Abstract
Currently, numerous hydrogels are under examination as potential nucleus replacements. The clinical success, however, depends on how well the mechanical function of the host structure is restored. This study aimed to evaluate the extent to and mechanisms by which surgery for nucleus replacements influence the mechanical behaviour of the disc. The effects of an annulus defect with and without nucleus replacement on disc height and nucleus pressure were measured using 24 ovine motion segments. The following cases were considered: intact; annulus incision repaired by suture and glue; annulus incision with removal and re-implantation of nucleus tissue repaired by suture and glue or plug. To identify the likely mechanisms observed in vitro, a finite-element model of a human disc (L4–L5) was employed. Both studies were subjected to physiological cycles of compression and recovery. A repaired annulus defect did not influence the disc behaviour in vitro, whereas additional nucleus removal and replacement substantially decreased disc stiffness and nucleus pressure. Model predictions demonstrated the substantial effects of reductions in replaced nucleus water content, bulk modulus and osmotic potential on disc height loss and pressure, similar to measurements. In these events, the compression load transfer in the disc markedly altered by substantially increasing the load on the annulus when compared with the nucleus. The success of hydrogels for nucleus replacements is not only dependent on the implant material itself but also on the restoration of the environment perturbed during surgery. The substantial effects on the disc response of disruptions owing to nucleus replacements can be simulated by reduced nucleus water content, elastic modulus and osmotic potential.
Keywords: intervertebral disc, hydrogels, disruptions, interface, finite-element method, in vitro
1. Introduction
More than 80 per cent of the population in industrialized countries have back pain at some time during their lives, which affects their quality of life and the workforce [1]. Low back pain most frequently occurs between ages 30 and 50 and therefore has enormous global socioeconomic and healthcare consequences. Back pain is strongly associated with degeneration of the intervertebral disc [2]. The intervertebral disc supports large loads and permits multi-axial motions of the spine. These demanding mechanical functions are supported by its complex structure that includes the nucleus pulposus at the centre surrounded by the highly organized lamellar annulus fibrosus at the periphery and cartilaginous endplates at both ends. With degeneration, the disc undergoes major changes in its composition that alter its normal mechanical function [3,4], which possibly leads to the emergence of low back pain. The disc degeneration process often starts in the nucleus space by decreasing its proteoglycan concentration and fluid content while increasing its unorganized collagen content.
Depending on the degree of degeneration, patients may face moderately invasive surgery, such as partial discectomy and laminectomy, or more invasive interventions, such as fusion or total disc replacements. Even moderate interventions are associated with complications involving disc height reduction and instabilities. Therefore, an optimal solution at the early stages of degeneration should focus on the structural and biomechanical restoration of the disc nucleus by minimally invasive surgery.
During the last decade, better understanding of the intervertebral disc function and technical advancements in the field of tissue engineering have resulted in numerous strategies to replace or regenerate the nucleus pulposus for the treatment of low back pain in the early stages of disc degeneration [5,6]. While hydrogels are considered as one of the most promising solutions [7], nevertheless no procedure has yet been recognized as a clinically proven effective therapy.
An optimal replacement or regeneration therapy of the nucleus should bring about an environment that closely replicates the physiological strains and stresses in a non-degenerate disc. As an example, fluid flow is essential for the normal biomechanical function of the disc as well as the survival of disc cells. Owing to the mechanical interactions between the disc nucleus and surrounding spinal structures, a successful strategy should aim to reproduce near-normal conditions for not only the nucleus itself but also the entire motion segment.
Located at the disc centre, the nucleus pulposus is enclosed by and integrated with strong annulus fibrosus layers on its periphery and endplates at the top and bottom. Material continuity at the interfaces allows for the support and transmission of tensile and shear stresses. After nucleotomy and implantation of a nucleus replacement, this interface is, however, destroyed. Furthermore, the mechanical function of the entire disc is directly dependent on the annulus integrity that is partially compromised during the replacement procedure. Extrusion of the implanted material is also of major concern for nucleus replacement strategies [8,9] as an effective sealant of the entire annulus defect width cannot be guaranteed, in particular in the inaccessible inner annulus regions. Owing to this inferior quality of the repaired annulus, forces caused by a high nucleus pressure may not be supported, in which case the implant material squeezes out of the nucleus cavity into the inner annulus defect. Both the destruction of the interface and the gaping of the inner annulus defect are likely, therefore, to compromise the mechanical integrity of the whole disc. Currently, however, the influence of each of these disruptions on the mechanical function of the disc remains unknown.
In this study, the extent of the foregoing alterations in disc biomechanics is investigated by both in vitro measurements and finite-element (FE) model studies. The in vitro part focuses on whether (i) a given annulus defect with or without the destruction of the annulus–nucleus interface has a mechanical effect on the disc height loss and nucleus pressure and (ii) the re-implantation of the native nucleus, representing the design of an ideal nucleus replacement strategy, has the ability to restore the biomechanical behaviour to a level comparable to that of the intact disc. To complement measurements, FE model studies simulating different re-implantation conditions are also performed in order to identify the underlying mechanisms observed in vitro.
