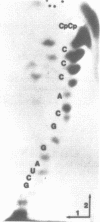
Abstract
Red clover necrotic mosaic virus, a member of the dianthovirus group, is characterized by a genome composed of two nonhomologous single-stranded RNAs of approximately 4.0 (RNA-1) and 1.4 kb (RNA-2). The complete nucleotide sequence of the RNA-2 has been determined. RNA-2 is 1448 nucleotides in length with a 5' terminal m7G cap and no 3' terminal poly-A tail or 5' terminal VPg. An open reading frame beginning at the first initiation codon at nucleotide 80 and ending at nucleotide 1030 has been identified which can encode a polypeptide of 35 kDa. RNA-2 directs the synthesis of a 35 kDa polypeptide in vitro. SP6 and T7 transcripts from full length RNA-2 cDNA clones directed the synthesis of a polypeptide with the same electrophoretic mobility as the polypeptide directed from authentic RNA-2. Clones with various 3' terminal deletions both outside and within the 35 kDa open reading frame were transcribed and translated in vitro to define the limits of the 35 kDa open reading frame. A second, small open reading frame capable of encoding a polypeptide of 4.9 kDa was also indicated from the sequence; however, there was no evidence for a protein product of that size. RNA-2 is presumed to be monocistronic and encode a cell-to-cell movement function. A small but significant amino acid sequence homology was observed with the brome mosaic virus RNA-3a polypeptide.
Full text
PDF







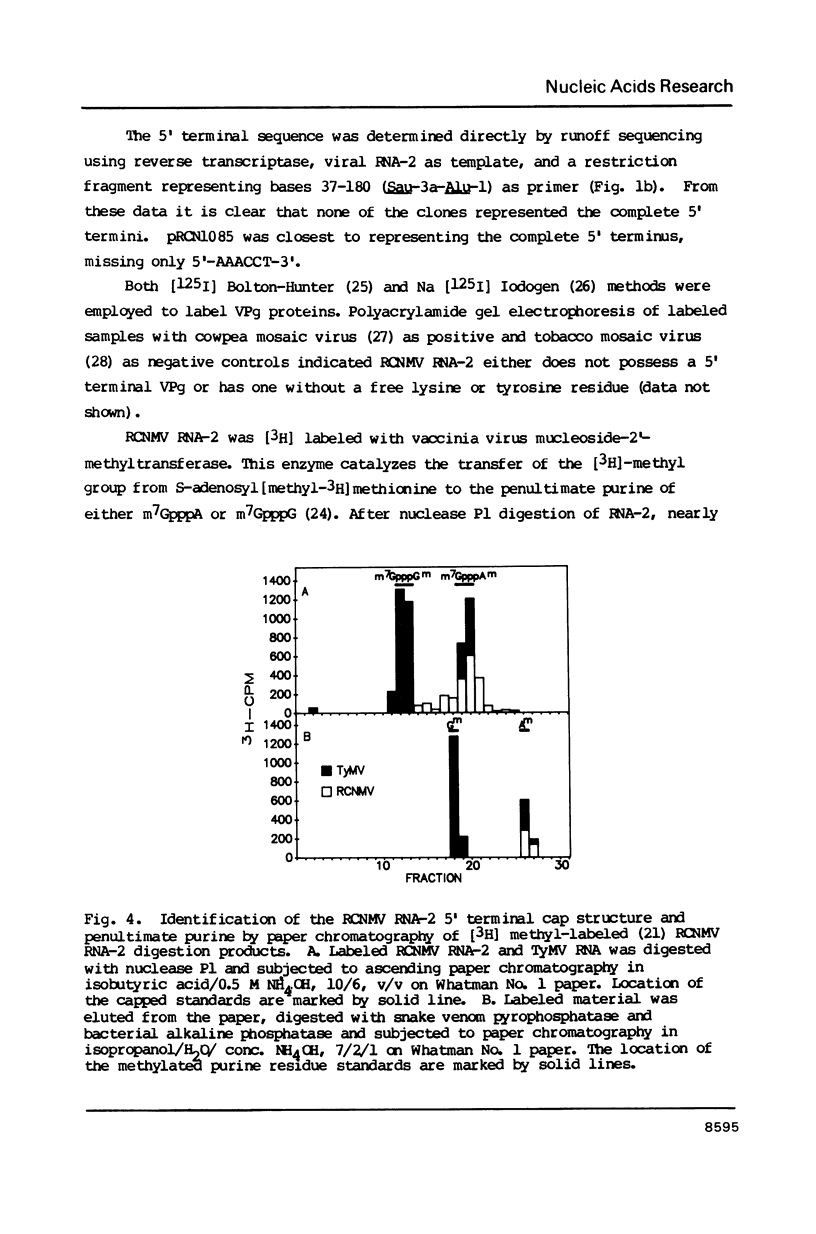

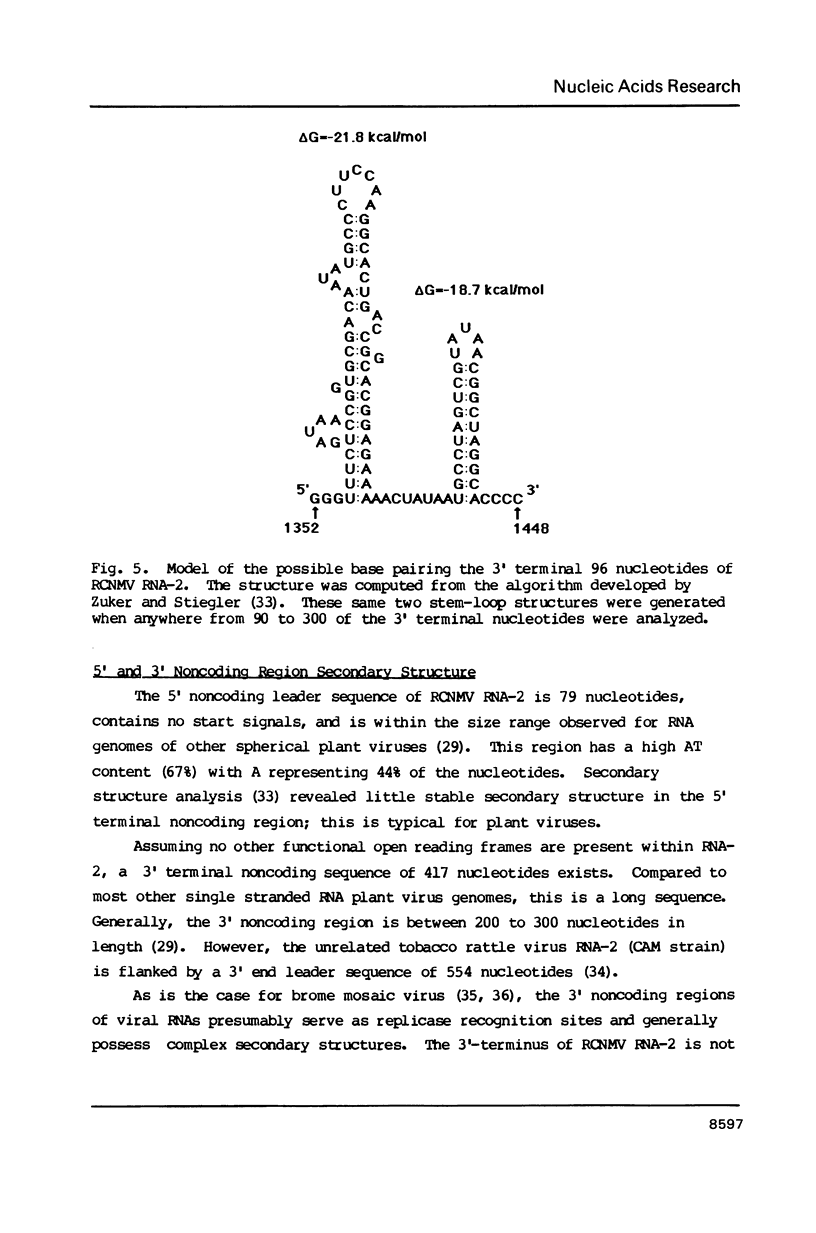
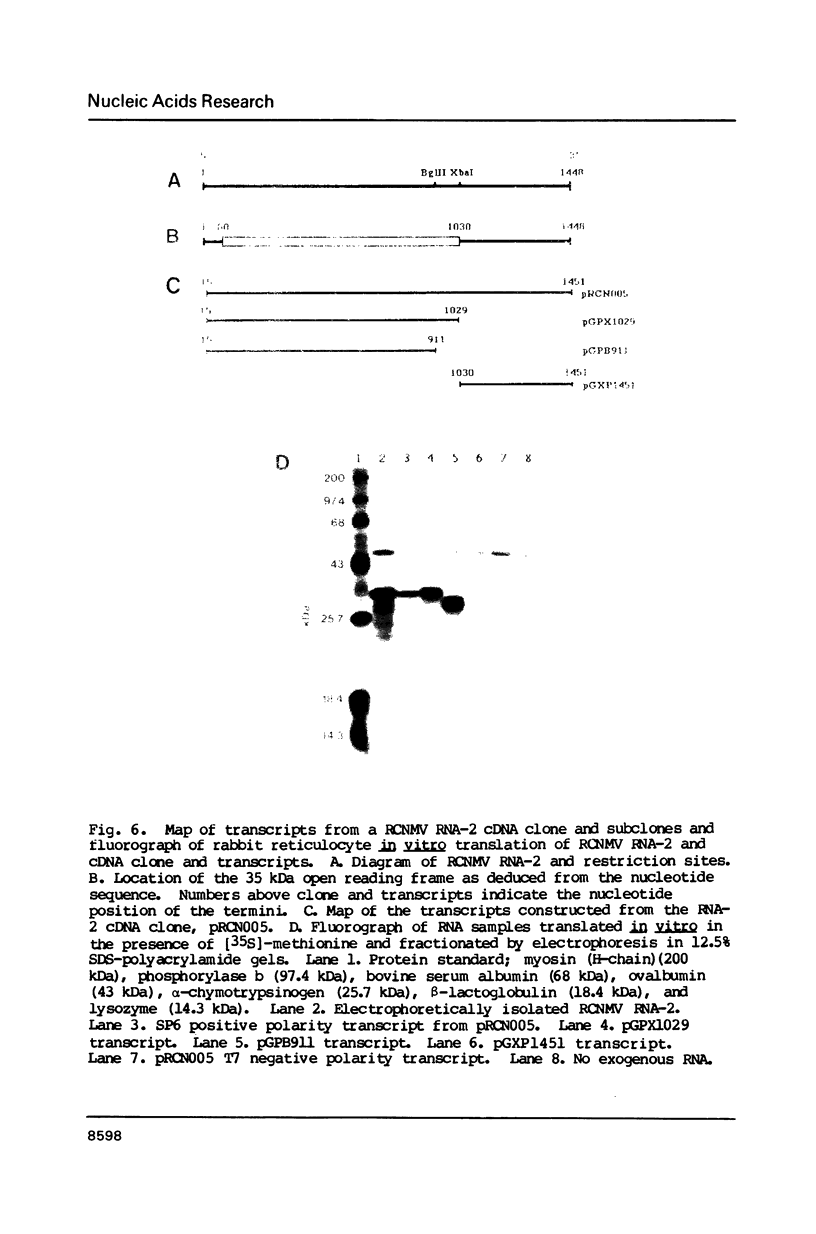




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlquist P., Luckow V., Kaesberg P. Complete nucleotide sequence of brome mosaic virus RNA3. J Mol Biol. 1981 Nov 25;153(1):23–38. doi: 10.1016/0022-2836(81)90524-6. [DOI] [PubMed] [Google Scholar]
