Abstract
For many species, there is broad-scale dispersal of juvenile stages and/or long-distance migration of individuals and hence the processes that drive these various wide-ranging movements have important life-history consequences. Sea turtles are one of these paradigmatic long-distance travellers, with hatchlings thought to be dispersed by ocean currents and adults often shuttling between distant breeding and foraging grounds. Here, we use multi-disciplinary oceanographic, atmospheric and genetic mixed stock analyses to show that juvenile turtles are encountered ‘downstream’ at sites predicted by currents. However, in some cases, unusual occurrences of juveniles are more readily explained by storm events and we show that juvenile turtles may be displaced thousands of kilometres from their expected dispersal based on prevailing ocean currents. As such, storms may be a route by which unexpected areas are encountered by juveniles which may in turn shape adult migrations. Increased stormy weather predicted under climate change scenarios suggests an increasing role of storms in dispersal of sea turtles and other marine groups with life-stages near the ocean surface.
Keywords: loggerhead sea turtles, mtDNA, Lagrangian buoy trajectories, particle tracking, storm tracks, mixed stock analysis
1. Introduction
Long-distance migration remains one of nature's wonders. Migratory animals exploit different locations at different stages in their life: a strategy so effective at optimizing resource use that the cost of travel is worthwhile [1]. The iconic questions of where eels go to spawn [2], and how sea turtles and salmonids navigate and the factors that shape their migratory routes [3–5] continue to drive scientific investigation. These studies go beyond curiosity, as anthropogenic changes to the environment are affecting large-scale processes (e.g. climate) that may have consequences for migratory behaviour and species survival [6]. It is therefore suggested that global migrators, such as transoceanic migratory birds, may be useful as biological indicators of climate and oceanic health [7].
For aerial organisms, global wind patterns are a strong determinant of long-distance migratory routes [8], but in the sea, it is the prevailing oceanographic features, such as circulation patterns, that are believed to be important in determining the distribution and connectivity of populations. Many species have been shown to use ocean currents as migratory pathways. For juvenile stages of marine organisms, small size may limit their capability to swim actively against currents, so ‘going with the flow’ would be an efficient means of migrating to distant foraging grounds while maximizing growth and development. For example, in the North Atlantic, the ‘subpolar gyre’ is used by Atlantic salmon [9] and the ‘subtropical gyre’ is used by sea turtles [10].
These gyres are major currents that occur at the ocean basin scale. At this scale in the subtropics, the ‘subtropical gyre’ is set up by the ‘Sverdrup transport’ [11], which is a broad equatorward flow across the subtropics. Northward return flow in the gyre is confined to a western boundary region, governed by frictional processes [12,13], and is consequently swift. These return flows comprise the ocean currents of leading importance for the long-distance migration of marine organisms. Such currents are quasi-steady, subject to some seasonality, particularly in wind forcing, e.g. [14] and dynamical instability (eddying). Current speeds are typically in the range of 10–100 cm s−1. Current width ranges considerably, from narrow swift flows spanning a few kilometres (e.g. the Florida Current) to broad weak flows spanning several hundred kilometres (e.g. the North Atlantic Current). Moving into mid-latitudes, some boundary currents (e.g. the Slope Current at the northwest European shelf break [15]) are principally driven by surface buoyancy forcing, owing to the combined effects of heat and freshwater exchange between ocean and atmosphere. In addition to the balanced upper circulation, surface ‘Ekman Currents’ arise through a balance between frictional forces associated with the wind and the Coriolis force, with the surface current oriented 45° to the right of the wind in the Northern Hemisphere [16]. Ekman Currents are most conspicuous in the ‘interior’ of the subtropical gyre, where the upper circulation is weak. Buoyant objects in the ocean, such as drifting organisms, thus move under the combined influence of quasi-steady and Ekman currents.
Although major migratory pathways of marine organisms appear to be fixed by ocean currents, frequent reports of ‘stranded’ or ‘vagrant’ individuals outside their ranges are common for many species, including seals [17], cetaceans [18] and sea turtles [19], indicating that animals can be displaced from normal migratory routes. Occasionally, displacement events are dramatic: for example, the thousands of kilometres displacement of an emperor penguin (http://www.bbc.co.uk/news/world-asia-pacific-13856024). Such dramatic displacements are rare, but in some locations, strandings of marine vertebrates are routine [18,19], and present opportunities to investigate whether there are factors other than currents that may influence dispersal.
Here, we focus on sea turtles, one of the paradigmatic long-distance migrators [20]. Adult turtles return to natal nesting beaches to breed and some species maintain fidelity to specific foraging grounds that may be thousands of kilometres from the breeding sites [21,22]. Our focal species, the loggerhead sea turtle (Caretta caretta), is one of the most well-studied of sea turtle species. After emerging from nests, hatchling loggerhead sea turtles enter the sea and the juveniles then spend several years in the open ocean, followed by a transition from pelagic to neritic habitats when individuals are around 40–50 cm in size [23], although exceptions to this general life cycle have been found [24]. Pelagic juveniles are therefore of relatively small size, and still subject to any oceanographic and meteorological forces that may alter their direction of dispersal in the open seas. In the North Atlantic, it is known that loggerhead juveniles spend 6.5–11.5 years within the oceanic zone [25]. These either remain around the American mainland, or are transported in the North Atlantic subtropical gyre towards the eastern Atlantic, where there are major foraging grounds for juvenile turtles, for example, around the Azores and the Canary Islands [26]. The trans-Atlantic drift from eastern USA to Europe is estimated to be 1.80–3.75 years [27]. Those transported further north from the normal foraging grounds towards northern Europe by the North Atlantic Current, may die from cold stunning [28]. Sea turtles are known to orientate in order to nest in their natal beaches and reach specific feeding areas [5]. This orientation is based, at least partly, on geomagnetic cues and may help loggerhead sea turtles to remain in warm waters [5]. However, when currents are strong or during extreme weather events, this ability may be reduced because of the limited swimming strength of juveniles that are small in size [26,29], particularly as they start to become cold stunned. Individuals failing to correct their drift might end up stranded far north of their normal foraging grounds, for example, in areas of northern Europe such as the Bay of Biscay or the English Channel.
