI] [PubMed] [Google Scholar]
- 30.Kirkwood T. B. L. 1985. Comparative and evolutionary aspects of longevity. In Handbook of the biology of ageing (eds Finch C. E., Schneider E. L.), pp. 27–44 New York, NY: Van Nostrand Reinhold [Google Scholar]
- 31.Austad S. N. 1993. Retarded senescence in an insular population of opossums. J. Zool. 229, 695–708 10.1111/j.1469-7998.1993.tb02665.x (doi:10.1111/j.1469-7998.1993.tb02665.x) [DOI] [Google Scholar]
- 32.Bate D. M. A. 1909. A new artiodactyle from Majorca. Geol. Mag. 543, 385–388 10.1017/S0016756800124665 (doi:10.1017/S0016756800124665) [DOI] [Google Scholar]
- 33.Köhler M., Moyà-Solà S. 2009. Physiological and life history strategies of a fossil large mammal in a resource-limited environment. Proc. Natl Acad. Sci. USA 106, 20 354–20 358 10.1073/pnas.0813385106 (doi:10.1073/pnas.0813385106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jordana X., Köhler M. 2011. Enamel microstructure in the fossil bovid Myotragus balearicus (Majorca, Spain): implications for life-history evolution of dwarf mammals in insular ecosystems. Palaeogeogr. Palaeoclimatol. Palaeoecol. 300, 59–66 10.1016/j.palaeo.2010.12.008 (doi:10.1016/j.palaeo.2010.12.008) [DOI] [Google Scholar]
- 35.Alcover J. A., Moyà-Solà S., Pons-Moyà J. 1981. Les quimeres del passat. Palma de Mallorca, Spain: Editorial Moll [Google Scholar]
- 36.Köhler M. 2010. Fast or slow? The evolution of life history traits associated with insular dwarfing. In Islands and evolution (eds Pérez-Mellado V., Ramon C.), pp. 261–280 Menorca, Spain: Institut Menorquí d'Estudis [Google Scholar]
- 37.Bover P., Alcover J. A. 1999. The evolution and ontogeny of the dentition of Myotragus balearicus Bate, 1909 (Artiodactyla, Caprinae): evidence from new fossil data. Biol. J. Linn. Soc. Lond. 68, 401–428 10.1006/bijl.1998.0294 (doi:10.1006/bijl.1998.0294) [DOI] [Google Scholar]
- 38.Alcover J. A., Pérez-Obiol R., Yll E. I., Bover P. 1999. The diet of Myotragus balearicus Bate 1909 (Artiodactyla, Caprinae), an extinct bovid from the Balearic islands: evidence from coprolites. Biol. J. Linn. Soc. Lond. 66, 57–74 10.1111/j.1095-8312.1999.tb01917.x (doi:10.1111/j.1095-8312.1999.tb01917.x) [DOI] [Google Scholar]
- 39.Fortelius M., Solounias N. 2000. Functional characterization of ungulate molars using the abrasion-attrition wear gradient: a new method for reconstructing paleodiets. Am. Mus. Novitat. 3301, 1–35 10.1206/00030082(2000)301 (doi:10.1206/00030082(2000)301) [DOI] [Google Scholar]
- 40.Kaiser T. M., Solounias N., Fortelius M., Bernor R. L., Schrenk F. 2000. Tooth mesowear analysis on Hippotherium primigenium from the Vallesian Dinotheriensande (Germany): a blind test study. Carolinea 58, 103–114 [Google Scholar]
- 41.Rivals F., Mihlbachler M.C., Solounias N. 2007. Effect of ontogenetic-age distribution in fossil and modern samples on the interpretation of ungulate paleodiets using the mesowear method. J. Vert. Paleontol. 27, 763–767 10.1671/0272-4634(2007)27[763:EOODIF]2.0.CO;2 (doi:10.1671/0272-4634(2007)27[763:EOODIF]2.0.CO;2) [DOI] [Google Scholar]
- 42.DeMiguel D., Azanza B., Morales J. 2010. Trophic flexibility within the oldest Cervidae lineage to persist through the Miocene Climatic Optimum. Palaeogeogr. Palaeoclimatol. Palaeoecol. 289, 81–92 10.1016/j.palaeo.2010.02.010 (doi:10.1016/j.palaeo.2010.02.010) [DOI] [Google Scholar]