2. Material and methods
2.1. In vitro: specimen preparation
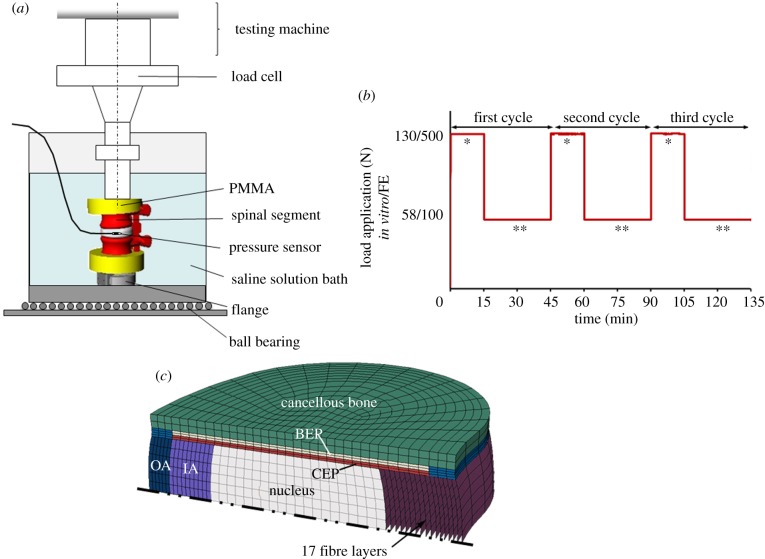
Twenty-four motion segments (12 × L2–L3, 12 × L4–L5) from adult merino sheep (3–6 years old) were harvested and stored at –20°C. These motion segments were stripped of muscles and soft tissues, preserving ligaments, capsules of facet joints and intervertebral discs. Following preparation, the motion segments were embedded in polymethylmethacrylate (PMMA, Technovit 3040, Heraeus Kulzer, Werheim, Germany) on the proximal end of the cranial and distal end of the caudal vertebrae. The upper and lower PMMA surfaces were aligned parallel to each other and to the disc midplane. Via a metal flange on the bottom of the lower PMMA block, specimens were fixed into an electromechanical material-testing machine (Z010, Zwick GmbH & Co. KG, Ulm, Germany; figure 1a). The axial force was regulated by a 1 kN load cell and applied without any sagittal rotation by a fixed-angle stamp. To measure the intradiscal pressure (IDP), a pressure sensor (Ø1.45 mm, FMSPEZ50, Mammendorfer Institut für Physik und Medizin GmbH, Mammendorf, Germany) was inserted into the centre of each nucleus. The output signal was transmitted via telemetry (Biotel 33 hybrid, Glonner Electronic GmbH, Martinsried, Germany), through a measuring amplifier (DMCplus; HBM Hottinger Baldwin Messtechnik, Darmstadt, Germany) and recorded at 50 Hz. During mechanical testing, specimens were placed in a bath with physiological buffered saline solution at room temperature. Owing to the long duration of experiments, each specimen was tested only once.
Figure 1.
(a) Experimental set-up for the in vitro compression tests on sheep motion segments. (b) Time history of the applied compressive force in vitro/in silico: three loading cycles were performed, each consisting of a loading period of 15 min at 130/500 N and a recovery period of 30 min at 58/100 N (single asterisks denote diurnal load resulting in a creep response; double asterisks denote night load resulting in a recovery response). (c) Symmetric finite-element model of the human lumbar intervertebral disc (IA, inner annulus; OA, outer annulus; CEP, cartilage endplate; BEP, bony endplate).
2.2. In vitro: test groups
The following cases were considered in the current in vitro studies (table 1):
INTACT (n = 6): the nucleus and annulus were left intact (except for the minor damage caused by inserting the pressure transducer that occurred in all specimen groups).
Annulus defect (DEF-ANN, n = 6): a small oblique incision through the whole annulus depth was set at the left lateral position (approx. 4 mm wide). The nucleus itself was not affected by this defect. The access was subsequently closed by sealing the incision with a mattress suture and cyanoacrylate glue.
Nucleus replacement (REPL-SG, n = 6): nucleus tissue was removed in pieces from the disc (total masses are given in table 1) using rongeurs with straight and flexed jaws of 1 and 1.5 mm size, respectively. The removed material was preserved for approximately 10 min in an airtight tube. Subsequently, the explanted nucleus tissue was completely re-implanted. The annulus defect was closed as in (ii) by suture and glue (SG).
Annulus closure devices (REPL-CD, n = 6): in contrast to (iii), the annulus defect was closed by a hollow mushroom-shaped balloon plug made of polyurethane that was inserted into the annulus opening until the head of the plug was inside the nucleus cavity. This was intended to avoid squeezing the nucleus material out into the defect. After fixation of the plug with SG, the closure device (CD) was filled with silicone.
Table 1.
Specimens used in different biomechanical tests.
| state | level | removed nucleus pulposus mass |
|---|---|---|
| INTACT | 6 × L2–L3 | — |
| DEF-ANN | 6 × L2–L3 | — |
| REPL-SG | 6 × L4–L5 | 0.14 g (0.1–0.15 g) |
| REPL-CD | 6 × L4–L5 | 0.14 g (0.1–0.15 g) |
2.3. In vitro: loading protocol
Specimens were subjected to three loading cycles. While the first cycle was used for pre-conditioning, the second and third cycles were used for data analysis. Each loading cycle consisted of a 15 min diurnal load under an axial compression of 130 N and a recovery period of 30 min (night load) at 58 N (figure 1b). These physiological loads were estimated based on an earlier in vivo study on the measurement of intervertebral disc (IVD) pressure in sheep [10]. Load application and release were performed at 30 N s−1.
2.4. In vitro: statistics
For the compression test, disc height loss was adjusted relative to 58 N at the end of the first loading cycle. Differences at the end of each loading and recovery phase were tested using the unpaired, two-sample Wilcoxon signed-rank test (p < 0.05). Statistics was performed using GNU R (R Development Core [11]).
2.5. In silico: mesh generation
A previously developed biphasic, nonlinear, symmetric FE model of the L4–L5 human lumbar intervertebral disc was used. The model includes nucleus pulposus, inner and outer annulus as well as cartilage and bony endplates (figure 1c). In the annulus, there are 17 criss-crossed fibre layers in the radial direction in which each layer is defined with a nonlinear stress–strain relationship [12].
2.6. In silico: material properties
The disc is modelled using an osmoelastic material consisting of a fluid phase and a fully saturated neo-Hookean porous solid phase. The material model was described in previous studies [13].
2.7. In silico: boundary and loading conditions
Because the model geometry and material properties are based on the human disc, the load magnitudes for the diurnal and night periods were adapted to the corresponding load magnitudes of 500 and 100 N, respectively, found in human lumbar spines in vivo [14]. Following a pre-conditioning cycle, which included a swelling phase until equilibrium (approx. 28 h), three loading cycles were applied similar to those in vitro (figure 1b) with the sagittal rotation constrained.
2.8. In silico: test groups
INTACTFE-PERM describes the above-mentioned FE model, which was initially validated with human in vivo pressure and displacement measurements [12]. Another intact model was created by blocking fluid inflow (INTACTFE-BLOCKED) in an attempt to simulate the observation in in vitro models of the virtual absence of fluid flow into the disc during recovery phases [15–17]. This was realized by defining the endplate as impermeable during the load release and recovery phases. Both foregoing models were subsequently modified as follows.
REPL-100FE-PERM/REPL-100FE-BLOCKED: the removed nucleus was completely re-implanted. This model aimed to study the effect of damage at the interface between the nucleus and surrounding tissues.
