Abstract
G protein-coupled receptors (GPCR) play central roles in almost all physiological functions, and mutations in GPCR are responsible for over 30 hereditary diseases associated with loss or gain of receptor function. Gain of function mutants are frequently described as having constitutive activity (CA), that is, they activate effectors in the absence of agonist occupancy. Although many GPCR have mutants with CA, the GnRH receptor (GnRHR) was not, until 2010, associated with any CA mutants. The explanation for the failure to observe CA appears to be that the quality control system of the cell recognizes CA mutants of GnRHR as misfolded and retains them in the endoplasmic reticulum. In the present study, we identified several human (h)GnRHR mutants with substitutions in transmembrane helix 6 (F272K, F272Q, Y284F, C279A, and C279S) that demonstrate varying levels of CA after being rescued by pharmacoperones from different chemical classes and/or deletion of residue K191, a modification that increases trafficking to the plasma membrane. The movement of the mutants from the endoplasmic reticulum (unrescued) to the plasma membrane (after rescue) is supported by confocal microscopy. Judging from the receptor-stimulated inositol phosphate production, mutants F272K and F272Q, after rescue, display the largest level of CA, an amount that is comparable with agonist-stimulated activation. Because mutations in other GPCR are, like the hGnRHR, scrutinized by the quality control system, this general approach may reveal CA in receptor mutants from other systems. A computer model of the hGnRHR and these mutants was used to evaluate the conformation associated with CA.
GnRH receptors (GnRHR) are G protein-coupled receptors (GPCR) that play a key role in regulating reproduction. Numerous point mutations, associated with the disease hypogonadotropic hypogonadism, were found in human GnRHR (hGnRHR) (1). These mutations are widely distributed over the receptor structure, including its N terminus (N10K, Q11K, and T32I), transmembrane helix (TM)2 (E90K), extracellular loop (EL)1 (Y104I, Q106R, and Y108C), TM3 (A129D and R139H), TM4 (A171T and S168R), EL2 (C200Y), TM5 (S217R), TM6 (R262Q, L266R, C279Y, and Y284C), and TM7 (L314Xstop and P320L). Most of these naturally occurring, as well as genetically engineered, mutants are misfolded and misrouted molecules (1, 2) that are retained in the endoplasmic reticulum (ER) (2). This has been shown by relief of the dominant negative effect, whereby misfolded mutants oligomerize with wild type (WT) and retain it in the ER, an effect that is reversed by pharmacoperone drugs (2). Additionally, in the case of two hGnRHR mutants labeled with green fluorescent protein (GFP), E90K, and D98N, confocal microscopy shows that these are ER retained and, after treatment with pharmacoperones, traffic to the plasma membrane (3, 4).
Pharmacoperone drugs are small molecules that enter cells and act as templates to allow correct folding (or refolding) of mutants. Correctly folded mutants traffic to the plasma membrane and show biological activity (1, 3, 5–8). The WT hGnRHR has a less than perfect chance of folding correctly. This is due to steric inhibition by residue K191 of the formation of the Cys14-Cys200 bridge. Deletion of K191 or the use of pharmacoperones increases the plasma membrane expression of WT hGnRHR (9, 10) by stabilizing the conformation that is acceptable to the cellular quality control system (QCS). Because the WT hGnRHR is balanced between the plasma membrane and the ER, the human receptor is highly susceptible to misrouting due to single point mutations (11).
The biochemical mechanism by which pharmacoperones engage and rescue mutants has been described (3, 4, 12). For the four different chemical classes of these drugs used in the present work (In3, Q89, A177775, and TAK-013), the drug creates a surrogate bridge between residues D98 and K121, stabilizing a relation between transmembrane segments 2 and 3, which is required for trafficking to the plasma membrane. The biochemical mechanism by which altered residues cause ER retention has been summarized for several misfolded mutants (13, 14). Because the efficiency of recognition by the cellular QCS differs, ER retention is different.
Among several hundreds of GnRHR mutants reported before 2010, none were known to show constitutive activity (CA) (15). This observation was surprising, because most GPCR have multiple mutants with CA (16). The absence of such proteins has been one of the impediments to understanding the active conformation of the GnRHR.
The first mutant identified with CA was E90K, which has been extensively studied in our laboratory (1, 3–5, 10, 17–19). This naturally occurring mutation, identified in patients with hypogonadotropic hypogonadism, affects a highly conserved structural feature of the GnRHR, a salt bridge between TM2 and TM3 (4), created by residues E90 and K121 in the human receptor sequence (20, 21). GnRH agonists, but not antagonists, bind both receptor residues D98 and K121 (20, 21) and interfere with the E90-K121 salt bridge, altering the physical relation between TM2 and TM3. Formation of this bridge is required for trafficking of the hGnRHR to the plasma membrane (4), and accordingly, this mutant is 100% retained in the ER and 100% rescuable by pharmacoperones (1, 3–5, 19).
We predicted that mutant E90K would result in charge repulsion between K90 and K121 and potentially lead to CA by breaking this salt bridge, as does the agonist. From confocal studies, we also knew that mutant E90K is recognized by the QCS as a misfolded protein (22), fully retained in the ER and unable to traffic to the plasma membrane (2). These same studies showed that pharmacoperone drugs rescue this mutant (4) and can, in fact, refold and rescue mutants previously retained in the ER (3). In accordance with this prediction, E90K mutant overexpressed in Cos-7 cells clearly demonstrated CA but only after treatment by pharmacoperones or by an additional genetic modification (deletion of residue K191), which increases receptor trafficking of hGnRHR to the plasma membrane (10, 19, 23). Unfortunately, the hGnRHR is among GPCRs, for which it has not been possible to generate antisera, so immunohistochemical localization of the WT or mutant forms of this receptor has not been possible.
In the present study, we suggested that mutations in regions that undergo conformational rearrangement during receptor activation may lead to CA. Therefore, we analyzed functional properties of mutants of residues from the largely movable TM6 located at the interfaces with TM3 (F272K, F272Q, and F272L), TM5 (Y284F), and TM7 (C279A and C279S). Here, we took advantage of mutant rescue by four different chemical classes of pharmacoperones (12) and K191 deletion to reveal previously undetected CA of particular mutants. We used these data to improve the model of the GnRHR in its active state.
Materials and Methods
General
pcDNA3.1 (Invitrogen, San Diego, CA), GnRH analog, D-tert-butyl-Ser6-des-Gly10-Pro9-ethylamide-GnRH (Buserelin; Hoechst-Roussel Pharmaceuticals, Somerville, NJ), myo-[2-3H(N)]-inositol (NET-114A; PerkinElmer, Waltham, MA), DMEM, OPTI-MEM, lipofectamine, PBS (GIBCO, Invitrogen), competent cells (Promega, Madison, WI), and Endofree maxi-prep kits (QIAGEN, Valencia, CA), were obtained as indicated.
Mutant receptors
WT and mutant GnRHR cDNA for transfection were prepared as reported (5); the purity and identity of plasmid DNA were verified by dye terminator cycle sequencing (Applied Biosystems, Foster City, CA). GnRHR mutants F272Q and F272K were tagged with GFP at the C terminus via a 14-residue spacer (TPSFRADLSRCFCW) derived from the sequence of the catfish GnRHR (24) as previously described (25). Pharmacoperones In3 and Q89 (Merck and Co., Darmstadt, Germany), A177775 (Abbott Laboratories, Abbott Park, IL), and TAK-013 (Takeda Pharmaceuticals, Deerfield, IL) were obtained as indicated (4, 12). The drugs are, respectively, from the following chemical classes: indoles, quinolones (both Q89 and Q103), erythromycin macrolides and (N-{4-[5-{[benzyl(methyl)amino]methyl}-1-(2,6-difluorobenzyl)-2,4-dioxo-3-phenyl-1,2,3,4-tetrahy-drothieno[2,3-d]pyrimidin-6-yl]phenyl}-N′-methoxyurea). Chemical structures (12, 26) and the mechanism of action of these on the hGnRHR (4) have been reported.
