Abstract
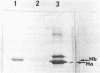
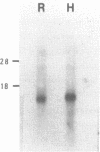
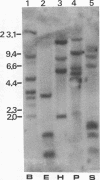
We have isolated from a lambda gt10 cDNA library a clone lambda GTH4 which encodes a human liver glutathione S-transferase Hb subunit, designated as subunit 4. Expression of this cDNA in E. coli and subsequent purification and immunoblotting analysis provided a definitive assignment of a structure and function relationship. RNA blot hybridization with human liver poly(A) RNA revealed a single band of approximately 1200 nucleotides, comparable in size to the rat brain Yb3 mRNA. Divergence analysis of amino acid replacement sites in subunit 4 relative to the four rat Yb subunits revealed that it is most closely related to the brain-specific Yb3 subunit. This conclusion is further substantiated by the nucleotide sequence homology between lambda GTH4 and the Yb3 cDNA in their 3' untranslated region. In situ chromosome mapping has located this glutathione S-transferase gene in the region of p31 on chromosome 1. Results from many laboratories, including ours, indicate that the human glutathione S-transferases are encoded by a gene superfamily which is located on at least two different chromosomes.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramovitz M., Listowsky I. Selective expression of a unique glutathione S-transferase Yb3 gene in rat brain. J Biol Chem. 1987 Jun 5;262(16):7770–7773. [PubMed] [Google Scholar]
- Awasthi Y. C., Singh S. V. Subunit structure of human and rat glutathione S-transferases. Comp Biochem Physiol B. 1985;82(1):17–23. doi: 10.1016/0305-0491(85)90121-x. [DOI] [PubMed] [Google Scholar]
- Balloul J. M., Sondermeyer P., Dreyer D., Capron M., Grzych J. M., Pierce R. J., Carvallo D., Lecocq J. P., Capron A. Molecular cloning of a protective antigen of schistosomes. Nature. 1987 Mar 12;326(6109):149–153. doi: 10.1038/326149a0. [DOI] [PubMed] [Google Scholar]
- Bennett C. F., Spector D. L., Yeoman L. C. Nonhistone protein BA is a glutathione S-transferase localized to interchromatinic regions of the cell nucleus. J Cell Biol. 1986 Feb;102(2):600–609. doi: 10.1083/jcb.102.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Board P. G. Biochemical genetics of glutathione-S-transferase in man. Am J Hum Genet. 1981 Jan;33(1):36–43. [PMC free article] [PubMed] [Google Scholar]
- Board P. G., Webb G. C. Isolation of a cDNA clone and localization of human glutathione S-transferase 2 genes to chromosome band 6p12. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2377–2381. doi: 10.1073/pnas.84.8.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130–7139. [PubMed] [Google Scholar]
- Harper M. E., Saunders G. F. Localization of single copy DNA sequences of G-banded human chromosomes by in situ hybridization. Chromosoma. 1981;83(3):431–439. doi: 10.1007/BF00327364. [DOI] [PubMed] [Google Scholar]
- Hirrell P. A., Hume R., Fryer A. A., Collins M. F., Drew R., Bradwell A. R., Strange R. C. Studies on the developmental expression of glutathione S-transferase isoenzymes in human heart and diaphragm. Biochim Biophys Acta. 1987 Oct 15;915(3):371–377. doi: 10.1016/0167-4838(87)90022-7. [DOI] [PubMed] [Google Scholar]
- Kamisaka K., Habig W. H., Ketley J. N., Arias M., Jakoby W. B. Multiple forms of human glutathione S-transferase and their affinity for bilirubin. Eur J Biochem. 1975 Dec 1;60(1):153–161. doi: 10.1111/j.1432-1033.1975.tb20987.x. [DOI] [PubMed] [Google Scholar]
- Kano T., Sakai M., Muramatsu M. Structure and expression of a human class pi glutathione S-transferase messenger RNA. Cancer Res. 1987 Nov 1;47(21):5626–5630. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai H. C., Grove G., Tu C. P. Cloning and sequence analysis of a cDNA for a rat liver glutathione S-transferase Yb subunit. Nucleic Acids Res. 1986 Aug 11;14(15):6101–6114. doi: 10.1093/nar/14.15.6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai H. C., Tu C. P. Rat glutathione S-transferases supergene family. Characterization of an anionic Yb subunit cDNA clone. J Biol Chem. 1986 Oct 15;261(29):13793–13799. [PubMed] [Google Scholar]
- Laisney V., Nguyen Van Cong, Gross M. S., Frezal J. Human genes for glutathione S-transferases. Hum Genet. 1984;68(3):221–227. doi: 10.1007/BF00418392. [DOI] [PubMed] [Google Scholar]