REPL-98FE-PERM/REPL-98FE-BLOCKED: a likely loss of nucleus into the annulus defect was modelled by assuming a smaller nucleus volume of 98 per cent resulting in a uniform initial gap of 0.1 mm at the boundary between the nucleus and surrounding tissues. Two different interface conditions were considered at the nucleus periphery: (i) the standard fluid contact condition that ensures continuity of pore pressures on opposite contacting surfaces at all times, which corresponds to a fully saturated gap, and (ii) a zero pore pressure at contacting surfaces that offers no resistance to the permeating fluid.
-
WC-80FE-PERM/WC-80FE-BLOCKED: the water content of the re-implanted nucleus tissue was reduced by 20 per cent to account for any desiccation or squeezing out of fluid from the nucleus material during removal and re-implantation in vitro. This was simulated by reducing the total implanted nucleus volume from 100 to 84 per cent, corresponding to a fluid loss of about 20 per cent and resulting in an initial uniform gap of 0.7 mm at the boundary between the nucleus and surrounding tissues.
It was further assumed that the collagen–proteoglycan compound making up the native nucleus tissue was mechanically damaged during the nucleotomy and re-implantation. For that purpose additional simulations were performed in which the material properties were separately modified.
EL-75FE-PERM/EL-75FE-BLOCKED: the elastic modulus was reduced by 25 per cent to account for the disruptions in material continuity during removal and re-implantation of the tissue.
K-75FE-PERM/K-75FE-BLOCKED: the drained solid matrix may become much more compressible after re-implantation. Therefore, the bulk modulus was decreased by 25 per cent.
OP-90FE-PERM/OP-90FE-BLOCKED: the osmotic potential of the nucleus material was reduced by decreasing the fixed charge density by 10 per cent.
It should be noted that all models in cases (5)–(7) also incorporated the 98 per cent volume model with contact interfaces because they all simulate re-implanted nucleus pulposus material.
2.9. In silico: contact conditions
For the interaction between the re-implanted material and the surrounding biological tissues (cases 2–7), a frictional contact model with a small friction coefficient of 0.01 was used.
3. Results
3.1. In vitro: INTACT
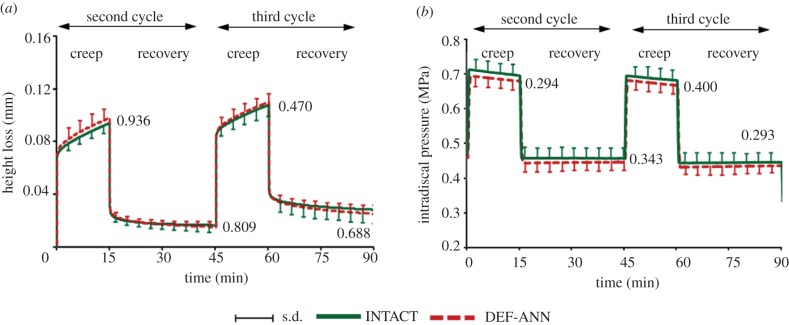
The diurnal load caused a creep response with a steady loss of specimen height. The night load led to only a partial recovery. Equilibrium was almost reached under night load but not under diurnal load. The average height loss after the complete loading protocol was 0.03 mm (approx. 1% of disc height), demonstrating that two recovery intervals of night load did not totally compensate for the loss of height under diurnal load periods (figure 2a). In the first pre-conditioning cycle, the application of 130 and 58 N initially caused IDPs of 0.75 and 0.5 MPa, respectively, that slightly decreased with time over the subsequent loading cycles. During the diurnal loads, the IDP slightly decreased by approximately 3 per cent, whereas it remained almost constant during the night loads (figure 2b).
Figure 2.
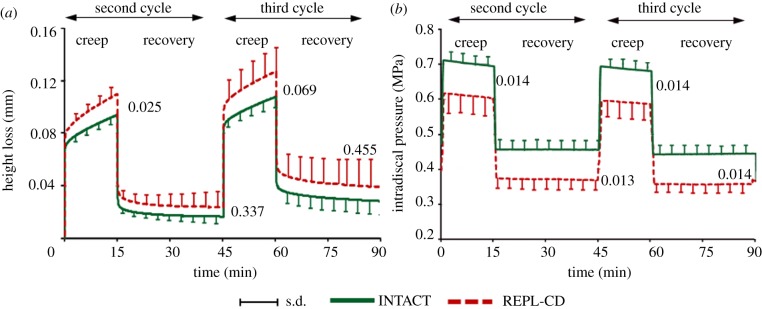
Temporal changes of averaged (a) specimen height loss and (b) intradiscal pressure measured in the centre of the nucleus for INTACT and DEF-ANN for the second and third loading cycles. Curves represent the mean and s.d. of six specimens. Specimen height loss is presented relative to the height loss under 58 N at the end of the first loading cycle. Numbers in the diagrams represent the p-values estimated at the end of each loading and recovery phase (Wilcoxon signed-rank test). DEF-ANN: a small oblique incision through the whole annulus depth was set.
3.2. In vitro: DEF-ANN versus INTACT
The annulus incision (DEF-ANN) caused no significant changes in specimen height or IDP over the two loading cycles (figure 2).
3.3. In vitro: REPL-SG versus INTACT
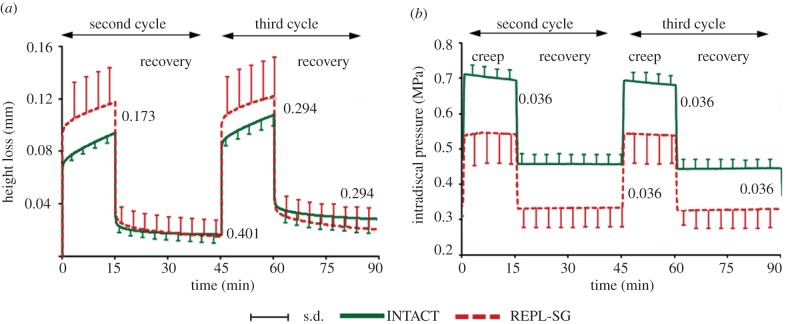
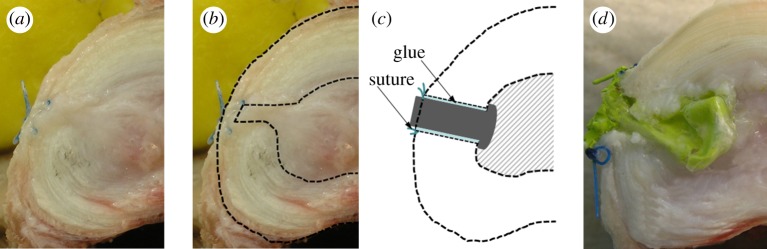
REPL-SG led to a greater mean height loss immediately after load application than did INTACT (figure 3a). This height loss slightly decreased over the two loading cycles; at the end, the height loss was comparable to that found for INTACT, with the differences remaining insignificant. During loading cycles, the IDP significantly dropped on average by approximately 26 per cent between REPL-SG and INTACT (figure 3b). Post-test macroscopic observations showed that the re-implanted nucleus material squeezed out of the nucleus cavity into the inner annulus defect all the way to the stitched outer fibrous annular layers (figure 4).