Transient transfection
Cos-7 cells were cultured in growth medium [DMEM, 10% fetal calf serum (FCS), and 20 μg/ml gentamicin] at 37 C in a 5% CO2 humidified atmosphere. For transfection of WT or mutant receptors into cells, 5 × 104 cells were plated in 0.25 ml of growth medium in 48-well Costar cell culture plates. Twenty-four hours after plating, the cells were washed with 0.5 ml of OPTI-MEM and then transfected with WT or mutant receptor DNA with pcDNA3.1 (empty vector) to keep the total DNA constant (100 ng/well). Lipofectamine was used according to the manufacturer's instructions. Five hours after transfection, 0.125 ml of DMEM with 20% FCS and 20 μg/ml gentamicin was added. Twenty-three hours after transfection, the medium was replaced with 0.25 ml of fresh growth medium. Where indicated, pharmacoperones (indicated concentration) in 1% DMSO (vehicle) was added for 4 h in respective media to the cells and then removed 18 h before agonist treatment (8). In the present study, we used trypan blue exclusion to show cell viability after drug exposure.
Inositol phosphate (IP) assays
Twenty-seven hours after transfection, cells were washed twice with 0.50 ml of DMEM, 0.1% BSA, and 20 μg/ml gentamicin and then “preloaded” for 18 h with 0.25 ml of 4 μCi/ml myo-[2-3H(N)]-inositol in inositol-free DMEM, then washed twice with 0.30 ml of DMEM (inositol free) containing 5 mm LiCl and treated for 2 h with 0.25 ml of a saturating concentration of Buserelin (10−7 m), a metabolically stable GnRHR agonist, in the same medium. When CA was assessed, Buserelin was omitted from the assessment period. Total IP was then determined (27). This assay has been validated as a sensitive measure of plasma membrane expression for functional receptors when expressed at low amounts of DNA (<100 ng/well) and stimulated by excess agonist (1, 6, 8, 11, 28–33).
Binding assays. Saturation and Scatchard analysis
Cells were cultured and plated in growth medium as described above, except 105 cells in 0.5 ml of growth medium were added to 24-well Costar cell culture plates (cell transfection and medium volumes were doubled accordingly). Twenty-three hours after transfection, the medium was replaced with 0.5 ml of fresh growth medium with or without pharmacoperone (1 μg/ml In3). We have reported the details of the Scatchard analysis. Twenty-seven hours after transfection, cells were washed twice with 0.5 ml of DMEM containing 0.1% BSA and 20 μg/ml gentamicin, and then 0.5 ml of DMEM were added. After 18 h, cells were washed twice with 0.5 ml of DMEM, 0.1% BSA, and 10 mm HEPES, then 2 × 106 cpm/ml of [125I]-Buserelin, prepared in our laboratory (specific activity, 700–800 μCi/μg; molecular weight, 1363.4), was added to the cells in 0.5 ml of the same medium and allowed to incubate at room temperature for 90 min, consonant with maximum binding (34). New receptor synthesis during this period is negligible at room temperature. After 90 min, the media were removed and radioactivity was measured (35). To determine nonspecific binding, the same concentrations of radioligand were added to similarly transfected cells in the presence of 10 μg/ml unlabeled GnRH.
Confocal microscopy
Cells (105 per well) were plated in 1 ml of DMEM, 10% FCS, and 20 μg/ml gentamicin in Lab-Tek-II Chamber no. 1.5 German Coverglass slides (Nalge Nunc, Naperville, IL) and transfected with 25 ng of GFP-tagged F272Q or F272K. Twenty-three hours after transfection, the cells were pretreated with 0.75 μm TAK-013 for 4 h, washed, and incubated for an additional 18 h with DMEM, 0.1% BSA, and gentamicin. ER-Tracker Red (glibenclamide BODIPY TR; Molecular Probes, Eugene, OR) was diluted in DMEM and 0.1% BSA, supplemented with 10 mm HEPES (pH 7.4), to a final concentration of 1 μm and added for 30 min at 37 C. Cells were washed once and then incubated for 10 min with WGA-Alexa Fluor 633 (Molecular Probes), which was diluted to a final concentration of 12.5 μg/ml. After approximately 10 min with the tracking stains, cells were imaged with a Leica SP5 AOBS confocal microscope (Leica Microsystems, Mannheim, GE) using a ×63/1.4 oil objective. ER tracker was excited at 561 nm, and emission was detected in a broad spectral domain from 575- to 630-nm interval. GFP was excited at 488 nm and detected in the 500- to 570-nm interval, and Alexa Fluor 633 was excited at 633 nm and detected at 650–720 nm. GFP and Alexa Fluor 633 were imaged simultaneously; ER tracker was imaged sequentially to eliminate the possibility of bleed through from the GFP channel. Images of single confocal planes were open and contrast enhanced using ImageJ 1.46 (Wayne Rasband; National Institutes of Health, Bethesda, MD).
Data analysis
Kd and Bmax (dissociation constant and maximum binding, respectively) values were determined by linear regression analysis using Scatchard plots. Data show means (n ≥ 3); sem are shown. Two-way ANOVA was used as indicated.
Modeling
The homology modeling of hGnRHR was based on crystal structures of the β2-adrenergic receptor in its inactive state (PDB ID, 2rh1) and in the active conformation bound to Gsα-βγ (PDB ID, 3sn6) as previously described (19). The models can be accessed at http://mosberglab.phar.umich.edu/resources/. The new hGnRHR models were compared with our previous models of hGnRHR (19), built using known crystal structures of the dark-adapted rhodopsin (PDB ID, 1u19) and opsin (PDB ID, 3dqb). The validity of the models was verified by docking of peptide agonists and antagonists into active and inactive conformations, respectively. Ligand docking was performed during distance geometry calculations to satisfy receptor-ligand structural constraints, as described (19).