We examine mitochondrial DNA (mtDNA) sequences of juvenile loggerhead sea turtles (Caretta caretta) stranding around the Bay of Biscay to estimate the origins of these turtles. The study area lies outside of the species’ normal range, with the nearest foraging areas in the Azores and southern Spain. These stranded juvenile loggerhead turtles may have been transported by prevailing ocean surface currents, or they may have been blown off-course by storms. The episodic passage of cyclonic storms can influence the subtropical gyre of the North Atlantic. The passage of such storms will excite a dynamical response of the upper ocean in the form of Ekman Currents [30]. While the steady Ekman response to wind forcing [16] is not easily observed in the ocean, divergent Ekman Currents of 1–2 m s−1 have been observed in the wake of hurricanes, weakening over a few days [30]. A degree of asymmetry in the currents, along and about the axis of the hurricane, depends on the storm trajectory. A pattern of residual surface currents may thus be associated with cyclonic storms moving clockwise around the North Atlantic.
Classically, storms are well known to cause unusual transport of terrestrial animals [31]. However, given that climate change models predict increasing storm activity [32], there is growing interest in understanding how storms impact on the dispersal and distribution of marine organisms [33,34]. In this study, we aim to consider both ocean currents and storm effects in understanding the factors driving the strandings of loggerhead turtles. Previous studies have found oceanographic data invaluable in interpreting the ecological and genetic structure of sea turtles [35–38], but in this study, we take the multi-disciplinary approach a stage further in using oceanographic modelling as well as oceanographic and meteorological data in understanding the movements of sea turtles inferred from genetic data.
2. Methods
2.1. Genetic analyses
A total of 89 juveniles stranded in the Bay of Biscay from 1995 to 2009 were analysed (figure 1). Blood samples or tissue samples from skin or pectoral muscle were taken and stored in 96 per cent ethanol at 4°C. Genomic DNA was isolated using DNeasy Tissue Kit (QIAGEN) and a 760 base pair (bp) fragment of the mtDNA control region was sequenced using established primers and protocols [39]. New haplotype sequences were submitted to the Archie Carr Center for Sea Turtle Research (http://accstr.ufl.edu/cclongmtdna.html) and GenBank. Arlequin v. 3.0 [40] was used to estimate haplotype (h) and nucleotide diversity (π), and to perform exact tests of population differentiation (spatial and temporal genetic structure). We added unpublished sequences for the Cape Verde and Canary Islands in Bayesian ‘many-to-many’ mixed stock analysis (MSA) [41,42] (electronic supplementary material, tables S1 and S2).
Figure 1.

Stranding locations of the individuals sampled in this study. Single strandings are represented by black circles; strandings of two, three, four, five and six individuals are represented by white circles, white triangles, grey triangles, black triangles and black squares, respectively. The inset map shows the location of loggerhead nesting populations in the Atlantic (stars).
We attempted to group individuals according to estimated origins. Haplotypes described for the Cape Verde population [43] were used to assign individuals to ‘Cape Verdean’ or ‘American’ groups. Haplotypes of uncertain assignment were excluded. Although not all individuals can be assigned and some errors could be introduced with this classification, it is useful as it allows testing for differences between the two groups. We tested for size and weight variation using the non-parametric U Mann–Whitney test (SPSS v. 15.0), and for temporal variation using the G-test of independence.
2.2. Particle track modelling
To evaluate whether hatchlings leaving the Cape Verde Islands might passively drift to the broader Bay of Biscay region, we use both satellite-tracked buoy data (see §2.3) and model-based trajectories. In this section, we describe the latter. The ocean model, for which we diagnose trajectories of passively drifting particles arriving in the Bay of Biscay, is based on NEMO (the Nucleus for European Modelling of the Ocean). We use fields from a global 1/4° implementation [44] that resolve the mesoscale variability of energetic currents and oceanic eddies of radii exceeding around 100 km. An efficient analytical method for computing large ensembles of offline trajectories [45] was customized as the Ariane software (http://stockage.univ-brest.fr/~grima/Ariane/) for use with NEMO datasets. We specified particle ‘endpoints’ in a regular grid spanning the Bay of Biscay (electronic supplementary material, figure S1). To cover the period during which the sampled turtles are likely to have been at sea and to account for interannual variability, a particle is back-tracked from each endpoint to obtain trajectory ensembles for the 3 years preceding 1995, 1998, 2001, 2004 and 2007. The trajectories are based on time-varying currents and are characterized by age (since release), depth (whether or not the particles are buoyant) and property (temperature and salinity). The spacing between adjacent endpoints was around 50 km. The end date for trajectories was mid-February of a selected year. Particles were constrained to remain at the uppermost NEMO depth level of 0.5 m, to mimic animal buoyancy. Advected by a surface velocity field that is updated every 30 days (as a monthly-mean field), a particle is back-tracked from each endpoint for 3 years or less (depending whether the particle originated from beyond the North Atlantic domain within 3 years). Positions of particles and associated water temperature are recorded every 5 days.
2.3. Lagrangian drifter and storm track data
To investigate the destination of turtles drifting away from the Cape Verde Islands, Lagrangian drifter data were downloaded from the NOAA-AOML global drifter program (http://www.aoml.noaa.gov/envids/gld/) with no restrictions on date or drogue attachment imposed. This dataset contains quality controlled data of over 14 500 satellite-tracked surface buoys deployed since the 1970s. Buoys are drogued at 15 m (i.e. a subsurface sea anchor, a ‘drogue’, is tethered to the surface buoy) to reduce wind effects and interpolated to provide fixes at 6 h intervals [46]. All buoys passing within 100 km of the coast of the Cape Verde Islands were selected, and upon first reaching this proximity, all subsequent fixes were used to investigate surface currents in this region.