Figure 3.
Temporal changes of averaged (a) specimen height loss and (b) intradiscal pressure measured in the centre of the nucleus for INTACT and REPL-SG for the second and third loading cycles. Numbers in the diagrams represent the p-values estimated at the end of each loading and recovery phase (Wilcoxon signed-rank test). REPL-SG: nucleus tissue was removed and subsequently completely re-implanted.
Figure 4.
(a,b) Axial view of the intervertebral disc with the re-implanted nucleus (REPL-SG) after three cycles of compression. Displaced nucleus material was observed underneath the stitched and glued outer fibrous annular layers. (c) Schematic and (d) photographic views of the intervertebral disc cross section depicting the annulus closure device in REPL-CD.
3.4. In vitro: REPL-CD versus INTACT
REPL-CD (figures 4c,d and 5a) behaved similar to REPL-SG regarding the height loss during the diurnal load. For the night load, however, REPL-CD caused a reduced recovery compared with both INTACT and REPL-SG, resulting in a greater height loss at the end of the loading protocol. The balloon plug, in this case, yielded a lower drop in the disc pressure (figure 5b) than did REPL-SG; the differences between REPL-SG and REPL-CD, however, were not significant (p = 0.163–0.456).
Figure 5.
Temporal changes of averaged (a) specimen height loss and (b) intradiscal pressure measured in the centre of the nucleus for INTACT and REPL-CD for the second and third loading cycles. Numbers in the diagrams represent the p-values estimated at the end of each loading and recovery phase (Wilcoxon signed-rank test). REPL-CD: the annulus defect was closed by a hollow mushroom-shaped balloon plug.
3.5. In silico: INTACTFE
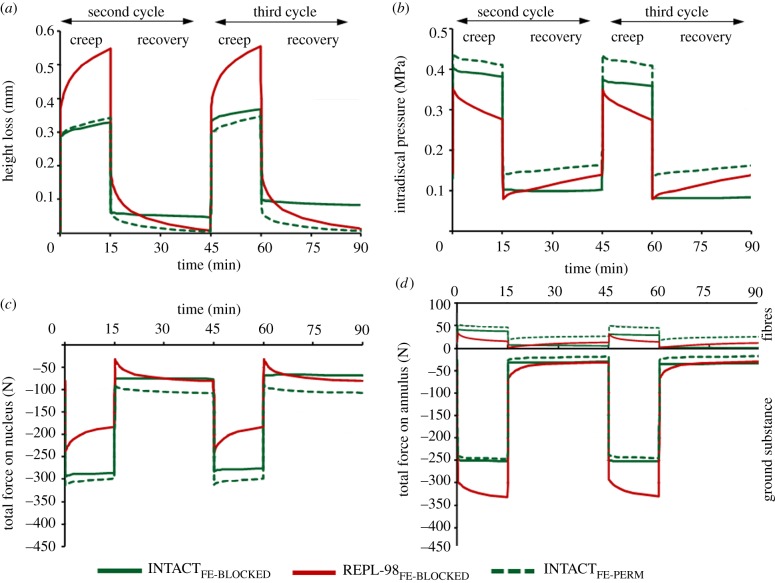
In contrast to INTACTFE-PERM, INTACTFE-BLOCKED showed a net loss of height (0.09 mm or approx. 0.7% of disc height) over the two loading and recovery periods (figure 6a). This indicates that, in accordance with measurements (figure 2a), a total of 60 min recovery with only a low-compression preload did not compensate for the loss of height under the 30 min of loading. During loading, the IDP (sum of water chemical potential and osmotic pressure) decreased more or less linearly in both INTACTFE models (figure 6b), while, during relaxation and in agreement with in vitro measurements (figure 2b), the IDP remained almost constant in INTACTFE-BLOCKED. In contrast, INTACTFE-PERM showed a slight IDP recovery. The differences in IDP between INTACTFE-PERM and INTACTFE-BLOCKED increased with time owing to a net loss of water content in INTACTFE-BLOCKED. The INTACTFE-BLOCKED model, as expected, is a better representation of the in vitro model than the INTACTFE-PERM, whose results are hence not shown hereafter for the sake of clarity in figures. About 60 per cent of the applied compression in INTACTFE-BLOCKED was transmitted through the nucleus (figure 6c) and the remainder through the annulus (figure 6d). Annulus collagen fibrils, while in tension, in turn increased the disc compression by approximately 10 per cent (figure 6d).
Figure 6.
Ninety-eight per cent nucleus replacement (REPL-98FE) versus intact disc model (INTACTFE). Temporal changes of the (a) height loss and (b) intradiscal pressure in the centre of the nucleus as well as the total axial forces transmitted through the (c) nucleus and (d) annulus (ground substance and collagen fibres). The fluid flows freely out of the nucleus into the gap assuming a zero boundary pore pressure at the outer nucleus surface.
3.6. In silico: REPL-100FE and REPL-98FE
REPL-100FE and INTACTFE yielded negligible differences in disc height loss. Immediately after the load application and throughout the creep phase, the IDP was approximately 10 per cent lower in REPL-100FE. While attempting to simulate the likely loss of nucleus into the interface gap and the annulus defect as observed in vitro, REPL-98FE yielded negligible differences compared with REPL-100FE when considering a fully saturated gap (standard contact) between the nucleus and surrounding tissues. In contrast, and with the free fluid flow out of the nucleus into the gap and not into the opposite tissue, results deviated from those of both INTACTFE and REPL-100FE and were found to be in better agreement with in vitro measurements (figure 6). The rise in IDP during recovery was, however, in contrast to in vitro data, which remained almost constant with time. Compared with INTACT, the compressive forces decreased by approximately 20 per cent on the nucleus (figure 6c) but increased on the annulus bulk by approximately 20 per cent (figure 6d) immediately after load application. Owing to the non-restricted fluid outflow, these changes further increased with time during loading periods.