Results
Radioligand binding of mutants rescued by deletion of residue K191
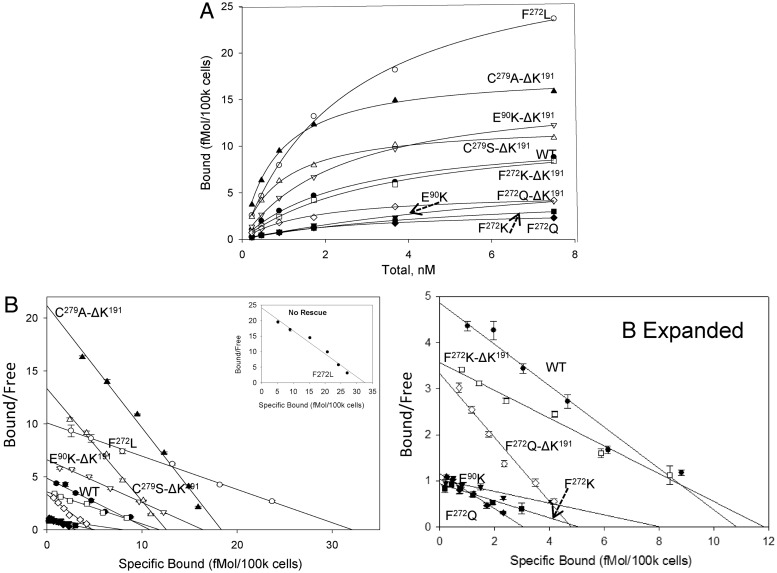
Specific binding of the radioligand, [125I]-Buserelin, was observed in Cos-7 cells expressing WT hGnRHR, and F272L hGnRHR, but not for mutants F272K, F272Q, C279A, C279S, or Y284F. After rescue by pharmacoperone In3, measurable binding of radioligand was detected for F272K, F272Q, C279A, C279S, and Y284F. After deletion of residue K191, a modification that increases WT and mutant hGnRHR trafficking to the plasma membrane (10), the maximal amount of radioligand binding is similar to, or higher than, that observed for the WT receptor except for F272QΔK191. Binding characteristics for WT and mutant receptors was determined after their rescue by In3 (Fig. 1 and Table 1). Consistent with previous observations (36), F272L showed substantially higher cell-surface expression level, compared with WT receptor. High expression levels were also observed for Y284F, Y284F-ΔK191, C272A-ΔK191, and C272S-ΔK191mutants rescued by In3. All mutants, after rescue by In3, demonstrate Kd values that are similar to those of WT receptors with variations that do not exceed 3-fold.
Fig. 1.
Saturation (A) and Scatchard (B) plots of binding of [125I]-buserelin to hGnRHR mutants. Cos-7 cells were transfected with 25 ng of DNA for WT receptor or mutant as described in Materials and Methods. After rescue with In3 or by deletion of K191, Scatchard analysis was performed on all mutants as indicated except for C279A, C279S, Y284F, and Y284F-desK191, for which saturation binding was used, in which case the Kd was not determined (nd). The Bmax and Kd values are from at least three independent experiments showing the variance between experiments as seen in Table 1. The inset to B shows mutant F272L that has not been rescued by pharmacoperone In3. The image marked B expanded shows the bottom portion of B, expanded for clarity.
Table 1.
Binding of [125I]-Buserelin to the WT and mutant hGnRHR
| Mutant | Bmax (fmol/105 cells) | Kd (nm) |
|---|---|---|
| WT | 7.7 ± 0.9 | 3.1 ± 1.5 |
| E90K | 3.9 ± 0.1 | 4.6 ± 2.2 |
| E90K-ΔK191 | 11.0 ± 0.9 | 2.2 ± 0.4 |
| F272L | 32.2 ± 0.5 | 3.2 ± 0.1 |
| F272K | 2.1 ± 0.3 | 5.6 ± 1.3 |
| F272K-ΔK191 | 6.8 ± 0.9 | 2.5 ± 0.4 |
| F272Q | 2.3 ± 0.5 | 4.4 ± 1.0 |
| F272Q-ΔK191 | 3.3 ± 0.7 | 1.7 ± 0.6 |
| C279A-ΔK191 | 14.7 ± 0.6 | 0.8 ± 0.1 |
| C279S-ΔK191 | 10.0 ± 1.1 | 0.9 ± 0.2 |
| C279A | 6.7 ± 0.5 | nd |
| C279S | 5.0 ± 0.9 | nd |
| Y284F | 7.3 ± 0.5 | nd |
| Y284F-ΔK191 | 15.6 ± 0.5 | nd |
Cos-7 cells were transfected with 25 ng of receptor or mutant DNA as described in Materials and Methods. After rescue with In3, Scatchard analysis was performed on all mutants as indicated except for C279A, C279S, Y284F, and Y284F-desK191, where saturation binding was utilized and the Kd was not determined (nd). The Bmax and Kd values are from at least three experiments showing the mean ± sem.
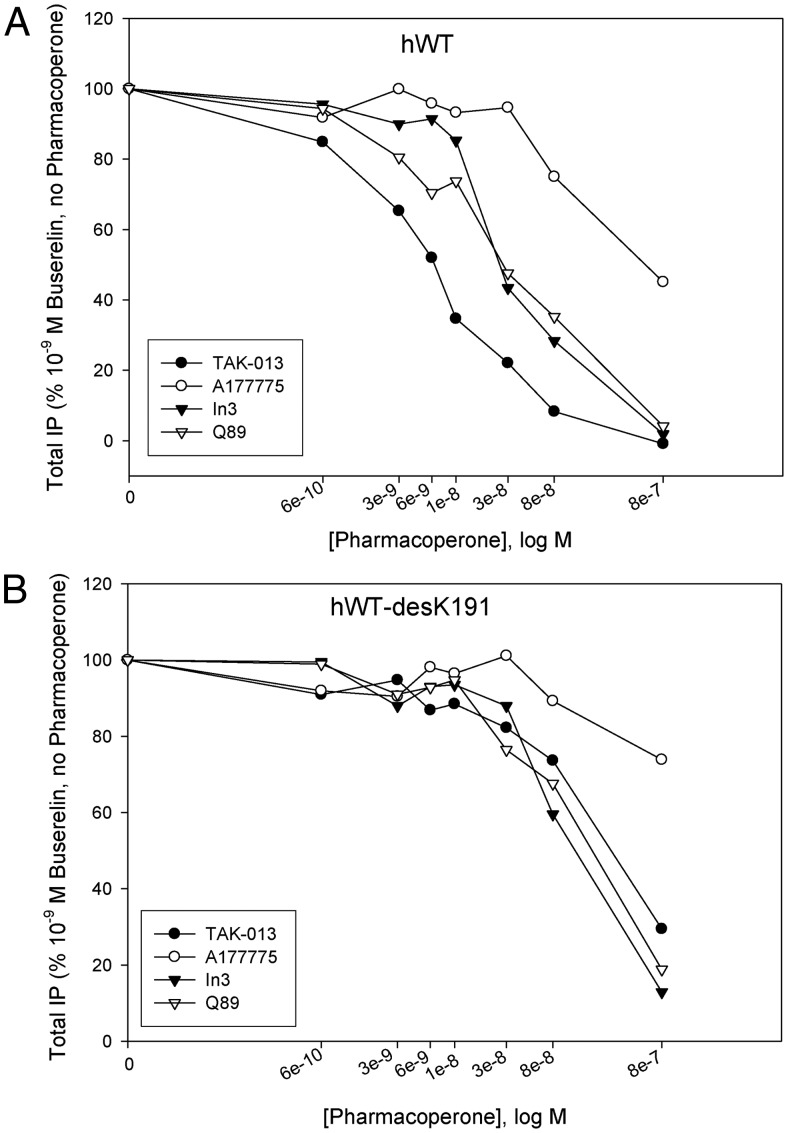
IC50 of pharmacoperones of the hGnRHR
We next evaluated the ability of four different chemical classes of pharmacoperones (12) to rescue CA. These included an indole (In3), quinolone (Q89), erythromycin macrolide (A177775), and TAK-013. All known pharmacoperones of the GnRHR are also receptor antagonists. The inhibitory potency of these antagonists (IC50) was evaluated by measuring the inhibition of buserelin-stimulated IP production in cells expressing WT and ΔK191-WT hGnRHR (Fig. 2). The IC50 values are, respectively: TAK-013, 5.9 × 10−9 m; In3, 3 × 10−8 m; Q89, 2.9 × 10−8 m; and A177775, 9.0 × 10−8 m. These values were significantly reduced (by up to 100-fold) in the receptor mutants lacking K191 (Fig. 2B): In3, 9.3 × 10−8 m; Q89, 1.7 × 10−7 m; TAK-013, 3.2 × 10−7 m; and A177775, more than 10−6 m. The IC50 values of different pharmacoperones to the receptor with K191 deletion correlate with our previous observations for Q89 (19). As we noted before, this effect may be related to the partial occlusion of the ligand binding pocket by the EL2 shifted inside the TM α-bundle after the K191 deletion (19).
