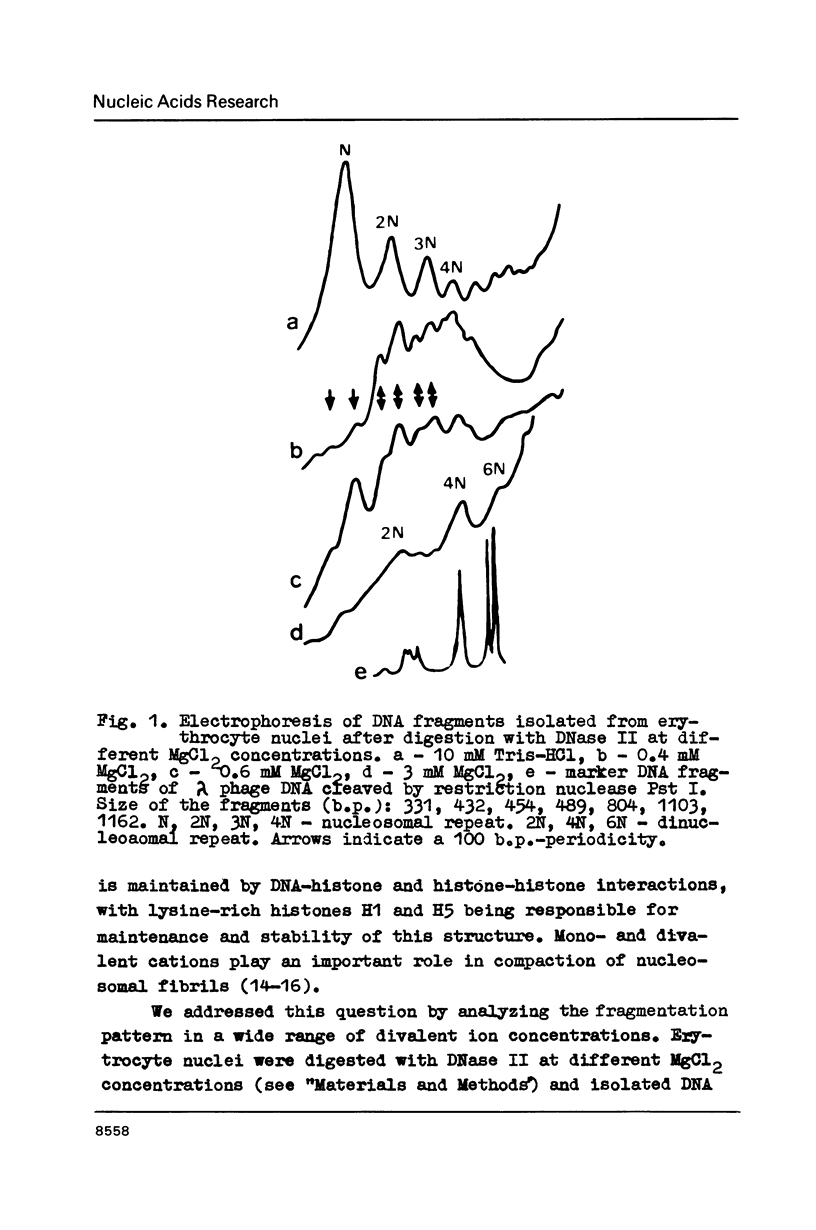
Abstract
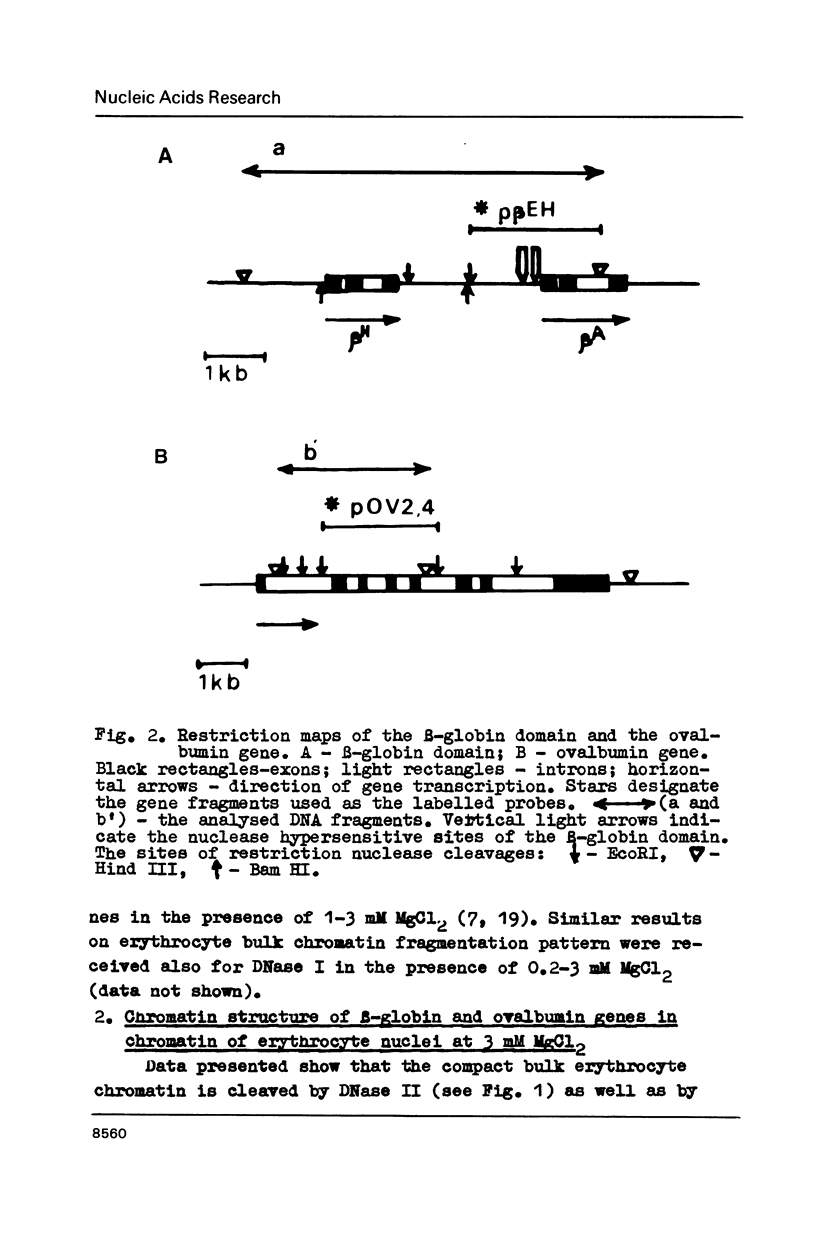
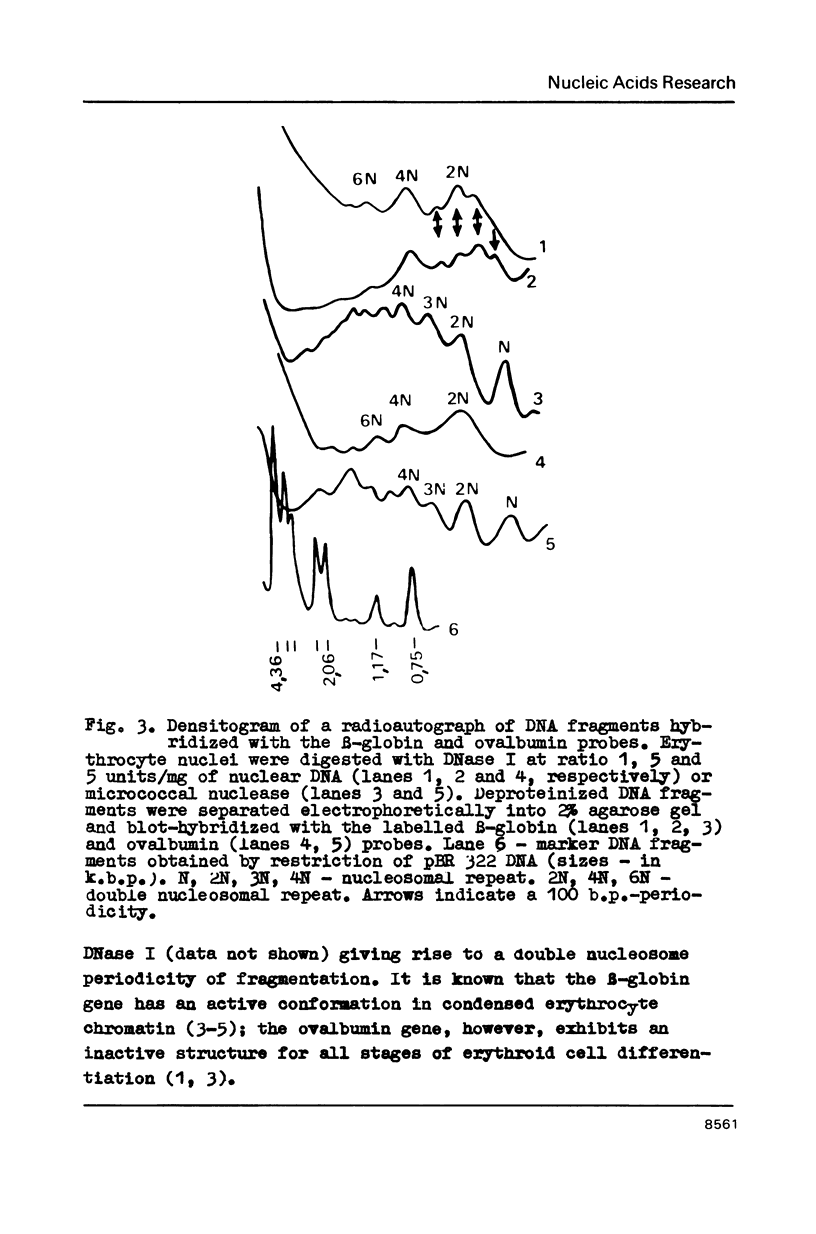
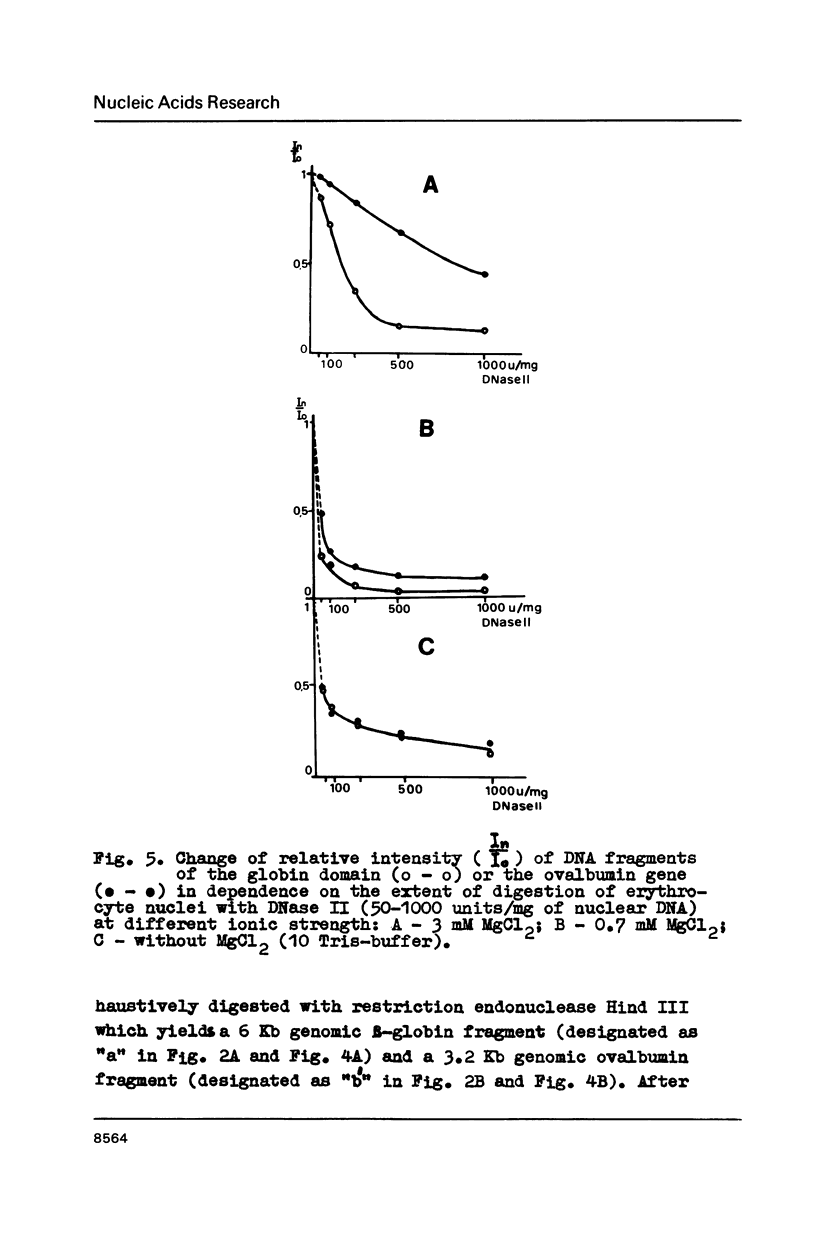
In the presence of 3 mM MgCl2 DNase I cleavage of bulk, globin and ovalbumin gene chromatin in chicken erythrocyte nuclei generates fragments which are multiples of a double-nucleosome repeat. However, in addition to the dinucleosomal periodicity beta-globin gene chromatin was fragmented into multiples of a 100 b.p. interval which is characteristic for partially unfolded chromatin. This distinction correlates with higher sensitivity of beta-globin domain to DNase I and DNase II as compared to the inactive ovalbumin gene. At 0.7 mM MgCl2 where these DNases fragment bulk chromatin into series of fragments with a 100 b.p. interval, the difference in digestibility of the investigated genes is dramatically decreased. When chromatin has been decondensed by incubation of nuclei in 10 mM Tris-buffer, DNase II generates a typical nucleosomal repeat, and the differential nuclease sensitivity of the analyzed genes is not observed. The data suggest that higher nuclease sensitivity of potentially active genes is due to irregularities in higher order chromatin structure.