Particle and buoy trajectories do not capture the influence of storm-induced displacement. While NEMO is forced by high-frequency winds, the particle trajectories are computed with monthly-averaged currents, and so storm-forced drift on time scales of hours to days is not explicitly included. Furthermore, the sampled buoys may not capture the relatively infrequent storm-induced drift, and being drogued to reduce wind effects, they will not experience the storm-induced fate of juveniles confined to the upper few metres. So to investigate storm trajectories, the tracks of major storms originating near Cape Verde Islands during our studied period were obtained from the National Hurricane Center website (http://www.nhc.noaa.gov/). This database is generated through the analyses of a wide variety of data, including a storm's life cycle (defined to include the tropical or subtropical depression stage, but does not include the extratropical stage) and maximum sustained (1 min average) surface (10 m) winds. For storms east of 55° W, the primary source of information was geostationary weather satellite imagery, with occasional in situ observations from ships and buoys. Only major storms (i.e. wind speeds of at least 17 m s−1) of the following classes were included in our data: ‘tropical storm’ with wind speed 17–32 m s−1; ‘hurricane’ with wind speed 33–49 m s−1; ‘major hurricane’ with wind speed 50 m s−1 or higher. We use the term ‘storm’ generically to refer to all classes.
3. Results
3.1. Genetic analyses
Our data included 13 previously described, and two novel haplotypes (CC-A63.1 and CC-A64.1; GenBank accession numbers JF957336 and JF957337, respectively; electronic supplementary material, table S3). Using a short version of haplotypes (380 bp), pairwise comparisons between the stranded group and rookeries revealed significant differences (exact p < 0.011) except for Lebanon (exact p = 0.149), which has a small sample size (n = 9). Foraging ground centric MSA with population sizes as prior information (table 1) showed that a high proportion of juveniles were from the south Florida population (51%; 95% CI = 0.67), but surprisingly, juveniles from Cape Verde, in the eastern Atlantic, were relatively frequent (26%; 95% CI = 0.40) and more abundant than juveniles from northeast (8%; 95% CI = 0.19) or northwest Florida (1%; 95% CI = 0.03). There was no correlation with geographical distance to the Gulf Stream using either foraging ground centric (r = 0.443, r2 = 0.197; p = 0.098) or rookery-centric MSA results (r = 0.398, r2 = 0.158; p = 0.329).
Table 1.
Mixed stock analysis (MSA) using ‘many-to-many’ model. The proportion of stranded juveniles in the Bay of Biscay originating from the different rookeries is estimated using foraging ground centric analysis, computed with and without population size information. The proportion of individuals from each rookery that ends up stranded in the Bay of Biscay is estimated with rookery-centric analysis. The latter excluded Mediterranean rookeries since foraging ground centric analysis showed little contribution from these populations. Mean and standard deviation (s.d.) values are shown. Br, Brazil; ES-RJ, Espírito Santo-Rio de Janeiro. Further details of datasets used in the MSA are in the electronic supplementary material, tables S1 and S2.
| rookery | relative population size | many-to-many foraging ground centric mean (s.d.) |
many-to-many rookery-centric mean (s.d.) | |
|---|---|---|---|---|
| no size | size | size | ||
| south Florida | 0.6863 | 0.0623 (0.0517) | 0.5107 (0.1041) | 0.0410 (0.0242) |
| northwest Florida | 0.0061 | 0.0791 (0.0601) | 0.0114 (0.0121) | 0.0862 (0.0765) |
| northeast Florida | 0.0634 | 0.0842 (0.0595) | 0.0775 (0.0596) | 0.0645 (0.0507) |
| Dry Tortugas | 0.0022 | 0.0587 (0.0474) | 0.0040 (0.0044) | 0.0881 (0.0812) |
| Mexico | 0.0184 | 0.1575 (0.0562) | 0.0718 (0.0351) | 0.1901 (0.0775) |
| Bahía-Sergipe (Br) | 0.0274 | 0.0117 (0.0114) | 0.0115 (0.0113) | 0.0225 (0.0266) |
| ES-RJ (Br) | 0.0199 | 0.0118 (0.0118) | 0.0104 (0.0104) | 0.0282 (0.0334) |
| Cape Verde | 0.1432 | 0.2242 (0.0687) | 0.2601 (0.0805) | 0.1038 (0.0697) |
| Greece | 0.0212 | 0.0347 (0.0326) | 0.0210 (0.0222) | — |
| Cyprus | 0.0058 | 0.0433 (0.0401) | 0.0095 (0.0111) | — |
| Lebanon | 0.0004 | 0.0434 (0.0384) | 0.0007 (0.0008) | — |
| Crete | 0.0040 | 0.0418 (0.0366) | 0.0066 (0.0078) | — |
| Israel | 0.0003 | 0.0381 (0.0332) | 0.0006 (0.0007) | — |
| eastern Turkey | 0.0010 | 0.0606 (0.0425) | 0.0019 (0.0021) | — |
| western Turkey | 0.0013 | 0.0486 (0.0444) | 0.0022 (0.0024) | — |
The global test of population differentiation did not reveal genetic structure among the stranded group and foraging groups of the eastern Atlantic (exact p = 0.135). The stranded samples presented the highest h-value (0.7043), but similar π (0.0342) to those of eastern Atlantic foraging grounds (electronic supplementary material, table S4). There were significant genetic differences among years (exact p = 0.001; electronic supplementary material, tables S5 and S6) but removal of 2001 data resulted in non-significance (exact p = 0.255). The greatest number of strandings occurred in 2001 and with a higher proportion of haplotype CC-A1.1 (0.40) than for other years (0.09–0.33). The number of strandings increased from December onwards with the highest proportion occurring in April (figure 2a) is consistent with other reports [28], and coincides with the months with lower sea surface temperature. Intra-annual genetic variation was detected for months with five or more samples (n = 8; exact p = 0.005).