Fig. 2.
Antagonist properties of pharmacoperones, In3, Q89, TAK-013, and A17777, assessed by inhibition of buserelin-stimulated IP production. Cos-7 cells were transfected with 10 ng of DNA for (A) hWT or (B) hWT-desK191 GnRHR as described in Materials and Methods. IP production was measured in response to 10−9 m Buserelin (a metabolically stable GnRH agonist) and in the presence of the indicated level of four different pharmacoperones as indicated. At least three independent experiments were performed in replicates of four to six. The optimal doses of pharmacoperones were selected from earlier studies (4).
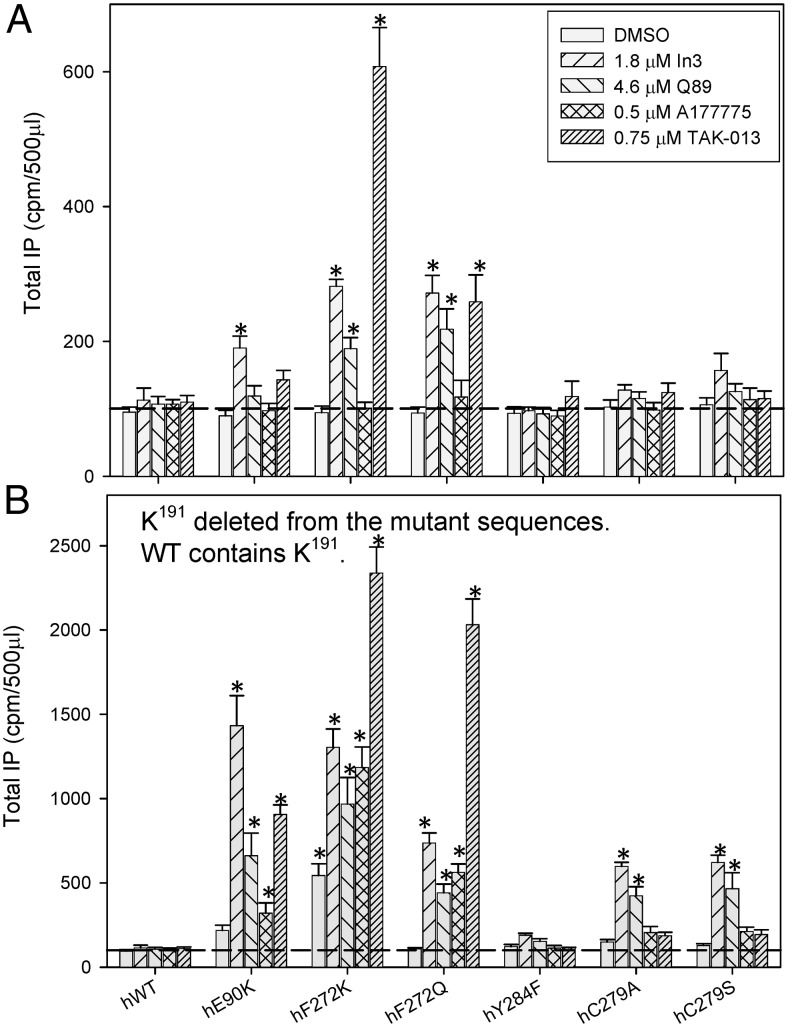
CA of mutants rescued by pharmacoperones
We observed that all four pharmacoperones were able to rescue cell-surface expression and stimulated CA in studied mutants but to different extents (Fig. 3A). The effect of pharmacoperones on the rescue of CA mutants generally correlates with their IC50 values (Fig. 2). The highly potent antagonist TAK-013 had the largest rescue effect in most cases, and the least potent A177775 macrolide had the lowest effect on CA. Overall, the highest level of CA was found for F272K and F272Q mutants rescued by TAK-013 and In3. These data indicate that the mutants can be functional after reaching the cell surface. WT receptor, which shows no CA, is included as a control, and the horizontal dashed line shows the measured IP background with vector-only transfections; this was not changed with different pharmacoperones. See Supplemental Figs. 1 and 2, published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org, show confocal data that are consistent with the movement of mutants F272K and F272Q from the ER (unrescued) to the plasma membrane (rescued by TAK-013).
Fig. 3.
CA of hGnRHR mutants rescued by pharmacoperones and assessed by agonist-independent IP production. IP production was assessed for mutants with K191 (the WT condition) (A) or without K191 (B). Cos-7 cells were transfected with 100 ng of WT (a control that lacks CA) or mutant cDNA as described in Materials and Methods. Mutants were incubated in media alone or rescued with each of four pharmacoperones; these drugs were then washed out, and IP production was measured in response to media alone (no agonist added). In all figures, sem are shown for least three independent experiments performed in replicates of four to six. The horizontal dashed line is IP production after transfection with vector only. Bars marked with an asterisk show values that differ from those obtained after transfection with vector only (two-way ANOVA). DMSO, Dimethylsulfoxide.
CA of mutants rescued by deletion of K191
Figure 3B shows the effect of an alternate method of rescue, the deletion of K191. When K191 was deleted, E90K and F272K mutants demonstrated some CA without pharmacoperone treatment (Fig. 3B). All mutants studied showed higher CA after treatment with at least one pharmacoperone, compared with untreated cells. In the case of mutations at positions 90 and 272, the amount of CA is quite substantial. The observation that K191 deletion alone (i.e. without pharmacoperone rescue) can produce CA suggests that the development of CA is not caused by pharmacoperone drugs and supports the view that each of these approaches enhances trafficking of the mutants to the plasma membrane, and the CA is then measurable.
