Abstract
Somatostatin receptor subtype 5 (SSTR5) mediates the inhibitory effect of somatostatin and its analogs on insulin expression/secretion and islet cell proliferation. We provide biochemical and genetic evidence that SSTR5 exerted its physiological actions via down-regulating pancreatic and duodenal homeobox-1 (PDX-1), a β-cell-specific homeodomain-containing transcription factor. Cotransfection of SSTR5 with PDX-1 resulted in dose-dependent inhibition of PDX-1 expression in human embryonic kidney 293 cells. SSTR5 agonist RPL-1980 inhibited PDX-1 expression and abolished glucagon-like peptide 1-stimulated PDX-1 expression in mouse insulinoma β-TC-6 cells. SSTR5 knockdown by short hairpin RNA led to increased PDX-1 expression that was accompanied by enhanced insulin secretion stimulated by high glucose in β-TC6 cells and alternated expressions of cell cycle proteins that favor cell proliferation in mouse insulinoma MIN6 cells. Quantitative RT-PCR analysis showed that cotransfected SSTR5 inhibited PDX-1 mRNA expression, whereas knockdown of SSTR5 increased PDX-1 mRNA expression. In addition, we found that cotransfected wild-type SSTR5 increased PDX-1 ubiquitination in human embryonic kidney 293 cells, whereas SSTR5 P335L, a hypofunctional single nucleotide polymorphism of SSTR5, inhibited PDX-1 ubiquitination. SSTR5 knockout resulted in increased expression of PDX-1, insulin, and proliferating cell nuclear antigen in the islets of sstr−/− mice. Immunohistochemistry analysis showed that SSTR5 P335L was associated with elevated expression of PDX-1 in human pancreatic neuroendocrine tumor. Taken together, our studies demonstrated that SSTR5 is a negative regulator for PDX-1 expression and that SSTR5 may mediate the inhibitory effects of somatostatin and its analogs on insulin expression/secretion and cell proliferation via down-regulating PDX-1 at both transcriptional and posttranslational levels.
Somatostatin (SST), a cyclic tetradecapeptide peptide hormone, acts as a suppressor of cell proliferation and endocrine and nervous system functions by inhibiting the secretion of numerous endocrine hormones, including insulin, glucagons, and gastrin (1). The characteristic that SST is an endogenous antiproliferative agent makes SST and its analogs promising antitumor agents in a variety of experimental tumor models (2–5). SST exerts its physiological actions by binding a group of G protein-coupled receptors known as SST receptors (6), followed by a series of signaling events including conformational changes, homo/heterodimerization, internalization, protein-protein interaction, and activation of downstream signaling pathways (3, 7). SST receptor type 5 (SSTR5) is one of major SSTR in the islets of Langerhans and plays an essential role in mediating the inhibitory effect of SST on insulin expression/secretion and cell proliferation (8). SSTR5 also contributes to decreased pancreatic carcinogenesis (9–11), decreased islet angiogenesis (12), and increased apoptosis (13). We have recently identified a group of SSTR5-regulated islet genes involved in cell proliferation, angiogenesis, apoptosis, and immunity based on their differential expressions in the pancreas of SSTR5-deficient mice (14).