- Li N. Q., Reddanna P., Thyagaraju K., Reddy C. C., Tu C. P. Expression of glutathione S-transferases in rat brains. J Biol Chem. 1986 Jun 15;261(17):7596–7599. [PubMed] [Google Scholar]
- Mannervik B. The isoenzymes of glutathione transferase. Adv Enzymol Relat Areas Mol Biol. 1985;57:357–417. doi: 10.1002/9780470123034.ch5. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Perler F., Efstratiadis A., Lomedico P., Gilbert W., Kolodner R., Dodgson J. The evolution of genes: the chicken preproinsulin gene. Cell. 1980 Jun;20(2):555–566. doi: 10.1016/0092-8674(80)90641-8. [DOI] [PubMed] [Google Scholar]
- Rhoads D. M., Zarlengo R. P., Tu C. P. The basic glutathione S-transferases from human livers are products of separate genes. Biochem Biophys Res Commun. 1987 May 29;145(1):474–481. doi: 10.1016/0006-291x(87)91345-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S. V., Kurosky A., Awasthi Y. C. Human liver glutathione S-transferase psi. Chemical characterization and secondary-structure comparison with other mammalian glutathione S-transferases. Biochem J. 1987 Apr 1;243(1):61–67. doi: 10.1042/bj2430061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S. V., Leal T., Ansari G. A., Awasthi Y. C. Purification and characterization of glutathione S-transferases of human kidney. Biochem J. 1987 Aug 15;246(1):179–186. doi: 10.1042/bj2460179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. B., Davern K. M., Board P. G., Tiu W. U., Garcia E. G., Mitchell G. F. Mr 26,000 antigen of Schistosoma japonicum recognized by resistant WEHI 129/J mice is a parasite glutathione S-transferase. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8703–8707. doi: 10.1073/pnas.83.22.8703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soma Y., Satoh K., Sato K. Purification and subunit-structural and immunological characterization of five glutathione S-transferases in human liver, and the acidic form as a hepatic tumor marker. Biochim Biophys Acta. 1986 Feb 14;869(3):247–258. doi: 10.1016/0167-4838(86)90064-6. [DOI] [PubMed] [Google Scholar]
- Stockman P. K., Beckett G. J., Hayes J. D. Identification of a basic hybrid glutathione S-transferase from human liver. Glutathione S-transferase delta is composed of two distinct subunits (B1 and B2). Biochem J. 1985 Apr 15;227(2):457–465. doi: 10.1042/bj2270457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange R. C., Davis B. A., Faulder C. G., Cotton W., Bain A. D., Hopkinson D. A., Hume R. The human glutathione S-transferases: developmental aspects of the GST1, GST2, and GST3 loci. Biochem Genet. 1985 Dec;23(11-12):1011–1028. doi: 10.1007/BF00499944. [DOI] [PubMed] [Google Scholar]
- Taylor J. B., Vidal A., Torpier G., Meyer D. J., Roitsch C., Balloul J. M., Southan C., Sondermeyer P., Pemble S., Lecocq J. P. The glutathione transferase activity and tissue distribution of a cloned Mr28K protective antigen of Schistosoma mansoni. EMBO J. 1988 Feb;7(2):465–472. doi: 10.1002/j.1460-2075.1988.tb02834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu C. P., Matsushima A., Li N. Q., Rhoads D. M., Srikumar K., Reddy A. P., Reddy C. C. Immunological and sequence interrelationships between multiple human liver and rat glutathione S-transferases. J Biol Chem. 1986 Jul 15;261(20):9540–9545. [PubMed] [Google Scholar]
- Tu C. P., Qian B. Human liver glutathione S-transferases: complete primary sequence of an Ha subunit cDNA. Biochem Biophys Res Commun. 1986 Nov 26;141(1):229–237. doi: 10.1016/s0006-291x(86)80358-8. [DOI] [PubMed] [Google Scholar]
- Vander Jagt D. L., Hunsaker L. A., Garcia K. B., Royer R. E. Isolation and characterization of the multiple glutathione S-transferases from human liver. Evidence for unique heme-binding sites. J Biol Chem. 1985 Sep 25;260(21):11603–11610. [PubMed] [Google Scholar]
- Warholm M., Guthenberg C., Mannervik B. Molecular and catalytic properties of glutathione transferase mu from human liver: an enzyme efficiently conjugating epoxides. Biochemistry. 1983 Jul 19;22(15):3610–3617. doi: 10.1021/bi00284a011. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]
- Zweig S. E. Analysis of large nucleic acid dot matrices on small computers. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):767–776. doi: 10.1093/nar/12.1part2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]