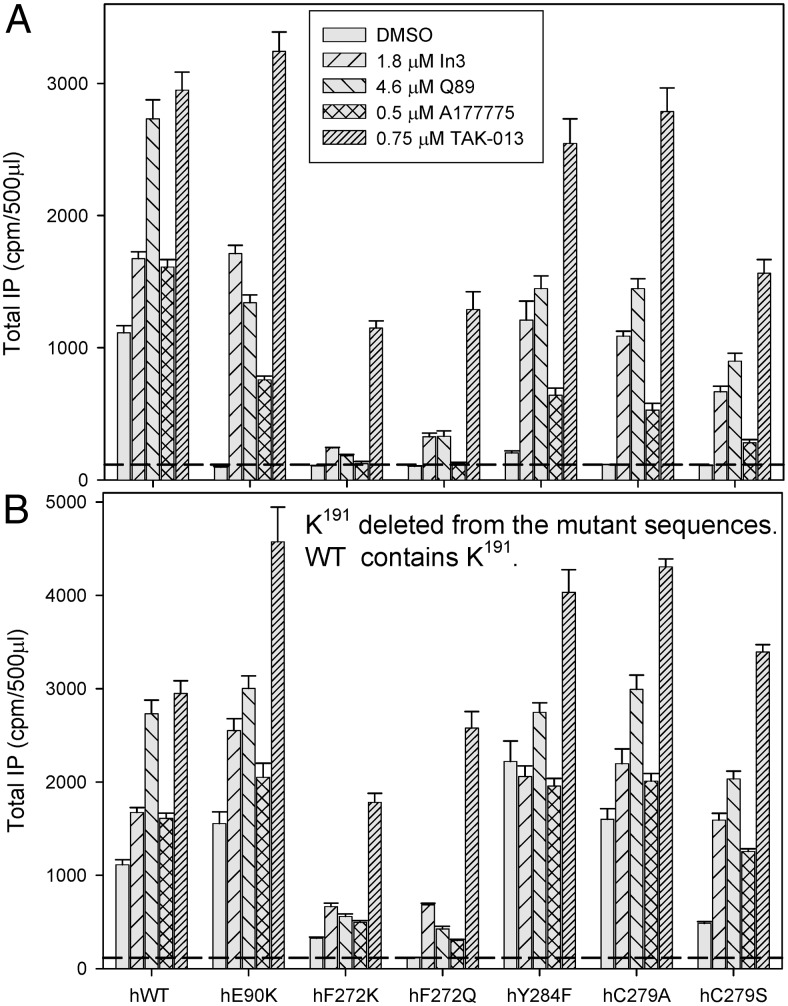
Stimulated response of mutants rescued by deletion of K191 or by pharmacoperones
As was the case for CA (and expectedly), buserelin-stimulated IP responses were increased by pharmacoperone rescue (Fig. 4A). Deletion of K191 in all studied hGnRHR mutants further enhances the IP production (Fig. 4B). These observations were consonant with increased trafficking to the plasma membrane due to deletion of K191 and/or the use of pharmacoperones. Examination of receptor numbers on the cell surface, assessed with radioactive tracer, [125I]-Buserelin, is also consistent with the view that both the pharmacoperone treatment and deletion of K191 were associated with increasing trafficking of the mutants to the plasma membrane (Fig. 1).
Fig. 4.
Agonist-stimulated IP production by mutants of the hGnRHR after pharmacoperone rescue. Functional activity was assessed for mutants with K191 (the WT condition) (A) or without K191 (B). Cos-7 cells were transfected with 10 ng of WT or mutant cDNA, with or without K191 as described in Materials and Methods. After rescue, IP production was measured in response to 10−7 m buserelin (metabolically stable GnRH agonist). In all figures, sem are shown for least three independent experiments performed in replicates of four to six.
Interestingly, F272K and F272Q mutants as well as F272KΔK191 and F272QΔK191 mutants demonstrated relatively low levels of buserelin-stimulated IP production that was almost equal to the level of agonist-independent IP produced by these mutants (Figs. 3 and 4). These results suggest that incorporation of polar Q272 or charged K272 likely creates similar distortions at the cytoplasmic end of TM6 as does the agonist binding. An alternative explanation may be related to the inability of these mutants to efficiently bind and respond to Buserelin treatment. However, the improved binding affinity of [125I]-Buserelin to these mutants (Kd decreased by 20–50%) (Table 1) made this possibility unlikely.
Modeling of hGnRHR rescued mutants with CA
Our present β2-adrenoreceptor-based models of the hGnRHR were similar to our previous rhodopsin-based models of hGnRHR within the seven TM α-helices (with ∼2 Å root-mean square deviation) but deviated in the loop regions. In particular, the structures of all intracellular loops and helical ends were corrected in accordance with the β2-adrenoreceptor template (PDB ID, 3sn6) to provide similar interactions with G protein trimer. In addition, we reconstructed N terminus by building an amphiphilic helix at residues 14–29 and omitting 1–11 region, as functionally unimportant (36). The hydrophobic surface of the amphiphilic helix 14–29 formed by residues C14, I17, I21, and M24 is facing the ligand-binding pocket. This structure and position of the N terminus may explain the known effect of mutations of C14 and M24 (10, 11, 13, 36, 37) on ligand binding. The side chain of K191 from the EL2 attached by two disulfides (C114-C196 and C14-C200) contacts the N-terminal helix near N18; glycosylation of this residue is essential for proper receptor trafficking to the plasma membrane (38, 39). The presence of K191 in hGnRHR may preclude N18 glycosylation in addition to its possible inhibitory effect on the formation of C14-C200 disulfide (17) or insertion of the EL2 between helices (19). All these factors would impair receptor trafficking to the plasma membrane. The absence of K191 and corresponding steric restrictions in rat and mouse receptors as well as existence of an additional glycosylation site at N4 would explain the much more efficient trafficking of rodent receptors to the plasma membrane (10, 11).
Experimental data obtained in the present study also allowed us to validate the improved hGnRHR models. Similarly to the structural templates, our models reproduced all consensus conformational changes associated with transition from the inactive to the active conformation (40–43). The major changes included the movement of TM5 toward TM6, counterclockwise rotation of TM6 (as viewed from the extracellular surface) and the inward shift of TM7 closer to TM2. Such helix rearrangement leads to the contraction of the extracellular ligand binding pocket (Fig. 5) but opened the large intracellular cavity between TM 3–5-6, enabling the insertion of C-terminal helix of Gα in this opening.
Fig. 5.
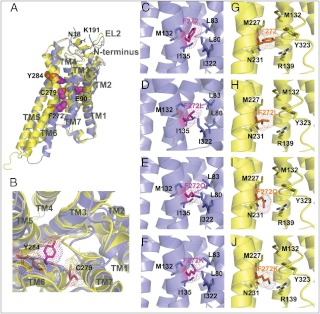
Location of mutations in the hGnRHR. Helices are shown by cartoons colored blue (inactive conformation) and yellow (active conformation). Substituted residues are shown by sticks, highlighted by dots, and colored purple (inactive conformation) and orange (active conformation). A, Positions of residues mutated in this work (E90K, F272Q, K, C279A, S, Y284F, and ΔK191) in inactive and active conformations of hGnRHR. Side chains of substituted residues (E90, F272, C279, and Y284) are shown by balls colored purple (for the inactive state) and orange (for the active state). K191 from the EL2 and proximal N18 from the N terminus are shown by sticks colored blue. B, Suggested changes in the ligand binding pocket on receptor activation. Positions of mutated residues (C279 and Y284) in the binding pocket of active (yellow) and inactive (blue) receptor conformations. Helix shift and rotamer change of Y284 and C279 during the receptor activation bring Y284 to the nonpolar lipid environment and C279 to the polar environment of the ligand binding pocket. Note the contraction of the ligand binding pocket in the active state caused by the inward movement of TM5, TM3, and TM7. C–J, Interactions between substituted residues in position 272 from the moving TM6 and adjacent residues from TM3, TM5, and TM7 in the model of the inactive and activated hGnRHR. Environment of F272 of hGnRHR (C and G) and its substitutions, F272L (D and H), F272Q (E and I), and F272K (F and J), in inactive (C–F, blue) or active (G–J, yellow) receptor states.
We assumed that structures of G protein-interacting regions should be rather similar in all GPCR. Intracellular ends of seven helices likely undergo similar shifts, which are accompanied by reorientation of residues from conserved GPCR sequence motifs that surround the G protein binding site, such as E/DRY in TM3, PxxxxxxCY in TM5, and D/NPxxY in TM7. On the contrary, rearrangement of residues from the extracellular part of helices, which form dissimilar ligand binding pockets, and from the FxxCWxP motif in the TM6, which acts as a ligand-activated conformational switch, are likely receptor specific and may depend on the ligand type (agonist, partial agonist, antagonist, inverse agonist). Indeed, as it can be noticed from comparison of conformational changes in different GPCR, the movement of the extracellular part of the TM6 was more pronounced during rhodopsin activation caused by cis-trans isomerization of retinal (PDB ID, 1u19 vs. 3pqr) than during agonist-induced activation of β2-adrenoreceptor (PDB ID, 2rh1 vs. 3sn6). Thus, we expected that identification and modeling of CA mutants would shed light on the conformation of extracellular part of the TM6 in the activated state of hGnRHR.