3.7. In silico: WC-80FE
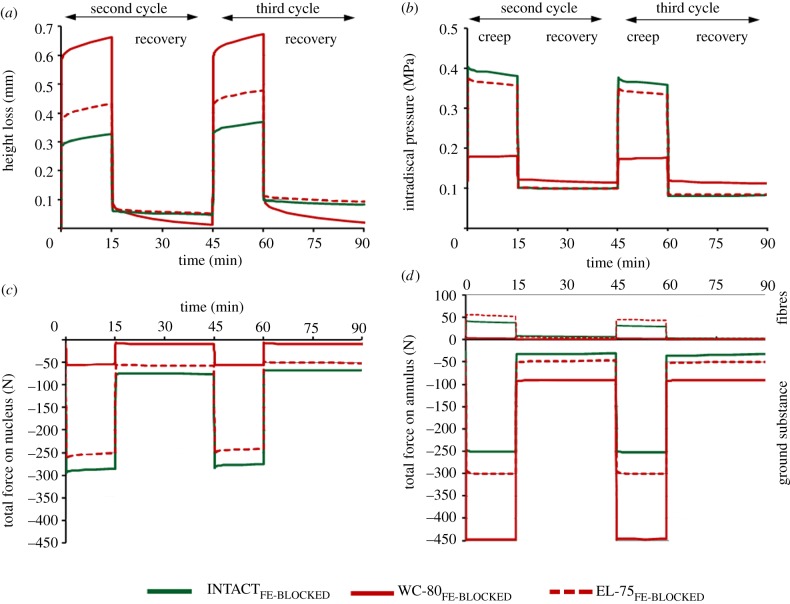
Reduced water content strongly increased the disc height loss (approx. two times larger than that calculated for INTACTFE; figure 7a) and decreased the IDP by approximately 60 per cent (figure 7b) during diurnal load. In contrast to REPL-98FE, the IDP remained almost constant during recovery, which is in agreement with in vitro measurements (figure 3). The axial compression force altered by nearly 80 per cent in the nucleus, where it decreased (figure 7c), and in the annulus, where it increased (figure 7d). The reduced water content appears to play a significant role in the disc mechanical function after re-implantation in vitro.
Figure 7.
Reduced water content of 20% (WC-80FE) and reduced elasticity of 25% (EL-75FE) of the replaced nucleus material versus intact disc model (INTACTFE). Temporal changes of the (a) height loss and (b) intradiscal pressure in the centre of the nucleus as well as the total axial forces transmitted through the (c) nucleus and (d) annulus (ground substance and collagen fibres).
3.8. In silico: EL-75FE
During the removal and insertion phases, the continuity of the intact nucleus pulposus is markedly compromised, which could alter its overall mechanical properties. Lower elastic moduli of the solid grains (EL-75FE) substantially decreased the disc overall stiffness compared with INTACTFE (figure 7a). The IDP and the compressive forces were also moderately affected (figure 7b–d).
3.9. In silico: K-75FE
The disc height loss and IDP were not markedly affected by a 25 per cent drop in the bulk modulus of the drained solid matrix of the re-implanted nucleus (κ : 5.2 → 3.9 MPa). Results remained nearly the same even with a much smaller bulk modulus (κ = 0.1 MPa).
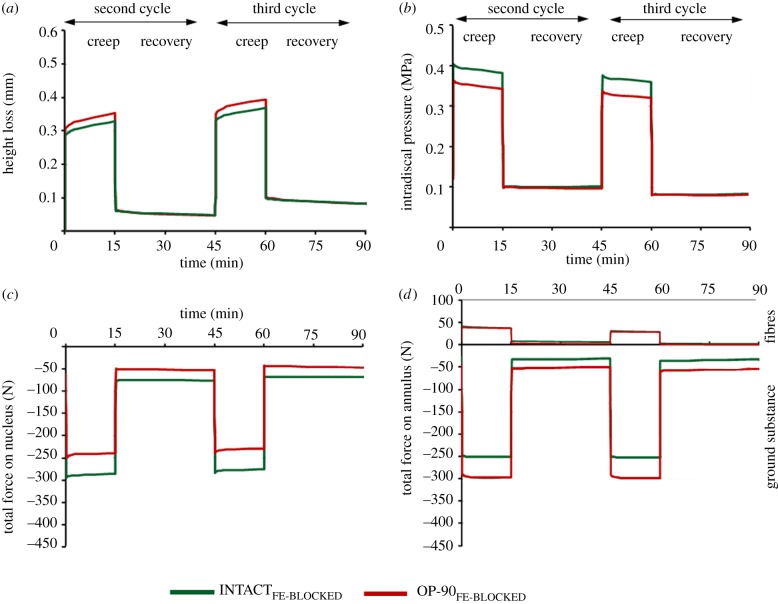
3.10. In silico: OP-90FE
Compared with INTACT, OP-90FE led to a slight increase in the disc height loss (figure 8a) and a moderate decrease in the IDP, both occurring only in the creep phase of loading (figure 8b). The compressive forces decreased within the nucleus (figure 8c) and increased within the annulus bulk (figure 8d) during the entire loading protocol.
Figure 8.
Reduced osmotic potential of 10% (OP-90FE) versus intact disc model (INTACTFE). Temporal changes of the (a) height loss and (b) intradiscal pressure in the centre of the nucleus as well as the total axial forces transmitted through the (c) nucleus and (d) annulus (ground substance and collagen fibres).
4. Discussion
Replacement of a degenerated or damaged nucleus in the presence of a relatively intact surrounding annulus fibrosus can allow for the restoration of the intervertebral disc and hence near-normal mechanical function of the entire motion segment. Being applicable to a limited population with the foregoing indications, this procedure can be applied either as an early measure to prevent degeneration or as a treatment modality in patients. This study aimed to evaluate the extent to and mechanisms by which such surgical interventions for nucleus replacement influence the mechanical behaviour of the disc. For this purpose and to avoid confounding effects associated with the alterations in the material and structural properties of the replaced material, the denucleated disc was filled in this study with the removed native nucleus material itself. This work hence concentrated on the likely alterations owing to the intervention itself. In vitro results demonstrated that a small annulus defect with an intact nucleus (DEF-ANN) did not affect the height loss and fluid pressurization of the disc. In contrast, subsequent removal and re-implantation of the native nucleus (REPL-SG and -CD) significantly increased the height loss during the creep loading phases and decreased the nucleus pressure in both creep and recovery loading phases.