Figure 2.
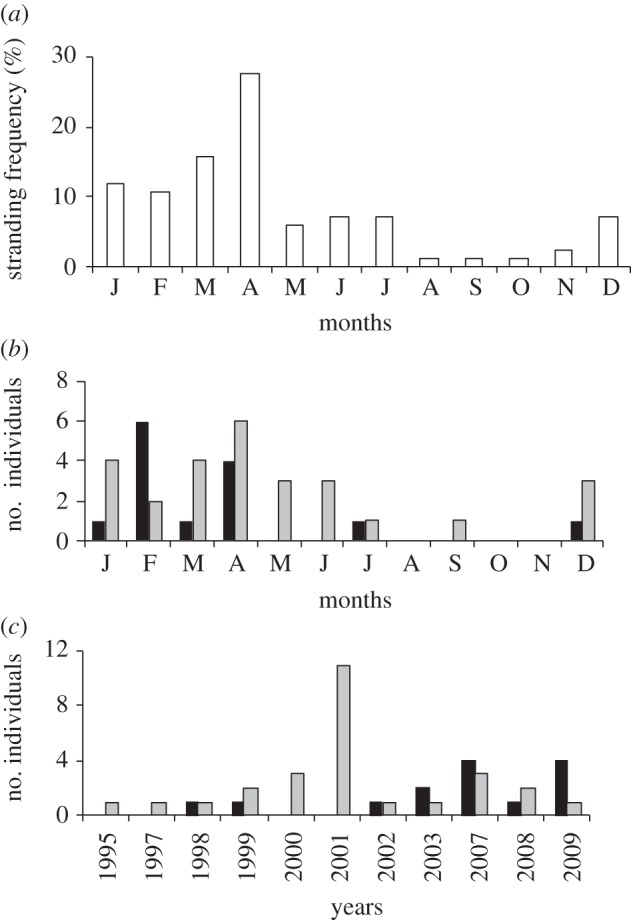
Temporal distribution of strandings of loggerhead sea turtle juveniles off the Bay of Biscay. (a) The monthly distribution of all 82 stranding records (white) between 1995 and 2009 showed the highest frequency occurring in April. Months are ordered as in the calendar, and coded with the first letter of the month (i.e. starting with J, January, and ending with D, December). (b) The monthly distribution of 14 Cape Verdean (black) and 27 American (grey) individuals studied during this period. The critical months for strandings in the Bay of Biscay appeared to be different for turtles of different origins. (c) The distribution of 14 Cape Verdean (black) and 27 American (grey) individuals across the years included in the study (excludes years for which data were not taken). The distribution was significantly different for turtles of different origins (n = 41; p = 0.021). Whereas individuals of American origin stranded in all the years studied, individuals from Cape Verde only stranded in some years. The high number of strandings that occurred during 2001 was all of American origin.
The ‘Cape Verdean group’ (haplotypes CC-A1.3 and CC-A17.1; n = 17) presented a higher proportion of dead animals (84%) than the ‘American group’ (29%; haplotypes CC-A1.1, CC-A3.1 and CC-A10.1; n = 30, p < 0.001; electronic supplementary material, table S3) but were not significantly different in size (n = 46, p = 0.767) or weight (p = 0.617). There were no genetic differences among months (n = 41; p = 0.299), but the critical months for strandings appear to be different (figure 2b). Additional differences could be observed in the stranding frequencies by year (n = 41; p = 0.021; figure 2c). ‘Cape Verdean’ individuals did not strand in every year that ‘American’ individuals stranded. For example, in 2001—the year with highest strandings—there were no ‘Cape Verdean’ individuals.
3.2. Analyses of physical data
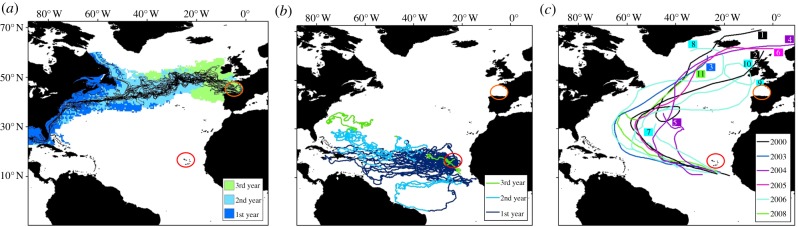
A total of 11 820, 3 year long Lagrangian hindcast trajectories were computed comprising a total of 2 588 580 particle locations. The general pattern of trajectories reflected the currents in this region: particles travelling to the Bay of Biscay would have originated from westwards in the North Atlantic Current after having streamed south/north in either the Labrador Current or Gulf Stream, respectively (figure 3a). The majority of particles originated from the south near the southeast USA, Gulf of Mexico, Caribbean and Sargasso Sea. After 3 years of drift, particles were still only tracked back as far as the western Atlantic, and no particles originated close to the Cape Verde Islands.
Figure 3.
Analysis of oceanographic and meteorological data for the North Atlantic to trace surface movements around northern Europe/the Bay of Biscay (orange circle) and the Cape Verde Islands (red circle). (a) Mean drift time in each 0.5° by 0.5° pixel from the full particle hindcast ensemble. Pixels are coloured to reflect the first (dark blue), second (light blue) and third year of drift (green) and the paths of a sample of 35 particle hindcast trajectories are plotted. (b) Trajectories of 53 Lagrangian drifter buoys passing within 100 km off the coast of the Cape Verde Islands. Pixels are coded as in (a) to reflect mean year of drift. (c) Tracks of 11 Atlantic basin major storms that originated near Cape Verde Islands during our studied period. These storms provided a more direct route from Cape Verde Islands to the northeast Atlantic than the prevailing ocean currents. Each colour represents a different year. Further information about each storm numbered 1–11 is in the electronic supplementary material, table S7.