Pancreatic and duodenal homeobox-1 (PDX-1), also known as insulin promoter factor-1 1) (15), SST-transactivating factor-1 (16), glucose-sensitive transcription factor (17), islet-duodenum homeobox gene-1 (18), glucose-sensitive factor (19), and insulin upstream factor 1 (20), is a homeodomain-containing transcription factor essential for pancreatic development, β-cell differentiation, and maintenance of normal β-cell function by regulating a variety of transcriptome targets involved in endocrine system and metabolism, various signaling pathways, and cellular survival (21). PDX-1 expression is restricted within the nuclei of approximately 90% of insulin-producing islet β-cells in adult pancreata, where it binds to the promoters of insulin, glucose transporter 2, islet amyloid polypeptide, and glucokinase, regulates their expression and, thus, plays a critical role in insulin expression/secretion and maitenance of glucose homeostasis (22, 23). Targeted inactivation of the PDX-1 gene in mice (24) and homozygosity for a nonsense mutation in the human PDX-1 gene (25) result in pancreatic agenesis. Targeted disruption of PDX-1 gene in β-cells of the mice leads to overt diabetes (26), whereas heterozygosity for the null mutation, and hence reduced PDX-1 expression levels, results in decreased insulin expression/secretion (26, 27) and predispose islets to apoptosis (28). In humans, mutations in the PDX-1 gene have been linked to diabetes, including type 4 maturity-onset diabetes of the young (MODY IV), an autosomal dominant form of diabetes mellitus affecting patients before the age of 25 yr, and non-mature-onset diabetes of the young type 2 in some populations (29). Recent studies show that PDX-1 is aberrantly overexpressed in a variety of human cancers including pancreatic, gastric, colon, breast, prostate, colorectal, kidney cancer, pediatric solid pseudopapillary tumor, and pancreatic neuroendocrine tumor (PNET) (30–37). Moreover, PDX-1 overexpression in patients with pancreatic cancer is significantly correlated with the pathological parameters (e.g. metastasis and histological grade) (35, 36), indicating that PDX-1 is functionally involved in tumorigenesis. Indeed, persistent expression of PDX-1 induces metaplasia (32), and PDX-1 is required for K-rasG12D to induce the development of PanIN, metaplasia, and pancreatic ductal adenocarcinoma (38). Furthermore, overexpression of PDX-1 results in an increased cell proliferation, invasion, and colony formation of human embryonic kidney (HEK)293 cells and an enhanced pancreatic Mia PaCa-2 tumor formation in severe combined immunodeficiency mice (39).