In this study, the isolated annulus defect did not have a significant influence on the disc axial stiffness and central nucleus pressure (figure 2), which could be due partly to the effectiveness of defect sealing by SG and partly also to the rather small loads of up to 130 N chosen in accordance with reported in vivo values [10]. The mean height loss in creep slightly increased whereas the disc pressure slightly decreased. Earlier measurements on the effect on the disc response of annular puncture and injury remain limited and inconclusive, probably because of the dependence of alterations on the specimen tested, needle size, penetration/injury depth, load magnitude, and mechanical parameter investigated [18–20].
The additional disruptions caused by removal and replacement of the host nucleus material, however, substantially altered the segmental response; the axial stiffness and nucleus pressure were both significantly decreased (figures 3 and 4). In the in vitro model, with the SG closure of the annular defect (figure 3), nucleus material was observed to have displaced into the defect during loading (figure 4), which is likely to be partially responsible for the foregoing alterations in measurements. Greater changes would have occurred had larger compression forces been applied on specimens. A more effective sealing of the defect by an additional CD in the model REPL-CD reduced the extent of the foregoing changes in the disc axial stiffness and nucleus pressure (figure 5). This demonstrates that the preservation of the disc's overall function following nucleus replacement is only partly dependent on the quality of the closure of the annulus defect in holding the pressurized nucleus in place under applied loads. The substantial changes in response even in the apparent absence of nucleus dislocation into the annular defect (case REPL-CD) in itself highlights additional disruptions that are also in play here.
Removal and replacement of the native nucleus material, even with maximum care to minimize material damage, loss and desiccation, cause major disruptions compared with the intact condition. The tissue continuity within the nucleus space and at the nucleus interfaces with the surrounding annulus and endplate materials is irreparably compromised, which affects, among others, the transfer of shear and tensile stresses at ruptured interfaces. Current in vitro data indicate the substantial fall in axial stiffness and disc pressure following such disruptions (figures 3 and 5). The FE model REPL-100FE that simulated disruption only at the outer nucleus periphery yielded, however, only a 10 per cent drop in IDP during the creep phases and negligible increases in the disc height loss. In contrast, REPL-98FE when combined with fluid loss resulted in substantial increases in disc height loss and reductions in IDP during the creep phases of loading. Reductions in the material elastic modulus and swelling potential also, although to a lower extent, had similar effects. The observations of substantial disruptions in disc overall axial stiffness and IDP generation are likely to be due to a cumulative effect of these deteriorations in material and structural properties that were simulated individually in the current model studies. Noteworthy is the substantial effect of such disruptions on the overall load sharing between the nucleus and annulus; the annulus share of applied compression substantially increases as the nucleus share drops as it relegates its important compression-carrying role.
The trends measured in vitro could not be seen in silico when considering an even smaller nucleus volume but with a fully saturated gap. On the contrary, with a free fluid flow into and out of the interface gap, the predictions were in better agreement with measurements. However, the rise in the IDP during recovery computed in REPL-98FE alone with no other modifications, which was found to disagree with in vitro data, is likely to be due to the unrestricted fluid inflow allowed into the nucleus at its bounding surfaces. It should be mentioned that the aim of the FE simulations was not to mimic the in vitro measurements on sheep motion segments. It was rather to determine the influence of single parameters (water content, osmotic potential, etc.) on the global segmental behaviour in isolation in order to avoid confounding effects when altering different variables together. For this reason and to gain insight into the role of various parameters in disc replacements, a model of a human disc, rather than a sheep motion segment, was chosen. Also, apart from differences in loading, material properties and anatomy, in in vitro studies, however, a large number of parameters acted altogether to cause the changes recorded between different cases. Ovine spines were used in vitro because they show less degeneration and more uniform mechanical properties than elderly human spines. The nucleus water and collagen content are comparable between ovine and human intervertebral discs [21–24]. However, one limitation could be the smaller size of sheep discs and the curvature of endplates in comparison with those of the human discs. Owing to the paucity of data on material properties and micro-anatomical characterizations of ovine specimens, for the FE analyses a human lumbar disc model was used. In order to enable a comparison between the results obtained in vitro and in silico, for both models associated physiological loads were applied. In the FE model, a compressive load of 500 N was assumed, representing the expected diurnal load for standing [14]. The spinal compression substantially increases in daily activities such as those involving forward flexed posture while carrying weights or under more dynamic diurnal activities [25,26]. The results of this study on the relative effect of various replacement parameters, both in vitro and in silico, could hence alter when larger single and combined loads are considered.
After three cycles of compressive creep and recovery loading INTACT specimens with no disruptions showed a net loss of height. This is consistent with earlier in vitro results on porcine specimens in which no or limited recovery was found [15,16]. This lack of full recovery may be due to a postmortem formation of blood clots within the endplates. In vivo IDP measurements in a human disc have, however, shown increases in nucleus pressure [27], water content [28] and disc height [29,30] during 7 h of rest. In the model studies, this in vitro phenomenon was taken into account by blocking the fluid inflow at endplates expected during the recovery phases of loading. It is to be noted that, with ageing and degeneration, the endplates calcify, which impedes free fluid flow. A replacement material for the nucleus, in vivo, should preferably be able to duplicate the intact nucleus in attracting fluid and regaining height during recovery periods.
The measurements and subsequent FE studies were performed under single axial compressive forces. Although the compression force is the primary component of load on spinal motion segments in various activities of daily living, sagittal, lateral and axial rotations are also present to various degrees in many tasks. These additional modes could influence the results directly by applying tension on the annulus defect or indirectly by increasing the disc pressure. In a preliminary test, we initially applied an unconstrained compressive force by combining flexion and compression loads together. Neither the combination of SG nor the annulus CD was found to withstand the stresses to seal the annulus defect and to avoid extrusion of the nucleus material. The interface between the annulus CD and the surrounding native tissue was in such cases of inferior quality to withstand the high nucleus pressure in combination with tensile forces. Therefore, pure compression loading was chosen to avoid nucleus extrusion. Future studies should focus on the annulus CDs to prevent extrusion under larger combined loads.