All 53 buoys that were found to pass within 100 km of the Cape Verde Islands drifted westwards in the North Atlantic Gyre with the North Equatorial Current, bar one which drifted south towards the coast of Brazil before looping back towards the Cape Verde Islands (figure 3b). The buoy that had travelled the furthest reached a longitude of ca 60° W and 30° N within 3 years, which corresponded to locations where particles back-tracked from the Bay of Biscay reached in 2–3 years.
Eleven major storms originated near the Cape Verde Islands during our study period (figure 3c; electronic supplementary material, table S7). Several occurred during the nesting and hatching season of loggerhead turtles at Cape Verde [47]. These major storms initially travelled north westwards from the Cape Verde Islands, but then travelled northwards and north eastwards to arrive in the northeast Atlantic.
4. Discussion
Here, we show that in addition to sea currents, storm-forcing may also impact on juvenile dispersion. The general importance of this is that it shows how stochastic weather effects may lead to drifting organisms arriving in areas that would not be predicted by dispersion on ocean currents alone. Increasingly, studies of various organisms, ranging from rock lobsters [48] to kelp [49], are showing that many factors aside from prevailing oceanographic conditions may influence dispersal trajectories.
A general hypothesis of oceanic transport with major currents would predict that the stranded turtles in the Bay of Biscay should all come from rookeries along the coasts of the American continent. It has been suggested that proximity to the Gulf Stream may be important [50], but we found no such association for the stranded turtles. MSA showed that the Atlantic nesting populations were indeed the main contributors with half of all individuals from south Florida. The more interesting result, however, was that a quarter of stranded turtles were apparently from the Cape Verde Islands, which is nowhere near currents that would take hatchlings to the Bay of Biscay. The analyses of particle and buoy trajectories demonstrated that juveniles from the northwestern Atlantic, but not from Cape Verde, could arrive at the Bay of Biscay in a few years by drifting with ocean currents.
We consider here the influence of storm-driven surface currents on juvenile sea turtles, and suggest that storms may move turtles into other current systems that deliver them to locations outside their expected distribution and where they are eventually stranded. During our study period, we identified 11 storms that could potentially influence the drift of juveniles from Cape Verde (figure 3). Interestingly, most of these storms occur around August–October (electronic supplementary material, table S7), while the highest frequency of strandings of Cape Verdean loggerhead turtles occur in February (figure 2). It should be noted that the database we used was designed for tracking major storms, and there will be many more less-intense storms that may similarly be influencing the trajectory of hatchling turtles. However, the storms we identified provide evidence of the general, predominant trajectories of storms in the Atlantic. Essentially, the predominant trajectory of storms provided a far more direct route from Cape Verde to the northeast Atlantic than that provided by prevailing ocean currents. Consequently, objects near the ocean surface moved by these storm winds would arrive in the northeast Atlantic much faster than objects carried by the current (figure 3). During the early stages, juveniles spend long periods at the ocean surface and storms could perhaps displace them sufficiently to end up on aberrant routes of migration. We suggest that juveniles would experience north westward drift in the vicinity of storms translating to the west in the tropics (10–25° N). If these juveniles move into the mid-gyre region (25–35° N), northward-translating storms will drive a north eastward drift. While these storm-induced ‘nudges’ are sporadic in nature (1–4 per year; see electronic supplementary material, table S7) and short-lived, they are individually strong, and against a weak background flow of a few centimetres per second, the net effect on trajectories may be substantial. Driven sufficiently far to the north, juveniles will drift with the North Atlantic Current towards the Bay of Biscay (implicit in figure 3a). Subsequent entrainment in the Slope Current, flowing polewards along the shelf break, may account for the distribution of strandings evident in figure 1.
Displacement by storms could explain the difference in survival and the more irregular occurrences of strandings for the Cape Verde turtles. These did not strand every year, even though loggerhead turtles are stranded in the Bay of Biscay regularly. For example, in 2001, there was an unusually high rate of loggerhead turtles stranding in Europe [51], but there were none in our data from Cape Verde. This would be consistent with stochastic events such as storms leading to a more irregular pattern of Cape Verde turtles reaching the Bay of Biscay.
Using multiple lines of evidence, we arrive at the conclusion that the loggerhead turtles that strand in the Bay of Biscay not only have different origins, but that their transport must have been driven by different factors. Prevailing oceanographic forces are thought to predominantly drive the direction of the dispersal of drifting organisms [52]. However, we show here that storm-forcing may perturb these regular patterns and although this may lead to novel dispersal or migration patterns, many individuals are also ‘lost at sea’ as a result. In our case, the turtles arrived in a sub-optimum area where cold temperatures can lead to death [27,28,53], but in other cases, we might expect the turtles could be blown to more favourable areas. Recently, it has been shown that variation in climate can influence the trajectory of storms in the Atlantic [54,55]. So if climate does change in the future, then the pattern of storm-forced dispersal may also change due to alterations to the overall directions of storms. Given that global warming models predict future increase in storm activity [32], we suggest that storm-forced dispersal will increase in importance, particularly for marine organisms with dispersive life-stages at the ocean surface.
Acknowledgements
We thank the Centre d'Etudes et de Soins pour les Tortues Marines de l'Aquarium La Rochelle and its sea turtle stranding network for samples, Jacques Fretey for putting us into contact with the aquarium, the French Ministry of Ecology for the financial support to analyse the samples, and Estación Biológica de Doñana and Fundación BBVA for helping with laboratory equipment. We are grateful to Jon Houghton and two anonymous referees for critical comments on the paper. F.D’.A., P.M., A.M., L.F.L.-J. provided samples and laboratory resources; G.C.H., R.S., R.M. provided oceanographic and meteorological analyses; C.M.-A. and P.L.M.L. conceived the study, analysed the genetic data and wrote the paper together, with contributions from all other authors.