- Bergh S. T., Koziel M. G., Huang S. C., Thomas R. A., Gilley D. P., Siegel A. The nucleotide sequence of tobacco rattle virus RNA-2 (CAM strain). Nucleic Acids Res. 1985 Dec 9;13(23):8507–8518. doi: 10.1093/nar/13.23.8507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccara M., Hamilton W. D., Baulcombe D. C. The organisation and interviral homologies of genes at the 3' end of tobacco rattle virus RNA1. EMBO J. 1986 Feb;5(2):223–229. doi: 10.1002/j.1460-2075.1986.tb04202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briand J. P., Keith G., Guilley H. Nucleotide sequence at the 5' extremity of turnip yellow mosaic virus genome RNA. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3168–3172. doi: 10.1073/pnas.75.7.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujarski J. J., Ahlquist P., Hall T. C., Dreher T. W., Kaesberg P. Modulation of replication, aminoacylation and adenylation in vitro and infectivity in vivo of BMV RNAs containing deletions within the multifunctional 3' end. EMBO J. 1986 Aug;5(8):1769–1774. doi: 10.1002/j.1460-2075.1986.tb04425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington J. C., Morris T. J. Complementary DNA cloning and analysis of carnation mottle virus RNA. Virology. 1984 Nov;139(1):22–31. doi: 10.1016/0042-6822(84)90326-x. [DOI] [PubMed] [Google Scholar]
- DeBorde D. C., Naeve C. W., Herlocher M. L., Maassab H. F. Resolution of a common RNA sequencing ambiguity by terminal deoxynucleotidyl transferase. Anal Biochem. 1986 Sep;157(2):275–282. doi: 10.1016/0003-2697(86)90626-3. [DOI] [PubMed] [Google Scholar]
- Dodds J. A., Tremaine J. H., Ronald W. P. Some properties of carnation ringspot virus single- and double-stranded ribonucleic acid. Virology. 1977 Dec;83(2):322–328. doi: 10.1016/0042-6822(77)90177-5. [DOI] [PubMed] [Google Scholar]