We have recently demonstrated that SSTR5 P335L, a nonsynonymous single-nucleotide polymorphism resulting from a C to T change at the 1004th nucleotide of the human SSTR5 gene, up-regulates PDX-1 expression (40). Given the hypofunctional nature of SSTR5 P335L compared with wild-type (WT) SSTR5 (40), it is likely that SSTR5 is a negative regulator for PDX-1 expression. The purpose of this study is to determine whether SST and its analogs regulate PDX-1 expression and whether SSTR5 mediates the inhibitory effects of SST on insulin expression/secretion and cell proliferation via a novel mechanism of down-regulating PDX-1.
Results
SSTR5 inhibits PDX-1 expression with an accompanied inhibition of PDX-1 mRNA expression
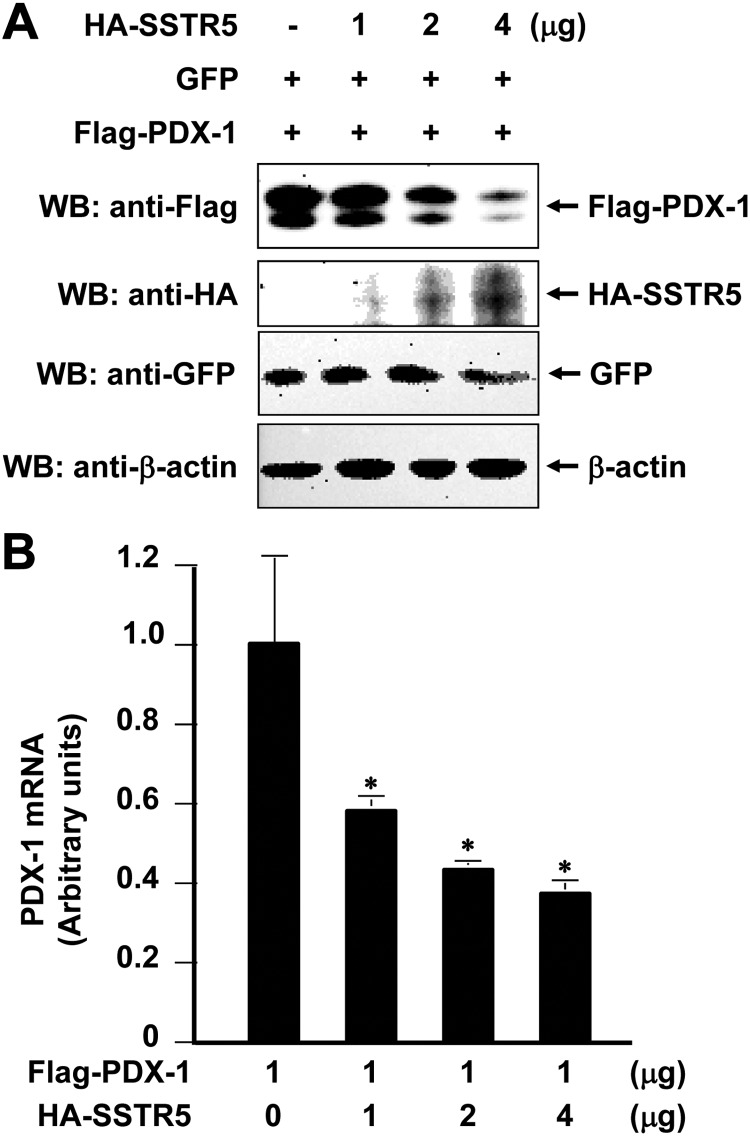
To determine the effect of SSTR5 on PDX-1, we first transfected Flag-PDX-1 into HEK293 cells with different amounts of hemagglutin (HA)-SSTR5. Western blot analysis of Flag-PDX-1 using an anti-Flag antibody showed that cotransfection of SSTR5 with PDX-1 resulted in a dose-dependent inhibition of PDX-1 expression (Fig. 1A, top panel). Under the same conditions, however, HA-SSTR5 had no such an inhibitory effect on the expression of cotransfected green fluorescent protein (GFP) (Fig. 1A, middle panel). These data demonstrated that SSTR5 was a negative regulator for PDX-1 expression and the down-regulating effect of SSTR5 on PDX-1 is specific. To understand the mechanism underlying the inhibition of PDX-1 by SSTR5, we performed quantitative RT-PCR (qRT-PCR) to determine whether SSTR5 affected PDX-1 mRNA expression levels. As shown in Fig. 1B, cotransfection of SSTR5 with PDX-1 resulted in a dose-dependent inhibition of PDX-1 mRNA expression. Thus, SSTR5 inhibited PDX-1 expression at least partially through inhibiting PDX-1 mRNA expression.
Fig. 1.
SSTR5 down-regulates PDX-1 with accompanied inhibition of PDX-1 mRNA. A, One microgram of Flag-PDX-1 and GFP was cotransfected with 1, 2, or 4 μg of HA-SSTR5 into HEK293 cells (4 × 105 in six-well plates) using Lipofectamine 2000. The cells were collected 40 h after transfection. A, Two thirds of the cells were used to make whole-cell lysates, which were subjected to SDS-PAGE, followed by Western blotting (WB) with an anti-Flag antibody for Flag-PDX-1, an anti-GFP antibody for GFP, an anti-HA antibody for HA-SSTR5, and an anti-β-actin antibody for β-actin. B, One third of the cells were used to isolate total RNA using TriZol reagent, followed by qRT-PCR analysis. The cDNA was prepared from the total RNA using qScript cDNA SuperMix. The mRNA levels of target genes in the samples were normalized against GAPDH. *, P < 0.05 showing significant difference.