References
- 1.Alexander R. M. 1998. When is migration worthwhile for animals that walk, swim or fly? J. Avian Biol. 29, 387–394 10.2307/3677157 (doi:10.2307/3677157) [DOI] [Google Scholar]
- 2.Avise J. C. 2011. Catadromous eels continue to be slippery research subjects. Mol. Ecol. 20, 1317–1319 10.1111/j.1365-294X.2011.05012.x (doi:10.1111/j.1365-294X.2011.05012.x) [DOI] [PubMed] [Google Scholar]
- 3.Sale A., Luschi P. 2009. Navigational challenges in the oceanic migrations of leatherback sea turtles. Proc. R. Soc. B 276, 3737–3745 10.1098/rspb.2009.0965 (doi:10.1098/rspb.2009.0965) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byrne R., Fish J., Doyle T. K., Houghton J. D. R. 2009. Tracking leatherback turtles (Dermochelys coriacea) during consecutive inter-nesting intervals: further support for direct transmitter attachment. J. Exp. Mar. Biol. Ecol. 377, 68–75 10.1016/j.jembe.2009.06.013 (doi:10.1016/j.jembe.2009.06.013) [DOI] [Google Scholar]
- 5.Lohmann K. J., Putman N. F., Lohmann C. M. F. 2008. Geomagnetic imprinting: a unifying hypothesis of long-distance natal homing in salmon and sea turtles. Proc. Natl Acad. Sci. USA 105, 19 096–19 101 10.1073/pnas.0801859105 (doi:10.1073/pnas.0801859105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilcove D., Wikelski M. 2008. Going, going, gone: is animal migration disappearing? PLoS Biol. 6, e188. 10.1371/journal.pbio.0060188 (doi:10.1371/journal.pbio.0060188) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaffer S. A., et al. 2006. Migratory shearwaters integrate oceanic resources across the Pacific Ocean in an endless summer. Proc. Natl Acad. Sci. USA 103, 12 799–12 802 10.1073/pnas.0603715103 (doi:10.1073/pnas.0603715103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Felicísimo A. M., Muñoz J., González-Solis J. 2008. Ocean surface winds drive dynamics of transoceanic aerial movements. PLoS ONE 3, e2928. 10.1371/journal.pone.0002928 (doi:10.1371/journal.pone.0002928) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dadswell M. J., Spares A. D., Reader J. M., Stokesbury M. J. W. 2010. The North Atlantic subpolar gyre and the marine migration of Atlantic salmon Salmo salar: the ‘Merry-Go-Round’ hypothesis. J. Fish Biol. 77, 435–467 10.1111/j.1095-8649.2010.02673.x (doi:10.1111/j.1095-8649.2010.02673.x) [DOI] [PubMed] [Google Scholar]
- 10.Luschi P., Hays G. C., Papi F. 2003. A review of long distance movements by sea turtles, and the possible role of ocean currents. Oikos 103, 293–302 10.1034/j.1600-0706.2003.12123.x (doi:10.1034/j.1600-0706.2003.12123.x) [DOI] [Google Scholar]
- 11.Sverdrup H. U. 1947. Wind driven currents in a baroclinic ocean with application to the equatorial currents in the eastern Pacific. Proc. Natl Acad. Sci. USA 33, 318–326 10.1073/pnas.33.11.318 (doi:10.1073/pnas.33.11.318) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stommel W. H. 1948. The westward intensification of wind-driven ocean currents. Trans. Am. Geophys. Union 29, 202–206 [Google Scholar]
- 13.Munk W. H. 1950. On the wind-driven ocean circulation. J. Meteorol. 7, 79–93 [Google Scholar]
- 14.Niiler P. P., Richardson W. S. 1973. Seasonal variability of the Florida Current. J. Mar. Res. 31, 144–167 [Google Scholar]
- 15.Huthnance J. M. 1995. Circulation, exchange and water masses at the ocean margin: the role of physical processes at the shelf edge. Prog. Oceanogr. 35, 353–431 10.1016/0079-6611 (doi:10.1016/0079-6611) [DOI] [Google Scholar]
- 16.Ekman V. W. 1905. On the influence of the earth's rotation on ocean currents. Ark. Mat. Astron. Fys. 2, 1–52 [Google Scholar]
- 17.Ferreira J. M., et al. 2008. Multiple origins of vagrant Subantarctic fur seals: a long journey to the Brazilian coast detected by molecular markers. Polar Biol. 31, 303–308 10.1007/s00300-007-0358-z (doi:10.1007/s00300-007-0358-z) [DOI] [Google Scholar]
- 18.Leeney R. H., Amies R., Broderick A. C., Witt M. J., Loveridge J., Doyle J., Godley B. J. 2008. Spatio-temporal analysis of cetacean strandings and bycatch in a UK fisheries hotspot. Biodivers. Conserv. 17, 2323–2338 10.1007/s10531-008-9377-5 (doi:10.1007/s10531-008-9377-5) [DOI] [Google Scholar]
- 19.Hart K. M., Mooreside P., Crowder L. B. 2006. Interpreting the spatio-temporal patterns of sea turtle strandings: going with the flow. Biol. Conserv. 129, 283–290 10.1016/j.biocon.2005.10.047 (doi:10.1016/j.biocon.2005.10.047) [DOI] [Google Scholar]
- 20.Hays G. C., Akesson S., Broderick A. C., Glen F., Godley B. J., Papi F., Luschi P. 2003. Island-finding ability of marine turtles. Proc. R. Soc. Lond. B 270, S5–S7 10.1098/rsbl.2003.0022 (doi:10.1098/rsbl.2003.0022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Avise J. C. 2007. Conservation genetics of marine turtles—ten years later. In Frontiers in wildlife science: linking ecological theory and management application (eds Hewitt D., Fulbright T.), pp. 295–314 Boca Raton, FL: CRC Press [Google Scholar]