- Dougherty W. G., Hiebertt E. Translation of potyvirus RNA in a rabbit reticulocyte lysate: reaction conditions and identification of capsid protein as one of the products of in vitro translation of tobacco etch and pepper mottle viral RNAs. Virology. 1980 Mar;101(2):466–474. doi: 10.1016/0042-6822(80)90460-2. [DOI] [PubMed] [Google Scholar]
- Dreher T. W., Bujarski J. J., Hall T. C. Mutant viral RNAs synthesized in vitro show altered aminoacylation and replicase template activities. Nature. 1984 Sep 13;311(5982):171–175. doi: 10.1038/311171a0. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Goelet P., Lomonossoff G. P., Butler P. J., Akam M. E., Gait M. J., Karn J. Nucleotide sequence of tobacco mosaic virus RNA. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5818–5822. doi: 10.1073/pnas.79.19.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould A. R., Francki R. I., Hatta T., Hollings M. The bipartite genome of red clover necrotic mosaic virus. Virology. 1981 Jan 30;108(2):499–506. doi: 10.1016/0042-6822(81)90457-8. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Guilley H., Briand J. P. Nucleotide sequence of turnip yellow mosaic virus coat protein mRNA. Cell. 1978 Sep;15(1):113–122. doi: 10.1016/0092-8674(78)90087-9. [DOI] [PubMed] [Google Scholar]
- Haidar M. A., Hirth L. 5'-terminal structure of tobacco rattle virus RNA: evidence for polarity of reconstitution. Virology. 1977 Jan;76(1):173–185. doi: 10.1016/0042-6822(77)90294-x. [DOI] [PubMed] [Google Scholar]
- Haseloff J., Goelet P., Zimmern D., Ahlquist P., Dasgupta R., Kaesberg P. Striking similarities in amino acid sequence among nonstructural proteins encoded by RNA viruses that have dissimilar genomic organization. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4358–4362. doi: 10.1073/pnas.81.14.