SSTR5 agonist RPL-1980 inhibits PDX-1 expression and abolishes glucagon-like peptide 1 GLP-1-stimulated PDX-1 expression
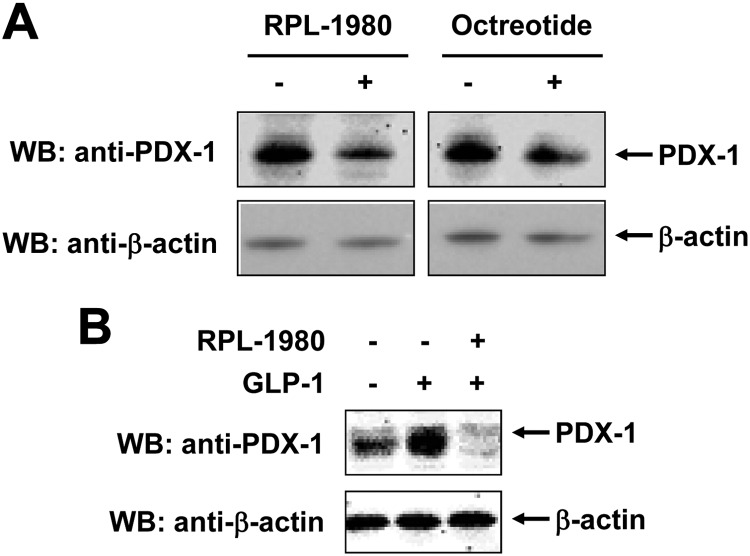
RPL-1980 is a SSTR5 agonist inhibiting cell proliferation and glucose-stimulated insulin secretion (40). To confirm the inhibitory effect of SSTR5 on PDX-1, we examined whether RPL-1980 or octreotide, an octapeptide that mimics natural SST pharmacologically, inhibited PDX-1 expression. Mouse insulinoma β-TC-6 cells were treated with 10−5 m of RPL-1980 or octreotide for 36 h. Western blotting analysis of endogenous PDX-1 using an anti-PDX-1 polyclonal antibody showed that PDX-1 expression levels were reduced with the treatment of both RPL-1980 (Fig. 2A, left panel) and octreotide (Fig. 2A, right panel). GLP-1 is a known stimulus for PDX-1 expression (41, 42). As expected, treatment of β-TC-6 cells with 10 nm GLP-1 for 12 h resulted in a significant increase of PDX-1 expression (Fig. 2B, lane 2 vs. lane 1). However, GLP-1-stimulated PDX-1 expression was abolished by pretreatment of β-TC-6 cells with 10−5 m RPL-1980 (Fig. 2B, lane 3 vs. lane 2). These data further demonstrate that PDX-1 expression is negatively regulated by SSTR5.
Fig. 2.
SSTR5 agonist RPL-1980 abolishes GLP-1-stimulated PDX-1 expression in β-TC-6 cells. A, β-TC-6 cells were treated with 10−5 m of RPL-1980 or octreotide for 36 h. The whole-cell lysates were subjected to SDS-PAGE, followed by Western blotting (WB) with an anti-PDX-1 antibody for PDX-1 and an anti-β-actin antibody for β-actin. B, β-TC6 cells were pretreated with 10−5 m of RPL-1980 for 30 min, followed by treatment with 10 nm GLP-1 for 16 h. The whole-cell lysates were subjected to SDS-PAGE, followed by Western blotting with an anti-PDX-1 antibody. The blot was then reprobed with an anti-β-actin antibody.
Knockdown of SSTR5 leads to increased PDX-1 expression with increased insulin secretion
SSTR5 mediates the inhibitory effect of SST on insulin expression/secretion (8, 43). On the other hand, PDX-1 is essential for insulin expression and secretion (26, 27). Given the inhibitory effect of SSTR5 on PDX-1 expression (Figs. 1 and 2), we speculated that SSTR5 may mediate the inhibitory effect of SST on insulin expression/secretion through inhibiting PDX-1. To test the hypothesis, we applied a short hairpin RNA (shRNA) approach to examine the effect of SSTR5 knockdown on PDX-1 expression and PDX-1-regulated insulin expression and secretion in β-TC-6 cells. β-TC-6 cells were transfected with a mouse SSTR5-specific shRNA or a scramble shRNA. Western blot analysis of endogenous SSTR5 and PDX-1 using an anti-SSTR5 and an anti-PDX-1 polyclonal antibody, respectively, showed that transfection of SSTR5 shRNA, but not scramble shRNA, resulted in a significant knockdown of SSTR5 (Fig. 3A, top panel), which was accompanied by an increased expression of PDX-1 (Fig. 3A, middle panel). These data further supported a negative role for SSTR5 in the regulation of expression of PDX-1. We then transfected SSTR5 shRNA or scramble shRNA into β-TC-6 cells, followed by treatment with 16.7 mm of glucose. Insulin ELISA analysis showed that a high concentration of glucose stimulated an increased insulin secretion in scramble shRNA-transfected cells (Fig. 3B, column 2 vs. 1), and SSTR5 knockdown resulted in an enhanced insulin secretion in response to high glucose (Fig. 3B, column 4 vs. 2). Moreover, basal insulin secretion was increased in SSTR5 knockdown cells compared with that in scramble shRNA-transfected cells (Fig. 3B, column 3 vs. 1). qRT-PCR analysis showed that, in comparison with scramble shRNA, SSTR5 shRNA-induced SSTR5 knockdown led to increased expression of PDX-1 mRNA under both basal and high glucose-stimulated conditions (Fig. 3C, column 3 vs. 1 and column 4 vs. 2, respectively). Taken together, these data indicate that PDX-1 is subject to negative regulation by SSTR5 and that SSTR5 exerts its inhibitory effect on insulin expression and secretion through a mechanism by inhibiting PDX-1 expression.