- 22.Bowen B. W., Karl S. A. 2007. Population genetics and phylogeography of sea turtles. Mol. Ecol. 16, 4886–4907 10.1111/j.1365-294X.2007.03542.x (doi:10.1111/j.1365-294X.2007.03542.x) [DOI] [PubMed] [Google Scholar]
- 23.Bowen B. W., et al. 2004. Natal homing in juvenile loggerhead turtles (Caretta caretta). Mol. Ecol. 13, 3797–3808 10.1111/j.1365-294X.2004.02356.x (doi:10.1111/j.1365-294X.2004.02356.x) [DOI] [PubMed] [Google Scholar]
- 24.McClellan C. M., Braun-McNeill J., Avens L., Wallace B. P., Read A. J. 2010. Stable isotopes confirm a foraging dichotomy in juvenile loggerhead sea turtles. J. Exp. Mar. Biol. Ecol. 387, 44–51 10.1016/j.jembe.2010.02.020 (doi:10.1016/j.jembe.2010.02.020) [DOI] [Google Scholar]
- 25.Bjorndal K. A., Bolten A. B., Martins H. R. 2000. Somatic growth model of juvenile loggerhead sea turtles Caretta caretta: duration of pelagic stage. Mar. Ecol. Prog. Ser. 202, 265–272 10.3354/meps202265 (doi:10.3354/meps202265) [DOI] [Google Scholar]
- 26.Monzón-Argüello C., Rico C., Carreras C., Calabuig P., Marco A., López-Jurado L. F. 2009. Variation in spatial distribution of juvenile loggerhead turtles in the eastern Atlantic and western Mediterranean Sea. J. Exp. Mar. Biol. Ecol. 373, 79–86 10.1016/j.jembe.2009.03.007 (doi:10.1016/j.jembe.2009.03.007) [DOI] [Google Scholar]
- 27.Hays G. C., Marsh R. 1997. Estimating the age of juvenile loggerhead sea turtles in the North Atlantic. Can. J. Zool./Rev. Can. Zool. 75, 40–46 10.1139/z97-005 (doi:10.1139/z97-005) [DOI] [Google Scholar]
- 28.Witt M. J., Penrose R., Godley B. J. 2007. Spatio-temporal patterns of juvenile marine turtle occurrence in waters of the European continental shelf. Mar. Biol. 151, 873–885 10.1007/s00227-006-0532-9 (doi:10.1007/s00227-006-0532-9) [DOI] [Google Scholar]
- 29.Revelles M., et al. 2007. Evidence for an asymmetrical size exchange of loggerhead sea turtles between the Mediterranean and the Atlantic through the Straits of Gibraltar. J. Exp. Mar. Biol. Ecol. 349, 261–271 10.1016/j.jembe.2007.05.018 (doi:10.1016/j.jembe.2007.05.018) [DOI] [Google Scholar]
- 30.Shay L. K. 2001. Upper ocean structure: response to strong forcing events. In Encyclopedia of ocean sciences (eds Weller R. A., Thorpe S. A., Steele J.), pp. 3100–3114 London, UK: Academic Press International [Google Scholar]
- 31.Gressitt J. L. 1960. The development of insect faunae in Oceania. In Proc. Centenary and Bicentenary Congress of Biology, Darwin-Wallace Centenary, Singapore, 2–9 December 1958, (ed. R. D. Purchon), pp. 58–62 Kuala Lumpur, Malaysia: University of Malaya Press [Google Scholar]
- 32.Webster P. J., Holland G. J., Curry J. A., Chang H. R. 2005. Changes in tropical cyclone number, duration and intensity in a warming environment. Science 309, 1844–1846 10.1126/science.1116448 (doi:10.1126/science.1116448) [DOI] [PubMed] [Google Scholar]
- 33.Lea M. A., Johnson D., Ream R., Sterling J., Melin S., Gelatt T. 2009. Extreme weather events influence dispersal of naive northern fur seals. Biol. Lett. 5, 252–257 10.1098/rsbl.2008.0643 (doi:10.1098/rsbl.2008.0643) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopez-Victoria M., Zea S. 2004. Storm-mediated coral colonization by an excavating Caribbean sponge. Clim. Res. 26, 251–256 10.3354/cr026251 (doi:10.3354/cr026251) [DOI] [Google Scholar]
- 35.Hays G. C., Fossette S., Katselidis K. A., Mariani P., Schofield G. 2010. Ontogenetic development of migration: Lagrangian drift trajectories suggest a new paradigm for sea turtles. J. R. Soc. Interface 7, 1319–1327 10.1098/rsif.2010.0009 (doi:10.1098/rsif.2010.0009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Godley B. J., Barbosa C., Bruford M., Broderick A. C., Catry P., Coyne M. S., Formia A., Hays G. C., Witt M. J. 2010. Unravelling migratory connectivity in marine turtles using multiple methods. J. Appl. Ecol. 47, 769–778 10.1111/j.1365-2664.2010.01817.x (doi:10.1111/j.1365-2664.2010.01817.x) [DOI] [Google Scholar]
- 37.Monzón-Argüello C., López-Jurado L. F., Rico C., Marco A., Lopez P., Hays G. C., Lee P. L. M. 2010. Evidence from genetic and Lagrangian drifter data for transatlantic transport of small juvenile green turtles. J. Biogeogr. 37, 1752–1766 10.1111/j.1365-2699.2010.02326.x (doi:10.1111/j.1365-2699.2010.02326.x) [DOI] [Google Scholar]
- 38.Okuyama J., Kitagawa T., Zenimoto K., Kimura S., Arai N., Sasai Y., Sasaki H. 2011. Trans-Pacific dispersal of loggerhead turtle hatchlings inferred from numerical simulation modeling. Mar. Biol. 158, 2055–2063 10.1007/s00227-011-1712-9 (doi:10.1007/s00227-011-1712-9) [DOI] [Google Scholar]