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman M. J., Sarachu A. N., Ablas F., Broxterman H. J., Van Vloten-Doting L., Bol J. F. Alfalfa mosaic virus temperature-sensitive mutants. III. Mutants with a putative defect in cell-to-cell transport. Virology. 1986 Oct 30;154(2):401–404. doi: 10.1016/0042-6822(86)90466-6. [DOI] [PubMed] [Google Scholar]
- Kamer G., Argos P. Primary structural comparison of RNA-dependent polymerases from plant, animal and bacterial viruses. Nucleic Acids Res. 1984 Sep 25;12(18):7269–7282. doi: 10.1093/nar/12.18.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Possible role of flanking nucleotides in recognition of the AUG initiator codon by eukaryotic ribosomes. Nucleic Acids Res. 1981 Oct 24;9(20):5233–5252. doi: 10.1093/nar/9.20.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Meshi T., Watanabe Y., Saito T., Sugimoto A., Maeda T., Okada Y. Function of the 30 kd protein of tobacco mosaic virus: involvement in cell-to-cell movement and dispensability for replication. EMBO J. 1987 Sep;6(9):2557–2563. doi: 10.1002/j.1460-2075.1987.tb02544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris-Krsinich B. A., Forster R. L., Mossop D. W. Translation of red clover necrotic mosaic virus RNA in rabbit reticulocyte lysate: identification of the virus coat protein cistron on the larger RNA strand of the bipartite genome. Virology. 1983 Jan 30;124(2):349–356. doi: 10.1016/0042-6822(83)90351-3. [DOI] [PubMed] [Google Scholar]
- Moss B. Utilization of the guanylyltransferase and methyltransferases of vaccinia virus to modify and identify the 5'-terminals of heterologous RNA species. Biochem Biophys Res Commun. 1977 Jan 24;74(2):374–383. doi: 10.1016/0006-291x(77)90314-x. [DOI] [PubMed] [Google Scholar]