Indeed, the observed CA of C279S, C279A, and Y284F mutants may indicate that a more polar residue at 279 position and less polar residue at 284 position favor the active state conformation. Therefore, we suggest that during receptor activation, side chains of C279 and Y284 may move together with the whole TM6 or undergo rotamer switch (χ1 change from −60° to 180°). Either helix or side chain rotation would relocate the C279 side chain inside the water-filled milieu of the ligand binding pocket and Y284 toward the hydrophobic lipid environment (Fig. 5). These conformational changes in the region of the ligand binding pocket are reminiscent to the large TM6 rotation observed in the metarhodopsin II, rather than to the minor TM6 shift seen in the activated β2-adrenoreceptor.
Data on CA of F272Q and F272K mutants (Fig. 3) and on the enhanced folding and cell-surface expression of F272L mutant (Table 1) confirm the structure of the TM3–TM6 interface in the inactive receptor and large conformational alterations in this region on receptor activation. Comparison of the position of F272 and its substituents in the receptor models showed that this residue is tightly packed in the nonpolar environment of the inactive receptor formed by aliphatic side chains of L80, L83, M132, I135, and I322 (Fig. 5, A–D, blue). However, F272 becomes more flexible in the more polar environment of the active receptor formed by loosely packed R139, N231, Y323, M132, and M227 side chains (Fig. 5, E–H, yellow). Aliphatic environment in the inactive receptor is favorable for accommodation of aliphatic L272 and, to a lesser extent, of aromatic F272. Therefore, presence of L272 may promote transition to the inactive receptor conformation and its stabilization. This would explain the higher cell-surface expression of the F272L mutant and lack of CA (44). On the other hand, presence of polar Q272 and K272 likely destabilizes the compact structure or the inactive conformation and switches it to the more open activated state, where these residues are surrounded by polar groups and are accessible to water molecules. This may explain the observed CA of F272Q and F272K mutants.
Discussion
The concept of CA for receptors was introduced in 1989 (45), and it has been theorized that virtually all GPCR have mutants that couple to effectors in the absence of agonist (16). It was curious, in light of the clinical importance of the hGnRHR and the many hundreds of mutants reported for this GPCR, that no mutant with CA was identified among either naturally occurring or designed mutants until recently (17). The present work supports the view that this CA has likely been missed, because the cell recognizes such mutants of the hGnRHR as misfolded and prevents them from being expressed at the plasma membrane.
Mutant E90K was the first constitutively active mutant identified for the GnRHR but only after rescue of this protein with pharmacoperones or by deletion of K191 from EL2 (10, 22). In the present study, the approach of revealing undetected CA by rescue was applied to mutants of residues from the movable TM6.
There is a wealth of data, relying on ligand binding and IP production (G protein coupling), that are consistent with the absence of mutants F272A, E, or K (44), C279A (46), and Y284F (19) at the plasma membrane and a lack of coupling of these mutants to the G protein activation. In contrast, it has been shown that F272L mutation has increased cell-surface expression (36) without CA (44). We confirmed elevated cell-surface expression of F272L mutant compared with WT (Fig. 1).
Studying these mutants, we detect CA for F272Q, F272K, C279S, C279A, and Y284F, whose trafficking from the ER to the plasma membrane was recovered by pharmacoperone treatment or by deletion of residue K191. The greatest effect was observed for Gln and Lys replacement of the F272 residue from the TM6, which undergoes the large shift from the TM7 toward the TM5 on receptor activation. This may indicate the high structural flexibility of these mutants, which potentially adopt misfolded or activated conformations. The comparable levels of agonist-dependent and agonist-independent IP production by F272Q and F272K mutants may indicate that their CA conformation is rather similar to that induced by agonists. The much smaller CA observed for C279A, S, and Y284F mutants coincides with smaller conformational changes in these regions. CA of these mutants may indicate that side chains of C279 and Y284 switch to polar and nonpolar environment, respectively. These observations allowed us to validate the proposed models of the active conformation of hGnRHR.
Previous studies of GPCR revealed CA of some receptors with substitutions at the orthologous position corresponding to F272 of hGnRHR. These include M257Y and M257N mutants of rhodopsin (47, 48), and the L250N, L250Q, L250E, and L250K mutants of melanocortin-4 receptor (49). Naturally occurring L250Q mutation of melanocortin-4 receptor was associated with morbid obesity. In the present studies, we observed CA of F272K and F272Q mutants only after their rescue by pharmacoperones or genetic modification. This indicates that the cell protects itself against CA hGnRHR by retaining them in the ER as misfolded proteins.
Like the hGnRHR, other GPCR receptors showed inefficient plasma membrane expression of some WT receptors and numerous mutants that is likely caused by misfolding and misrouting of these proteins (11). Examples include the δ-opioid receptor, V2 vasopressin receptor, GluR1, α1D adrenoreceptor, odorant, and LH receptors (50–55). It was shown that cell-permeable antagonists, presumably behaving as pharmacoperones, facilitated posttranslational processing and increased export of the ligand-stabilized receptor from the ER to the cell surface of these mutants (5, 55, 56). This suggested that restricted trafficking and the inability to observe CA mutants in the absent of rescue may be a more commonly occurring means of regulating protein availability at the plasma membrane than is presently appreciated and that CA may be revealed for mutants of other receptors by techniques that rescue ER-retained proteins.
Supplementary Material
Acknowledgments
We thank Jo Ann Binkerd for formatting the manuscript and Rachele Schuyler for technical assistance. We also thank Dr. B. Park in the Oregon National Primate Research Center Biostatistics Unit for guidance.
This work was supported by National Institutes of Health Grants DK85040, RR030229, RR000163 (to P.M.C), and DA003910 (to H.I.M. and I.D.P.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Bmax
- Maximum binding
- CA
- constitutive activity
- EL
- extracellular loop
- ER
- endoplasmic reticulum
- FCS
- fetal calf serum
- GFP
- green fluorescent protein
- GnRHR
- GnRH receptor
- GPCR
- G protein-coupled receptor
- hGnRHR
- human GnRHR
- IP
- inositol phosphate
- Kd
- dissociation constant
- QCS
- quality control system
- TM
- transmembrane helix
- WT
- wild type.