Fig. 3.
SSTR5 knockdown results in increased PDX-1 expression and enhanced glucose-stimulated insulin secretion in insulinoma β-TC6 cells. A, β-TC6 cells were transfected with a scramble or SSTR5 shRNA using Lipofectamine 2000. Forty eight housrs after transfection, whole-cell lysates were subjected to Western blotting (WB) using an anti-SSTR5, an anti-PDX-1, and anti-β-actin antibody, respectively. B, β-TC6 cells were transfected with scramble or SSTR5 shRNA using Lipofectamine 2000 and 48 h after transfection, the cells were washed twice with Krebs-Ringer bicarbonate (KRB) buffer and incubated in KRB-BSA for 1 h. The cells were then added KRB buffer containing 16.7 mm of glucose for 4 h. After incubation, the media were collected and centrifuged at 600 × g for 5 min. The insulin concentrations in the media were measured by ELISA assay (*, P < 0.05 showing significant difference). C, The cell pellets from panel B were used to isolate total RNA using TriZol reagent, followed by qRT-PCR analysis. The cDNA was prepared from the total RNA using qScript cDNA SuperMix. The mRNA levels of target genes in the samples were normalized against GAPDH.
Knockdown of SSTR5 leads to increased PDX-1 expression along with alterations of cell cycle proteins
Next, we sought to examine the effect of SSTR5 knockdown on PDX-1 expression and cell proliferation in mouse insulinoma MIN6 cells. MIN6 cells were transfected with SSTR5 shRNA or scramble shRNA. Western blot analysis showed that SSTR5 was significantly knocked down by SSTR5 shRNA, but not a scramble shRNA (Fig. 4A, top panel), and that knockdown of SSTR5 led to an increased expression of PDX-1 (Fig. 4A, panel 2). Moreover, SSTR5 knockdown-induced increase of PDX-1 expression was accompanied by an increased expression of cyclin E and cyclin-dependent kinase 4 (CDK4) (Fig. 4A, panels 3 and 4, respectively) and a decreased expression of p21, p27, and p53 (Fig. 4A, panels 5, 6, and 7, respectively). Cyclin E and CDK4 are both essential for the transition from cell cycle G1 to S phase. p21 and p27 are two CDK inhibitors. Tumor suppressor protein p53 induces growth arrest by holding the cell cycle at the G1/S. Thus, these data further support a negative role for SSTR5 in regulation of PDX-1 expression. These data also demonstrated that SSTR5 exerted its inhibitory effect on cell proliferation through a mechanism by inhibiting PDX-1 expression, leading to alterations of expression of cell cycle proteins and subsequent inhibition of cell cycle progression.
Fig. 4.