In this study, an intact annulus structure was assumed. FE simulation of an annulus defect and its subsequent repair attempts is very complex and requires specially designed measurements for adequate validation. Because the initial in vitro measurements confirmed the negligible effects of such isolated repaired annulus defects on disc height and IDP, no changes in the intact annulus were represented in the second part of the study on the FE replacement models. Moreover, the annulus–nucleus boundary is not clearly demarcated (especially in degenerate discs) and hence any attempt to remove the nucleus, even with care, can inevitably cause some damage and disruption to the inner annulus layers. The original nucleus material can also be left in place in some regions. Such considerations were neglected as they would require numerous studies with complex geometries. The overall trends, nevertheless, are expected to persist as in the current simulations. To simplify the analysis, the posterior bony elements, facet articulations and all ligaments were neglected. Under the posture and loading considered in this work, the facet joints play a relatively minor role by transmitting only about 10 per cent of the applied compression loads [31,32]. The stresses and strains in the disc are hence expected to decrease by nearly the same proportion. Ligaments, on the other hand, are expected to play no or only a negligible mechanical role in pure compression.
In summary, in vitro results did not show any noticeable changes in the annulus defect when repaired. Significant changes in disc height and IDP were, however, measured when the nucleus material was also removed and replaced. While mimicking the nucleus replacement, subsequent model studies confirmed the substantial effects of reductions in nucleus water content, elastic modulus and osmotic potential on disc height loss and IDP, in overall agreement with measurements. They also demonstrated the marked alterations in overall load sharing between the nucleus and annulus regions of the disc. The success of hydrogels for nucleus replacements is not only dependent on the proper functioning of the implant material itself but also on the restoration of the overall disc environment, which is perturbed during removal and replacement operations.
Acknowledgements
This work is funded by the EU project Disc regeneration (NMP-2007-CPIP 213904) and by the German Research Foundation (WI 1352/14-1).
References
- 1.Connelly L. B., Woolf A., Brooks P. 2006. Cost-effectiveness of interventions for musculoskeletal conditions. In Disease control priorities in development countries, 2nd edn (eds Jamison D. T., Breman J. G., Measham A. R., Alleyne G., Claeson M., Evans D. B., Jha P., Mills A., Musgrove P.), pp. 963–980. New York, NY: Oxford University Press; [PubMed] [Google Scholar]
- 2.Luoma K., Riihimaki H., Luukkonen R., Raininko R., Viikari-Juntura E., Lamminen A. 2000. Low back pain in relation to lumbar disc degeneration. Spine (Phila Pa 1976) 25, 487–492 10.1097/00007632-200002150-00016 (doi:10.1097/00007632-200002150-00016) [DOI] [PubMed] [Google Scholar]
- 3.Adams M. A., Roughley P. J. 2006. What is intervertebral disc degeneration, and what causes it? Spine 31, 2151–2161 10.1097/01.brs.0000231761.73859.2c (doi:10.1097/01.brs.0000231761.73859.2c) [DOI] [PubMed] [Google Scholar]
- 4.Osti O. L., Vernon-Roberts B., Moore R., Fraser R. D. 1992. Annular tears and disc degeneration in the lumbar spine. A post-mortem study of 135 discs. J. Bone Joint Surg. Br. 74, 678–682 [DOI] [PubMed] [Google Scholar]
- 5.Hegewald A. A., Ringe J., Sittinger M., Thome C. 2008. Regenerative treatment strategies in spinal surgery. Front. Biosci. 13, 1507–1525 10.2741/2777 (doi:10.2741/2777) [DOI] [PubMed] [Google Scholar]
- 6.Sebastine I. M., Williams D. J. 2007. Current developments in tissue engineering of nucleus pulposus for the treatment of intervertebral disc degeneration. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2007, 6401–6406 [DOI] [PubMed] [Google Scholar]
- 7.Bergknut N., et al. 2010. The performance of a hydrogel nucleus pulposus prosthesis in an ex vivo canine model. Biomaterials 31, 6782–6788 10.1016/j.biomaterials.2010.05.032 (doi:10.1016/j.biomaterials.2010.05.032) [DOI] [PubMed] [Google Scholar]
- 8.Ahlgren B. D., Lui W., Herkowitz H. N., Panjabi M. M., Guiboux J. P. 2000. Effect of annular repair on the healing strength of the intervertebral disc: a sheep model. Spine (Phila Pa 1976) 25, 2165–2170 10.1097/00007632-200009010-00004 (doi:10.1097/00007632-200009010-00004) [DOI] [PubMed] [Google Scholar]
- 9.Heuer F., Ulrich S., Claes L., Wilke H. J. 2008. Biomechanical evaluation of conventional annulus fibrosus closure methods required for nucleus replacement. Laboratory investigation. J. Neurosurg. Spine 9, 307–313 10.3171/SPI/2008/9/9/307 (doi:10.3171/SPI/2008/9/9/307) [DOI] [PubMed] [Google Scholar]
- 10.Reitmaier S., et al. Submitted. Hydrogels for nucleus replacement: facing the biomechanical challenge. J. Mech. Behav. Biomed. Mater. [DOI] [PubMed] [Google Scholar]
- 11.Team RDC. 2011. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.R-project.org/. [Google Scholar]
- 12.Schmidt H., Shirazi-Adl A., Galbusera F., Wilke H. J. 2011. Response analysis of the lumbar spine during regular daily activities: a finite element analysis. J. Biomech. 43, 1849–1856 10.1016/j.jbiomech.2010.03.035 (doi:10.1016/j.jbiomech.2010.03.035) [DOI] [PubMed] [Google Scholar]