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azorín F., Pérez-Grau L., Subirana J. A. Supranucleosomal organization of chromatin. Electron microscopic visualization of long polynucleosomal chains. Chromosoma. 1982;85(2):251–260. doi: 10.1007/BF00294969. [DOI] [PubMed] [Google Scholar]
- Burgoyne L. A., Skinner J. D. Chromatin superstructure: the next level of structure above the nucleosome has an alternating character. A two-nucleosome based series is generated by probes armed with DNAase-I acting on isolated nuclei. Biochem Biophys Res Commun. 1981 Apr 15;99(3):893–899. doi: 10.1016/0006-291x(81)91247-x. [DOI] [PubMed] [Google Scholar]
- Caplan A., Kimura T., Gould H., Allan J. Perturbation of chromatin structure in the region of the adult beta-globin gene in chicken erythrocyte chromatin. J Mol Biol. 1987 Jan 5;193(1):57–70. doi: 10.1016/0022-2836(87)90626-7. [DOI] [PubMed] [Google Scholar]
- Godson G. N., Vapnek D. A simple method of preparing large amounts of phiX174 RF 1 supercoiled DNA. Biochim Biophys Acta. 1973 Apr 11;299(4):516–520. doi: 10.1016/0005-2787(73)90223-2. [DOI] [PubMed] [Google Scholar]
- Hörz W., Zachau H. G. Deoxyribonuclease II as a probe for chromatin structure. I. Location of cleavage sites. J Mol Biol. 1980 Dec 15;144(3):305–327. doi: 10.1016/0022-2836(80)90093-5. [DOI] [PubMed] [Google Scholar]