- 43.DeMiguel D., Fortelius M., Azanza B., Morales J. 2008. Ancestral feeding state of ruminants reconsidered: earliest grazing adaptation claims a mixed condition for Cervidae. BMC Evol. Biol. 8, 1–13 10.1186/1471-2148-8-13 (doi:10.1186/1471-2148-8-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klevezal G. A. 1996. Recording structures of mammals: determination of age and reconstruction of life history. Rotterdam, The Netherlands: A.A. Balkema [Google Scholar]
- 45.Jones K. E., et al. 2009. PanTHERIA: a species-level database of life history, ecology, and geography of extant and recently extinct mammals. Ecology 90, 2648. 10.1890/08-1494.1 (doi:10.1890/08-1494.1) [DOI] [Google Scholar]
- 46.Grant A. 1982. The use of tooth wear as a guide to the age of domestic animals. In Ageing and sexing animal bones from archaeological sites, British Series 109 (eds Wilson B., Grigson C., Payne S.), pp. 91–108 Oxford, UK: BAR [Google Scholar]
- 47.Hamilton J. 1984. The population structure of Myotragus balearicus from the cave of Muleta (Mallorca). In The Deya Conf. of Prehistory. Early settlement in the western Mediterranean Islands and their peripheral areas, International Series 229(i) (eds Waldren W. H., Chapman R., Lewthwaite J., Kennard R. C.), pp. 71–97 Oxford, UK: BAR [Google Scholar]
- 48.Pianka E. R. 2000. Evolutionary ecology, 6th edn. San Francisco, CA: Addison-Wesley Longman Inc [Google Scholar]
- 49.Deevey E. S., Jr 1947. Life tables for natural populations of animals. Q. Rev. Biol. 22, 283–314 10.1086/395888 (doi:10.1086/395888) [DOI] [PubMed] [Google Scholar]
- 50.Ahrestani F. S., Iyer S., Heitkönig I. M. A., Prins H. H. T. 2011. Life-history traits of gaur Bos gaurus: a first analysis. Mammal. Rev. 41, 75–84 10.1111/j.1365-2907.2010.00166.x (doi:10.1111/j.1365-2907.2010.00166.x) [DOI] [Google Scholar]
- 51.Sinclair A. R. E. 1977. The African buffalo. A study of resource limitation of populations. Chicago, IL: The University of Chicago Press [Google Scholar]
- 52.Caughley G. 1966. Mortality patterns in mammals. Ecology 47, 906–918 10.2307/1935638 (doi:10.2307/1935638) [DOI] [Google Scholar]
- 53.Lubinski P. M. 2001. Estimating age and season of death of pronghorn antelope (Antilocapra americana Ord) by means of tooth eruption and wear. Int. J. Osteoarchaeol. 11, 218–230 10.1002/oa.536 (doi:10.1002/oa.536) [DOI] [Google Scholar]
- 54.Marín-Moratalla N., Jordana X., García-Martínez R., Köhler M. 2011. Tracing the evolution of fitness components in fossil bovids under different selective regimes. C. R. Palevol. 10, 469–478 10.1016/j.crpv.2011.03.007 (doi:10.1016/j.crpv.2011.03.007) [DOI] [Google Scholar]
- 55.Bover P. 2004. Noves aportacions al coneixement del gènere Myotragus Bate 1909 (Artiodactyla, Caprinae) de les Illes Balears. PhD thesis, Universitat de les Illes Balears, Mallorca, Spain [Google Scholar]
- 56.Davis M. H. L. A. 2002. Putting meat on the bone: an investigation into palaeodiet in the Balearic Islands using carbon and nitrogen stable isotope analysis. In World islands in prehistory, International Series S1095 (eds Waldren W. H., Ensenyat J.), pp. 198–216 Oxford, UK: BAR [Google Scholar]
- 57.Stearns S. C. 1992. The evolution of life histories. New York, NY: Oxford University Press [Google Scholar]
- 58.Austad S. N., Fischer K. E. 1991. Mammalian aging, metabolism, and ecology: evidence from the bats and marsupials. J. Gerontol. 46, B47–B53 10.1093/geronj/46.2.B47 (doi:10.1093/geronj/46.2.B47) [DOI] [PubMed] [Google Scholar]
- 59.McNab B. 2002. Minimizing energy expenditure facilitates vertebrate persistence on oceanic islands. Ecol. Lett. 5, 693–704 10.1046/j.1461-0248.2002.00365.x (doi:10.1046/j.1461-0248.2002.00365.x) [DOI] [Google Scholar]