SSTR5 knockdown-induced increase of PDX-1 is accompanied by alterations of expression of cell cycle proteins in mouse insulinoma MIN6 cells. MIN6 cells were transfected with scramble shRNA or SSTR5 shRNA using Lipofectamine 2000. Forty eight hours after transfection, the whole-cell lysates were subjected to SDS-PAGE, followed by Western blotting (WB) using an antibody against SSTR5, PDX-1, cyclin E, Cdk4, p21, p27, and p53, respectively. A, A representative Western blotting experiment. B, Densitometric analysis of three independent Western blotting experiments (*, P < 0.05 showing significant difference).
SSTR5 enhances PDX-1 ubiquitination
PDX-1 expression is controlled at both transcriptional (44–46) and posttranslational levels (47–50). Ubiquitin-targeted protein degradation is one mechanism that controls cellular expression levels of PDX-1 (47–49). Therefore, we sought to examine whether SSTR5 down-regulates PDX-1 via affecting its ubiquitination in addition to inhibition of PDX-1 mRNA expression. Flag-PDX-1 was transfected into HEK293T cells with HA-SSTR5 or HA-SSTR5 P335L in the presence of HA-ubiquitin. Immunoprecipitation/Western blotting analysis showed that cotransfected SSTR5 resulted in enhanced PDX-1 ubiquitination (Fig. 5, left panel), whereas hypofunctional SSTR5 P335L inhibited PDX-1 ubiquitination (Fig. 5, right panel). These data, thus, indicated that SSTR5 exerted its inhibitory effect on PDX-1 expression through another mechanism by enhancing PDX-1 ubiquitination and degradation in addition to inhibiting PDX-1 mRNA expression.
Fig. 5.
WT SSTR5 enhances, whereas SSTR5 P335L inhibits, PDX-1 ubiquitination. Flag-PDX-1 (8 μg) was cotransfected into HEK293 cells with or without 8 μg of HA-SSTR5 or HA-SSTR5 P335L in the presence or absence of 5 μg HA-ubiquitin (Ubi). Empty vector was used to normalize the amount of transfected DNA. Thirty hours after transfection, the cells were added to 5 μm MG132 for 12 h. Flag-PDX-1 was immunoprecipitated (IP) with an anti-Flag antibody, followed by immunoblotting with an anti-HA antibody. Expression levels of Flag-PDX-1, HA-SSTR5, HA-SSTR5 P335L, and HA-ubiquitin were examined by Western blotting (WB) using an anti-Flag and an anti-HA antibody, respectively.
Genetic ablation of SSTR5 results in increased islet expression of PDX-1, insulin, and proliferating cell nuclear antigen (PCNA)
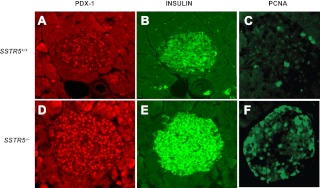
To further confirm the negative role for SSTR5 in regulation of PDX-1 expression, we took advantage of the availability of SSTR5 knockout mouse models generated in our laboratory (51–53). By performing immunohistochemistry analysis, we found that genetic ablation of SSTR5 resulted in a significant increase of PDX-1 expression in the islets of SSTR5 knockout mice in comparison with those of WT mice (Fig. 6, A vs. D). Moreover, we found that increased islet PDX-1 expression was accompanied by an increased expression of insulin (Fig. 6, B vs. E) and PCNA (Fig. 6, C vs. F). These data further support a concept that SSTR5 is a negative regulator for PDX-1 expression and that SSTR5 exerts its inhibitory effect on insulin expression and cell proliferation through inhibiting PDX-1 expression.
Fig. 6.
Genetic ablation of SSTR5 results in overexpression of PDX-1 in islet with accompanied increase of expression of insulin and PCNA. Whole pancreatic tissues isolated from WT and SSTR5−/− mice were fixed in 4% (vol/vol) paraformaldehyde and embedded in paraffin. Immunohistochemistry analyses were performed using antibodies against PDX-1 (1:200), insulin (1:75), or PCNA (1:100). Fluorescence was developed using cy3- or FITC-conjugated secondary antibody. Photomicrographs were taken under ×100 magnification. A, B and C were islets isolated from SSTR5 WT mice immunostained with an antibody against PDX-1, insulin and PCNA, respectively. D, E and F were islets isolated from SSTR5 knockout mice immunostained with an antibody against PDX-1, insulin and PCNA, respectively.