References
- 1. Janovick JA, Goulet M, Bush E, Greer J, Wettlaufer DG, Conn PM. 2003. Structure-activity relations of successful pharmacologic chaperones for rescue of naturally occurring and manufactured mutants of the gonadotropin-releasing hormone receptor. J Pharmacol Exp Ther 305:608–614 [DOI] [PubMed] [Google Scholar]
- 2. Brothers SP, Cornea A, Janovick JA, Conn PM. 2004. Human loss-of-function gonadotropin-releasing hormone receptor mutants retain wild-type receptors in the endoplasmic reticulum: molecular basis of the dominant-negative effect. Mol Endocrinol 18:1787–1797 [DOI] [PubMed] [Google Scholar]
- 3. Janovick JA, Brothers SP, Cornea A, Bush E, Goulet MT, Ashton WT, Sauer DR, Haviv F, Greer J, Conn PM. 2007. Refolding of misfolded mutant GPCR: post-translational pharmacoperone action in vitro. Mol Cell Endocrinol 272:77–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Janovick JA, Patny A, Mosley R, Goulet MT, Altman MD, Rush TS, 3rd, Cornea A, Conn PM. 2009. Molecular mechanism of action of pharmacoperone rescue of misrouted GPCR mutants: the GnRH receptor. Mol Endocrinol 23:157–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Janovick JA, Maya-Nunez G, Conn PM. 2002. Rescue of hypogonadotropic hypogonadism-causing and manufactured GnRH receptor mutants by a specific protein-folding template: misrouted proteins as a novel disease etiology and therapeutic target. J Clin Endocrinol Metab 87:3255–3262 [DOI] [PubMed] [Google Scholar]
- 6. Leaños-Miranda A, Janovick JA, Conn PM. 2002. Receptor-misrouting: an unexpectedly prevalent and rescuable etiology in gonadotropin-releasing hormone receptor-mediated hypogonadotropic hypogonadism. J Clin Endocrinol Metab 87:4825–4828 [DOI] [PubMed] [Google Scholar]
- 7. Leaños-Miranda A, Ulloa-Aguirre A, Janovick JA, Conn PM. 2005. In vitro coexpression and pharmacological rescue of mutant gonadotropin-releasing hormone receptors causing hypogonadotropic hypogonadism in humans expressing compound heterozygous alleles. J Clin Endocrinol Metab 90:3001–3008 [DOI] [PubMed] [Google Scholar]
- 8. Leaños-Miranda A, Ulloa-Aguirre A, Ji TH, Janovick JA, Conn PM. 2003. Dominant-negative action of disease-causing gonadotropin-releasing hormone receptor (GnRHR) mutants: a trait that potentially coevolved with decreased plasma membrane expression of GnRHR in humans. J Clin Endocrinol Metab 88:3360–3367 [DOI] [PubMed] [Google Scholar]
- 9. Conn PM, Knollman PE, Brothers SP, Janovick JA. 2006. Protein folding as posttranslational regulation: evolution of a mechanism for controlled plasma membrane expression of a G protein-coupled receptor. Mol Endocrinol 20:3035–3041 [DOI] [PubMed] [Google Scholar]
- 10. Janovick JA, Knollman PE, Brothers SP, Ayala-Yáñez R, Aziz AS, Conn PM. 2006. Regulation of G protein-coupled receptor trafficking by inefficient plasma membrane expression: molecular basis of an evolved strategy. J Biol Chem 281:8417–8425 [DOI] [PubMed] [Google Scholar]
- 11. Knollman PE, Janovick JA, Brothers SP, Conn PM. 2005. Parallel regulation of membrane trafficking and dominant-negative effects by misrouted gonadotropin-releasing hormone receptor mutants. J Biol Chem 280:24506–24514 [DOI] [PubMed] [Google Scholar]
- 12. Conn PM, Janovick JA. 2009. Drug development and the cellular quality control system. Trends Pharmacol Sci 30:228–233 [DOI] [PubMed] [Google Scholar]
- 13. Maya-Núñez G, Janovick JA, Aguilar-Rojas A, Jardón-Valadez E, Leaños-Miranda A, Zariñan T, Ulloa-Aguirre A, Conn PM. 2011. Biochemical mechanism of pathogenesis of human gonadotropin-releasing hormone receptor mutants Thr104Ile and Tyr108Cys associated with familial hypogonadotropic hypogonadism. Mol Cell Endocrinol 337:16–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Conn PM, Ulloa-Aguirre A. 2011. Pharmacological chaperones for misfolded gonadotropin-releasing hormone receptors. Adv Pharmacol 62:109–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tao YX. 2008. Constitutive activation of G protein-coupled receptors and diseases: insights into mechanisms of activation and therapeutics. Pharmacol Ther 120:129–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bond RA, Ijzerman AP. 2006. Recent developments in constitutive receptor activity and inverse agonism, and their potential for GPCR drug discovery. Trends Pharmacol Sci 27:92–96 [DOI] [PubMed] [Google Scholar]
- 17. Janovick JA, Conn PM. 2010. Salt bridge integrates GPCR activation with protein trafficking. Proc Natl Acad Sci USA 107:4454–4458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Janovick JA, Park BS, Conn PM. 2011. Therapeutic rescue of misfolded mutants: validation of primary high throughput screens for identification of pharmacoperone drugs. PLoS One 6:e22784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Janovick JA, Pogozheva ID, Mosberg HI, Conn PM. 2011. Salt bridges overlapping the gonadotropin-releasing hormone receptor agonist binding site reveal a coincidence detector for g protein-coupled receptor activation. J Pharmacol Exp Ther 338:430–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Flanagan CA, Rodic V, Konvicka K, Yuen T, Chi L, Rivier JE, Millar RP, Weinstein H, Sealfon SC. 2000. Multiple interactions of the Asp(2.61(98)) side chain of the gonadotropin-releasing hormone receptor contribute differentially to ligand interaction. Biochemistry 39:8133–8141 [DOI] [PubMed] [Google Scholar]
- 21. Zhou W, Rodic V, Kitanovic S, Flanagan CA, Chi L, Weinstein H, Maayani S, Millar RP, Sealfon SC. 1995. A locus of the gonadotropin-releasing hormone receptor that differentiates agonist and antagonist binding sites. J Biol Chem 270:18853–18857 [DOI] [PubMed] [Google Scholar]
- 22. Maya-Núñez G, Janovick JA, Ulloa-Aguirre A, Söderlund D, Conn PM, Méndez JP. 2002. Molecular basis of hypogonadotropic hypogonadism: restoration of mutant (E(90)K) GnRH receptor function by a deletion at a distant site. J Clin Endocrinol Metab 87:2144–2149 [DOI] [PubMed] [Google Scholar]
- 23. Janovick JA, Brothers SP, Knollman PE, Conn PM. 2007. Specializations of a G-protein-coupled receptor that appear to aid with detection of frequency-modulated signals from its ligand. FASEB J 21:384–392 [DOI] [PubMed] [Google Scholar]
- 24. Lin X, Janovick JA, Brothers S, Blömenrohr M, Bogerd J, Conn PM. 1998. Addition of catfish gonadotropin-releasing hormone (GnRH) receptor intracellular carboxyl-terminal tail to rat GnRH receptor alters receptor expression and regulation. Mol Endocrinol 12:161–171 [DOI] [PubMed] [Google Scholar]
- 25. Lin X, Cornea A, Janovick JA, Conn PM. 1998. Visualization of unoccupied and occupied gonadotropin-releasing hormone receptors in living cells. Mol Cell Endocrinol 146:27–37 [DOI] [PubMed] [Google Scholar]
- 26. Conn PM, Janovick JA. 2009. Trafficking and quality control of the gonadotropin releasing hormone receptor in health and disease. Mol Cell Endocrinol 299:137–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huckle WR, Conn PM. 1987. Use of lithium ion in measurement of stimulated pituitary inositol phospholipid turnover. Methods Enzymol 141:149–155 [DOI] [PubMed] [Google Scholar]