- Khachatrian A. T., Pospelov V. A., Svetlikova S. B., Vorob'ev V. I. Nucleodisome - a new repeat unit of chromatin revealed in nuclei of pigeon erythrocytes by DNase I digestion. FEBS Lett. 1981 Jun 1;128(1):90–92. doi: 10.1016/0014-5793(81)81087-3. [DOI] [PubMed] [Google Scholar]
- Khachatrian A. T., Pospelov V. A., Vorob'ev V. I. Neravnomernoe raspredelenie gistonov N1 i N5 v khromatine éritrotsitov golubia. Mol Biol (Mosk) 1983 Jan-Feb;17(1):72–81. [PubMed] [Google Scholar]
- Lebkowski J. S., Laemmli U. K. Evidence for two levels of DNA folding in histone-depleted HeLa interphase nuclei. J Mol Biol. 1982 Apr 5;156(2):309–324. doi: 10.1016/0022-2836(82)90331-x. [DOI] [PubMed] [Google Scholar]
- Makarov V., Dimitrov S., Smirnov V., Pashev I. A triple helix model for the structure of chromatin fiber. FEBS Lett. 1985 Feb 25;181(2):357–361. doi: 10.1016/0014-5793(85)80292-1. [DOI] [PubMed] [Google Scholar]
- McGhee J. D., Wood W. I., Dolan M., Engel J. D., Felsenfeld G. A 200 base pair region at the 5' end of the chicken adult beta-globin gene is accessible to nuclease digestion. Cell. 1981 Nov;27(1 Pt 2):45–55. doi: 10.1016/0092-8674(81)90359-7. [DOI] [PubMed] [Google Scholar]