- Rezaian M. A., Williams R. H., Gordon K. H., Gould A. R., Symons R. H. Nucleotide sequence of cucumber-mosaic-virus RNA 2 reveals a translation product significantly homologous to corresponding proteins of other viruses. Eur J Biochem. 1984 Sep 3;143(2):277–284. doi: 10.1111/j.1432-1033.1984.tb08370.x. [DOI] [PubMed] [Google Scholar]
- Rhoads D. D., Roufa D. J. Emetine resistance of Chinese hamster cells: structures of wild-type and mutant ribosomal protein S14 mRNAs. Mol Cell Biol. 1985 Jul;5(7):1655–1659. doi: 10.1128/mcb.5.7.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberklang M., Prochiantz A., Haenni A. L., Rajbhandary U. L. Studies on the sequence of the 3'-terminal region of turnip-yellow-mosaic-virus RNA. Eur J Biochem. 1977 Feb;72(3):465–478. doi: 10.1111/j.1432-1033.1977.tb11270.x. [DOI] [PubMed] [Google Scholar]
- Stanley J., Rottier P., Davies J. W., Zabel P., Van Kammen A. A protein linked to the 5' termini of both RNA components of the cowpea mosaic virus genome. Nucleic Acids Res. 1978 Dec;5(12):4505–4522. doi: 10.1093/nar/5.12.4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley J., Van Kammen A. Nucleotide sequences adjacent to the proteins covalently linked to the cowpea mosaic virus genome. Sequence determination after labelling in vitro using RNA ligase. Eur J Biochem. 1979 Nov 1;101(1):45–49. doi: 10.1111/j.1432-1033.1979.tb04214.x. [DOI] [PubMed] [Google Scholar]
- Zuker M., Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981 Jan 10;9(1):133–148. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vloten-Doting L., Francki R. I., Fulton R. W., Kaper J. M., Lane L. C. Tricornaviridae - a proposed family of plant viruses with tripartite, single-stranded RNA genomes. Intervirology. 1981;15(4):198–203. doi: 10.1159/000149232. [DOI] [PubMed] [Google Scholar]