- 39.Abreu-Grobois F. A., Horrocks J., Formia A., LeRoux R., Velez-Zuazo X., Dutton P., Soares L., Meylan P., Browne D. 2006. New mtDNA Dloop primers which work for a variety of marine turtle species may increase the resolution capacity of mixed stock analysis. In Book of abstracts of the 26th Annual Symp. on Sea Turtle Biology and Conservation, Crete, Greece, 3–8 April 2006 (eds Frick M., Panagopoulou A., Rees A. F., Williams K.), p. 179 [Google Scholar]
- 40.Excoffier L., Laval L. G., Schneider S. 2005. Arlequin v. 3.0: an integrated software package for population genetics data analysis. Evol. Bioinform. Online 1, 47–50 [PMC free article] [PubMed] [Google Scholar]
- 41.Bolker B. M., Okuyama T., Bjorndal K. A., Bolten A. B. 2007. Incorporating multiple mixed stocks in mixed stock analysis: ‘many-to-many’ analyses. Mol. Ecol. 16, 685–695 10.1111/j.1365-294X.2006.03161.x (doi:10.1111/j.1365-294X.2006.03161.x) [DOI] [PubMed] [Google Scholar]
- 42.Spiegelhalter D. J., Thomas A., Best N. G., Lunn D. 2004. WinBUGS v. 2.0 users manual. Cambridge, UK: MRC Biostatistics Unit [Google Scholar]
- 43.Monzón-Argüello C., Rico C., Naro-Maciel E., Varo-Cruz N., López P., Marco A., López-Jurado L. F. 2010. Population structure and conservation implications for the loggerhead sea turtle of the Cape Verde Islands. Conserv. Genet. 11, 1871–1884 10.1007/s10592-010-0079-7 (doi:10.1007/s10592-010-0079-7) [DOI] [Google Scholar]
- 44.Grist J. P., Josey S. A., Marsh R., Good S., Coward A. C., de Cuevas B. A., Alderson S. G., New A. L., Madec G. 2010. The roles of surface heat flux and ocean heat transport during four decades of Atlantic Ocean temperature variability . Ocean Dyn. 60, 771–790 10.1007/s10236-010-0292-4 (doi:10.1007/s10236-010-0292-4) [DOI] [Google Scholar]
- 45.Blanke B., Speich S., Madec G., Doos K. 2001. A global diagnostic of interocean mass transfers. J. Phys. Oceanogr. 31, 1623–1632 10.1175/1520-0485(2001)031%3C1623:AGDOIM%3E2.0.CO;2 (doi:10.1175/1520-0485(2001)031<1623:AGDOIM>2.0.CO;2) [DOI] [Google Scholar]
- 46.Lumpkin R., Pazos M. 2006. Measuring surface currents with Surface Velocity Program drifters: the instrument, its data, and some recent results. In Lagrangian analysis and prediction of coastal and ocean dynamics (LAPCOD) (eds Griffa A., Kirwan A. D., Mariano A. J., Ozgokmen T., Rossby T.), pp. 39–65 London, UK: Cambridge University Press [Google Scholar]
- 47.Varo N. 2010. Reproductive biology of the loggerhead turtle (Caretta caretta Linnaeus, 1758) in the island of Boavista, Cape Verde archipelago. Doctoral dissertation University of Las Palmas de Gran Canaria, Spain [Google Scholar]
- 48.Chiswell S.M., Wilkin J., Booth J. D., Stanton B. 2003. Trans-Tasman Sea larval transport: is Australia a source for New Zealand rock lobsters? Mar. Ecol. Prog. Ser. 247, 173–182 10.3354/meps247173 (doi:10.3354/meps247173) [DOI] [Google Scholar]
- 49.Collins C. J., Fraser C. I., Ashcroft A., Waters J. M. 2010. Asymmetric dispersal of southern bull-kelp (Durvillaea antarctica) adults in coastal New Zealand: testing an oceanographic hypothesis. Mol. Ecol. 19, 4572–4580 10.1111/j.1365-294X.2010.04842.x (doi:10.1111/j.1365-294X.2010.04842.x) [DOI] [PubMed] [Google Scholar]
- 50.Putman N. F., Bane J. M., Lohmann K. J. 2010. Sea turtle nesting distributions and oceanographic constraints on hatchling migration. Proc. R. Soc. B 277, 3631–3637 10.1098/rspb.2010.1088 (doi:10.1098/rspb.2010.1088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bellido J. J., Báez J. C., Sánchez R. F., Castillo J. J., Martín J. J., Mons J. L., Real R. 2008. Mass strandings of cold-stunned loggerhead turtles in the south Iberian Peninsula: ethological implications . Ethol. Ecol. Evol. 20, 401–405 10.1080/08927014.2008.9522520 (doi:10.1080/08927014.2008.9522520) [DOI] [Google Scholar]
- 52.Fraser C. I., Nikula R., Waters J. M. 2011. Oceanic rafting by a coastal community. Proc. R. Soc. B 278, 649–655 10.1098/rspb.2010.1117 (doi:10.1098/rspb.2010.1117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dell'Amico F., Morinière P. 2010. Observations de tortues marines en 2008 et 2009 (Côtes atlantiques françaises). Ann. Soc. Sci. Nat. Charente-Maritime 10, 69–76 [Google Scholar]
- 54.Wang C., Liu H., Lee S.-K., Atlas R. 2011. Impact of the Atlantic warm pool on United States landfalling hurricanes. Geophys. Res. Lett. 38, L19702. 10.1029/2011GL049265 (doi:10.1029/2011GL049265) [DOI] [Google Scholar]
- 55.Kossin J. P., Camargo S. J., Sitkowski M. 2010. Climate modulation of North Atlantic hurricane tracks. J. Clim. 23, 3057–3076 10.1175/2010JCLI3497.1 (doi:10.1175/2010JCLI3497.1) [DOI] [Google Scholar]