- 28. Conn PM, Leaños-Miranda A, Janovick JA. 2002. Protein origami: therapeutic rescue of misfolded gene products. Mol Interv 2:308–316 [DOI] [PubMed] [Google Scholar]
- 29. Ulloa-Aguirre A, Janovick JA, Brothers SP, Conn PM. 2004. Pharmacologic rescue of conformationally-defective proteins: implications for the treatment of human disease. Traffic 5:821–837 [DOI] [PubMed] [Google Scholar]
- 30. Castro-Fernández C, Maya-Núñez G, Conn PM. 2005. Beyond the signal sequence: protein routing in health and disease. Endocr Rev 26:479–503 [DOI] [PubMed] [Google Scholar]
- 31. Cook JV, Eidne KA. 1997. An intramolecular disulfide bond between conserved extracellular cysteines in the gonadotropin-releasing hormone receptor is essential for binding and activation. Endocrinology 138:2800–2806 [DOI] [PubMed] [Google Scholar]
- 32. Janovick JA, Ulloa-Aguirre A, Conn PM. 2003. Evolved regulation of gonadotropin-releasing hormone receptor cell surface expression. Endocrine 22:317–327 [DOI] [PubMed] [Google Scholar]
- 33. Ulloa-Aguirre A, Janovick JA, Leaños-Miranda A, Conn PM. 2003. Misrouted cell surface receptors as a novel disease aetiology and potential therapeutic target: the case of hypogonadotropic hypogonadism due to gonadotropin-releasing hormone resistance. Expert Opin Ther Targets 7:175–185 [DOI] [PubMed] [Google Scholar]
- 34. Brothers SP, Janovick JA, Maya-Nunez G, Cornea A, Han XB, Conn PM. 2002. Conserved mammalian gonadotropin-releasing hormone receptor carboxyl terminal amino acids regulate ligand binding, effector coupling and internalization. Mol Cell Endocrinol 190:19–27 [DOI] [PubMed] [Google Scholar]
- 35. Brothers SP, Janovick JA, Conn PM. 2003. Unexpected effects of epitope and chimeric tags on gonadotropin-releasing hormone receptors: implications for understanding the molecular etiology of hypogonadotropic hypogonadism. J Clin Endocrinol Metab 88:6107–6112 [DOI] [PubMed] [Google Scholar]
- 36. Kohout TA, Xie Q, Reijmers S, Finn KJ, Guo Z, Zhu YF, Struthers RS. 2007. Trapping of a nonpeptide ligand by the extracellular domains of the gonadotropin-releasing hormone receptor results in insurmountable antagonism. Mol Pharmacol 72:238–247 [DOI] [PubMed] [Google Scholar]
- 37. Betz SF, Reinhart GJ, Lio FM, Chen C, Struthers RS. 2006. Overlapping, nonidentical binding sites of different classes of nonpeptide antagonists for the human gonadotropin-releasing hormone receptor. J Med Chem 49:637–647 [DOI] [PubMed] [Google Scholar]
- 38. Davidson JS, Flanagan CA, Zhou W, Becker II, Elario R, Emeran W, Sealfon SC, Millar RP. 1995. Identification of N-glycosylation sites in the gonadotropin-releasing hormone receptor: role in receptor expression but not ligand binding. Mol Cell Endocrinol 107:241–245 [DOI] [PubMed] [Google Scholar]
- 39. Davidson JS, McArdle CA, Davies P, Elario R, Flanagan CA, Millar RP. 1996. Asn102 of the gonadotropin-releasing hormone receptor is a critical determinant of potency for agonists containing C-terminal glycinamide. J Biol Chem 271:15510–15514 [DOI] [PubMed] [Google Scholar]
- 40. Standfuss J, Edwards PC, D'Antona A, Fransen M, Xie G, Oprian DD, Schertler GF. 2011. The structural basis of agonist-induced activation in constitutively active rhodopsin. Nature 471:656–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Salon JA, Lodowski DT, Palczewski K. 2011. The significance of G protein-coupled receptor crystallography for drug discovery. Pharmacol Rev 63:901–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Katritch V, Abagyan R. 2011. GPCR agonist binding revealed by modeling and crystallography. Trends Pharmacol Sci 32:637–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lebon G, Warne T, Edwards PC, Bennett K, Langmead CJ, Leslie AG, Tate CG. 2011. Agonist-bound adenosine A2A receptor structures reveal common features of GPCR activation. Nature 474:521–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Myburgh DB, Pawson AJ, Davidson JS, Flanagan CA, Millar RP, Hapgood JP. 1998. A single amino acid substitution in transmembrane helix VI results in overexpression of the human GnRH receptor. Eur J Endocrinol 139:438–447 [DOI] [PubMed] [Google Scholar]
- 45. Costa T, Herz A. 1989. Antagonists with negative intrinsic activity at δ opioid receptors coupled to GTP-binding proteins. Proc Natl Acad Sci USA 86:7321–7325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. White CD. 2009. Dissection of GnRH receptor-G protein coupling. In: Medical research council human reproductive sciences unit. Edinburgh: University of Aberdeen; 1–281 [Google Scholar]
- 47. Han M, Smith SO, Sakmar TP. 1998. Constitutive activation of opsin by mutation of methionine 257 on transmembrane helix 6. Biochemistry 37:8253–8261 [DOI] [PubMed] [Google Scholar]
- 48. Kim JM, Altenbach C, Kono M, Oprian DD, Hubbell WL, Khorana HG. 2004. Structural origins of constitutive activation in rhodopsin: role of the K296/E113 salt bridge. Proc Natl Acad Sci USA 101:12508–12513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Proneth B, Xiang Z, Pogozheva ID, Litherland SA, Gorbatyuk OS, Shaw AM, Millard WJ, Mosberg HI, Haskell-Luevano C. 2006. Molecular mechanism of the constitutive activation of the L250Q human melanocortin-4 receptor polymorphism. Chem Biol Drug Des 67:215–229 [DOI] [PubMed] [Google Scholar]
- 50. Saito H, Kubota M, Roberts RW, Chi Q, Matsunami H. 2004. RTP family members induce functional expression of mammalian odorant receptors. Cell 119:679–691 [DOI] [PubMed] [Google Scholar]
- 51. Pietilä EM, Tuusa JT, Apaja PM, Aatsinki JT, Hakalahti AE, Rajaniemi HJ, Petäjä-Repo UE. 2005. Inefficient maturation of the rat luteinizing hormone receptor. A putative way to regulate receptor numbers at the cell surface. J Biol Chem 280:26622–26629 [DOI] [PubMed] [Google Scholar]
- 52. Petrovska R, Kapa I, Klovins J, Schiöth HB, Uhlén S. 2005. Addition of a signal peptide sequence to the α1D-adrenoceptor gene increases the density of receptors, as determined by [3H]-prazosin binding in the membranes. Br J Pharmacol 144:651–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Morello JP, Bichet DG. 2001. Nephrogenic diabetes insipidus. Annu Rev Physiol 63:607–630 [DOI] [PubMed] [Google Scholar]
- 54. Uberti MA, Hague C, Oller H, Minneman KP, Hall RA. 2005. Heterodimerization with β2-adrenergic receptors promotes surface expression and functional activity of α1D-adrenergic receptors. J Pharmacol Exp Ther 313:16–23 [DOI] [PubMed] [Google Scholar]
- 55. Petäjä-Repo UE, Hogue M, Bhalla S, Laperrière A, Morello JP, Bouvier M. 2002. Ligands act as pharmacological chaperones and increase the efficiency of δ opioid receptor maturation. EMBO J 21:1628–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bichet DG. 2006. Nephrogenic diabetes insipidus. Adv Chronic Kidney Dis 13:96–104 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.