- 13.Galbusera F., Schmidt H., Noailly J., Malandrino A., Lacroix D., Wilke H.-J., Shirazi-Adl A. 2011. Comparison of four methods to simulate swelling in poroelastic finite element models of intervertebral discs. J. Mech. Behav. Biomed. Mater. 4, 1234–1241 10.1016/j.jmbbm.2011.04.008 (doi:10.1016/j.jmbbm.2011.04.008) [DOI] [PubMed] [Google Scholar]
- 14.Rohlmann A., Zander T., Rao M., Bergmann G. 2009. Applying a follower load delivers realistic results for simulating standing. J. Biomech. 42, 1520–1526 10.1016/j.jbiomech.2009.03.048 (doi:10.1016/j.jbiomech.2009.03.048) [DOI] [PubMed] [Google Scholar]
- 15.van der Veen A. J., Mullender M., Smit T. H., Kingma I., van Dieen J. H. 2005. Flow-related mechanics of the intervertebral disc: the validity of an in vitro model. Spine (Phila Pa 1976) 30, E534–E539 10.1097/01.brs.0000179306.40309.3a (doi:10.1097/01.brs.0000179306.40309.3a) [DOI] [PubMed] [Google Scholar]
- 16.van Deursen D. L., Snijders C. J., Kingma I., van Dieen J. H. 2001. In vitro torsion-induced stress distribution changes in porcine intervertebral discs. Spine (Phila Pa 1976) 26, 2582–2586 10.1097/00007632-200112010-00011 (doi:10.1097/00007632-200112010-00011) [DOI] [PubMed] [Google Scholar]
- 17.Kingma I., van Dieen J. H., Nicolay K., Maat J. J., Weinans H. 2000. Monitoring water content in deforming intervertebral disc tissue by finite element analysis of MRI data. Magn. Reson. Med. 44, 650–654 (doi:10.1002/1522-2594(200010)44:4<650::AID-MRM21>3.0.CO;2-0) [DOI] [PubMed] [Google Scholar]
- 18.Holm S., Ekstrom L., Kaigle Holm A., Hansson T. 2007. Intradiscal pressure in the degenerated porcine intervertebral disc. Vet. Comp. Orthop. Traumatol. 20, 29–33 [PubMed] [Google Scholar]
- 19.Elliott D. M., Yerramalli C. S., Beckstein J. C., Boxberger J. I., Johannessen W., Vresilovic E. J. 2008. The effect of relative needle diameter in puncture and sham injection animal models of degeneration. Spine (Phila Pa 1976) 33, 588–596 10.1097/BRS.0b013e318166e0a2 (doi:10.1097/BRS.0b013e318166e0a2) [DOI] [PubMed] [Google Scholar]
- 20.Wang J. L., Tsai Y. C., Wang Y. H. 2007. The leakage pathway and effect of needle gauge on degree of disc injury post annular puncture: a comparative study using aged human and adolescent porcine discs. Spine (Phila Pa 1976) 32, 1809–1815 10.1097/BRS.0b013e31811ec282 (doi:10.1097/BRS.0b013e31811ec282) [DOI] [PubMed] [Google Scholar]
- 21.Reid J. E., Meakin J. R., Robins S. P., Skakle J. M., Hukins D. W. 2002. Sheep lumbar intervertebral discs as models for human discs. Clin. Biomech. 17, 312–314 10.1016/S0268-0033(02)00009-8 (doi:10.1016/S0268-0033(02)00009-8) [DOI] [PubMed] [Google Scholar]
- 22.Meakin J. R., Hukins D. W. 2000. Effect of removing the nucleus pulposus on the deformation of the annulus fibrosus during compression of the intervertebral disc. J. Biomech. 33, 575–580 10.1016/S0021-9290(99)00215-8 (doi:10.1016/S0021-9290(99)00215-8) [DOI] [PubMed] [Google Scholar]
- 23.Leahy J. C., Hukins D. W. 2001. Viscoelastic properties of the nucleus pulposus of the intervertebral disk in compression. J. Mater. Sci. 12, 689–692 10.1023/A:1011212425029 (doi:10.1023/A:1011212425029) [DOI] [PubMed] [Google Scholar]
- 24.Lyons G., Eisenstein S. M., Sweet M. B. 1981. Biochemical changes in intervertebral disc degeneration. Biochim. Biophys. Acta 673, 443–453 10.1016/0304-4165(81)90476-1 (doi:10.1016/0304-4165(81)90476-1) [DOI] [PubMed] [Google Scholar]
- 25.Arjmand N., Gagnon D., Plamondon A., Shirazi-Adl A., Lariviere C. 2009. Comparison of trunk muscle forces and spinal loads estimated by two biomechanical models. Clin. Biomech. 24, 533–541 10.1016/j.clinbiomech.2009.05.008 (doi:10.1016/j.clinbiomech.2009.05.008) [DOI] [PubMed] [Google Scholar]
- 26.Wilke H. J., Neef P., Hinz B., Seidel H., Claes L. 2001. Intradiscal pressure together with anthropometric data: a data set for the validation of models. Clin. Biomech. 16(Suppl. 1), S111–S126 10.1016/S0268-0033(00)00103-0 (doi:10.1016/S0268-0033(00)00103-0) [DOI] [PubMed] [Google Scholar]
- 27.Wilke H. J., Neef P., Caimi M., Hoogland T., Claes L. E. 1999. New in vivo measurements of pressures in the intervertebral disc in daily life. Spine 24, 755–762 10.1097/00007632-199904150-00005 (doi:10.1097/00007632-199904150-00005) [DOI] [PubMed] [Google Scholar]
- 28.Malko J. A., Hutton W. C., Fajman W. A. 2002. An in vivo MRI study of the changes in volume (and fluid content) of the lumbar intervertebral disc after overnight bed rest and during an 8-hour walking protocol. J. Spinal Disord. Tech. 15, 157–163 10.1097/00024720-200204000-00012 (doi:10.1097/00024720-200204000-00012) [DOI] [PubMed] [Google Scholar]
- 29.McGill S. M., Axler C. T. 1996. Changes in spine height throughout 32 hours of bedrest. Arch. Phys. Med. Rehabil. 77, 1071–1073 10.1016/S0003-9993(96)90071-4 (doi:10.1016/S0003-9993(96)90071-4) [DOI] [PubMed] [Google Scholar]
- 30.Reilly T., Tyrrell A., Troup J. D. 1984. Circadian variation in human stature. Chronobiol Int. 1, 121–126 10.3109/07420528409059129 (doi:10.3109/07420528409059129) [DOI] [PubMed] [Google Scholar]
- 31.Adams M. A., Hutton W. C. 1980. The effect of posture on the role of the apophysial joints in resisting intervertebral compressive forces. J. Bone Joint Surg. Br. 62, 358–362 [DOI] [PubMed] [Google Scholar]
- 32.Shirazi-Adl A., Drouin G. 1987. Load-bearing role of facets in a lumbar segment under sagittal plane loadings. J. Biomech. 20, 601–613 10.1016/0021-9290(87)90281-8 (doi:10.1016/0021-9290(87)90281-8) [DOI] [PubMed] [Google Scholar]