SSTR5 P335L contributes to elevated PDX-1 expression in human PNET
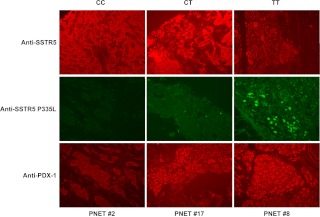
SSTR are widely expressed in human PNET (54, 55). We have recently found that hypofunctional SSTR5 P335L single-nucleotide polymorphism is expressed in more than 50% of PNET (56). We also found that PDX-1 is markedly overexpressed in PNET (37). Given the negative role for SSTR5 in regulation of PDX-1 expression, we were prompted to examine whether PDX-1 expression levels in PNET were related to the genotype of SSTR5. We performed immunohistochemistry analysis of total 22 PNET specimens using an anti-PDX-1 polyclonal antibody, an anti-SSTR5 polyclonal antibody, and a SSTR5 P335L-specific monoclonal antibody. Among the 22 PNET specimens, six with a homologous TT genotype expressing SSTR5 P335L only, six with a homologous CC genotype expressing WT SSTR5 only, and 10 with a heterozygous CT genotype expressing both WT SSTR5 and SSTR5 P335L. The PNET specimens with TT genotype had strongest PDX-1 immunostaining signal, whereas PNET specimens with CT genotype had mid-level PDX-1 signals, and PNET specimens with CC genotype had the weakest PDX-1 immunostaining signals. Representative immunostaining results from each genotype of PNET specimens are shown in Fig. 7. WT SSTR5 immunostaining signals were detected in all three genotype specimens with comparable levels (Fig. 7, top panel). SSTR5 P335L immunostaining signals were detected only in CT (patient 17) and TT (patient 8) genotype specimens (Fig. 7, middle panel). PDX-1 was detected as follows: greatest level of immunostaining signal from patient 8 (TT genotype), mid-level of immunostaining signal from patient 17 (CT genotype), and weakest level of immunostaining signal from patient 2 (CC genotype). These data showed that hypofunctional mutation of SSTR5 contributed to higher expression of PDX-1 in patients with PNET, further supporting a negative role for SSTR5 in regulation of PDX-1 expression.
Fig. 7.
Representative micrographs showing the expression levels of SSTR5 P335L and PDX-1 in PNET specimens with different SSTR5 genotypes. Human PNET specimens with different SSTR5 genotypes were fixed in 4% (vol/vol) paraformaldehyde and embedded in paraffin. After deparaffinization in xylene and rehydration through graded alcohol, tissue sections were incubated with an anti-SSTR5 (1:100), an anti-SSTR5 P335L monoclonal antibody (15 μg/ml), or an anti-PDX-1 antibody (1:200) overnight at 4 C. Fluorescence was developed using FITC- or Cy3-conjugated secondary antibody. Simultaneous fluorescence microscopy observation and photography were carried out using an Olympus IX70 microscope (×200).
Discussion
In this study, we provided biochemical and genetic evidence showing that PDX-1 was subject to negative regulation by SSTR5. The evidence includes: 1) cotransfection of SSTR5 with PDX-1 resulted in inhibition of PDX-1 expression in a dose-dependent manner in HEK293 cells; 2) SSTR5 agonist RPL-1980 inhibited PDX-1 expression and abolished GLP-1-stimulated increase of PDX-1 expression in β-TC6 cells; 3) knockdown of SSTR5 by SSTR5 shRNA resulted in enhanced PDX-1 expression in both β-TC6 and MIN6 cells; 4) genetic ablation of SSTR5 led to elevated expression of islet PDX-1 in sstr5−/− mice; and 5) hypofunctional mutation of SSTR5 contributed to the elevated expression of PDX-1 in patients with PNET. To our knowledge, this is the first time that SST/SSTR5 signaling is linked to PDX-1, a β-cell-specific transcription factor essential for insulin expression/secretion and cell proliferation. It has been reported that SST analog octreotide inhibits mRNA expression of transcription factors c-fos and AP-1 binding activity stimulated by dibutyryl-cAMP and serum in isolated gastric parietal cells and in GH3 pituitary cell line (57). Octreotide also inhibits phorbol ester 12-O-tetradecanoylphorbol-13-acetate-stimulated both AP-1 binding and transcriptional activity in GH3 cells through SSTR2 and SSTR5 (58). Thus, one mechanism by which SST exerts its physiological actions is to inhibit the expression of transcription factors. Our studies identified PDX-1 as another transcription factor that is subject to the negative regulation by SSTR5-mediated SST signaling.