- Pospelov V. A., Svetlikova S. B. On the mechanism of nucleodisome splitting off by nucleases. FEBS Lett. 1982 Sep 6;146(1):157–160. doi: 10.1016/0014-5793(82)80725-4. [DOI] [PubMed] [Google Scholar]
- Pospelov V. A., Svetlikova S. B., Vorob'ev V. I. Nucleosome packing in chromatin as revealed by nuclease digestion. Nucleic Acids Res. 1979 Jan;6(1):399–419. doi: 10.1093/nar/6.1.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridsdale J. A., Davie J. R. Chicken erythrocyte polynucleosomes which are soluble at physiological ionic strength and contain linker histones are highly enriched in beta-globin gene sequences. Nucleic Acids Res. 1987 Feb 11;15(3):1081–1096. doi: 10.1093/nar/15.3.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rocha E., Davie J. R., van Holde K. E., Weintraub H. Differential salt fractionation of active and inactive genomic domains in chicken erythrocyte. J Biol Chem. 1984 Jul 10;259(13):8558–8563. [PubMed] [Google Scholar]
- Sperling L., Tardieu A., Weiss M. C. Chromatin repeat length in somatic hybrids. Proc Natl Acad Sci U S A. 1980 May;77(5):2716–2720. doi: 10.1073/pnas.77.5.2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalder J., Larsen A., Engel J. D., Dolan M., Groudine M., Weintraub H. Tissue-specific DNA cleavages in the globin chromatin domain introduced by DNAase I. Cell. 1980 Jun;20(2):451–460. doi: 10.1016/0092-8674(80)90631-5. [DOI] [PubMed] [Google Scholar]
- Sun Y. L., Xu Y. Z., Bellard M., Chambon P. Digestion of the chicken beta-globin gene chromatin with micrococcal nuclease reveals the presence of an altered nucleosomal array characterized by an atypical ladder of DNA fragments. EMBO J. 1986 Feb;5(2):293–300. doi: 10.1002/j.1460-2075.1986.tb04212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma F., Koller T., Klug A. Involvement of histone H1 in the organization of the nucleosome and of the salt-dependent superstructures of chromatin. J Cell Biol. 1979 Nov;83(2 Pt 1):403–427. doi: 10.1083/jcb.83.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H., Groudine M. Chromosomal subunits in active genes have an altered conformation. Science. 1976 Sep 3;193(4256):848–856. doi: 10.1126/science.948749. [DOI] [PubMed] [Google Scholar]
- Weintraub H. Histone-H1-dependent chromatin superstructures and the suppression of gene activity. Cell. 1984 Aug;38(1):17–27. doi: 10.1016/0092-8674(84)90522-1. [DOI] [PubMed] [Google Scholar]
- Zasloff M., Camerini-Otero R. D. Limited DNase I nicking as a probe of gene conformation. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1907–1911. doi: 10.1073/pnas.77.4.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]