SSTR5 is one of the major SSTR that mediate the inhibitory effect of SST on insulin expression/secretion and cell proliferation (8, 43). Our studies presented here showed that SSTR5 knockdown-induced increase of PDX-1 expression was accompanied by enhanced insulin secretion in response to high concentration of glucose in β-TC6 cells. We also found that SSTR5 knockdown-induced increase of PDX-1 expression was accompanied by an increased expression of cyclin E and Cdk4, which favor the progression of cell cycle, and a decreased expression of p53, p21, and p27, which inhibits cell cycle progression, in MIN6 cells. This is consistent with a previous study showing that SSTR5 contributes to the induction of cyclin- dependent kinase inhibitor p27 (59). Moreover, genetic ablation of SSTR5 resulted in increased expression of PDX-1, insulin, and PCNA in sstr5−/− islets compared with WT pancreata. Given the essential role of PDX-1 in insulin expression (22, 23) and cell proliferation (60), our studies support a concept that SSTR5 may exert its inhibitory effect on insulin expression and cell proliferation via inhibiting PDX-1 expression. This is consistent with our previous finding that SSTR5−/−, β-cell-specific SSTR5−/− and SSTR1/5−/− mice develop islet hyperplasia, increased numbers of islets, and islet neogenesis compared with littermate WT controls (51–53).
PDX-1 is a β-cell-specific member of the homeodomain-containing transcription factors and plays an essential role in pancreatic development, islet β-cell differentiation, and maintenance of mature β-cell function (22, 23). PDX-1 expression is regulated by a variety of stimuli such as glucose (21), glucagon-like peptide 1 (GLP-1) (61, 62), GLP-1R agonist (63), peroxisome proliferator-activated receptor-α (64), and epidermal growth factor (65). Our studies identified SSTR5 as a novel regulator for PDX-1 expression, providing a potential novel mechanism by which SST exerts its cellular functions. That is, SSTR5 may mediate the inhibitory effects of SST on insulin expression/secretion and cell proliferation via down-regulating PDX-1PDX-1 expression that is temporally and spatially controlled at both transcriptional and posttranslational levels. Our studies showed that cotransfection of SSTR5 with PDX-1 resulted in inhibition of PDX-1 protein expression that was accompanied by reduced PDX-1 mRNA expression, whereas knockdown of SSTR5 led to increased PDX-1 protein expression that was accompanied by increased PDX-1 mRNA expression. Therefore, inhibition of PDX-1 mRNA expression is one potential mechanism underlying the down-regulation of PDX-1 by SSTR5, although it is not clear at this point whether the inhibition is due to inhibited transcription of PDX-1 gene or instability of PDX-1 mRNA. Our studies also showed that cotransfection of WT SSTR5 with PDX-1 resulted in an increased ubiquitination of PDX-1, whereas SSTR5 P335L, a hypofunctional mutation of SSTR5, inhibited PDX-1 ubiquitination, suggesting that SSTR5 may also be involved in posttranslational modification of PDX-1 and, through which regulate its ubiquitination and stability.
It has been shown that PDX-1 ubiquitination is subject to regulation by phosphorylation. DNA-dependent protein kinase phosphorylates PDX-1 on Thr 11 and drives PDX-1 degradation by proteasome in response to DNA damage stimulation (49). Oxidative stress (H2O2)-stimulated, GSK-3-mediated phosphorylation of Ser 61 and Ser 66 also drive PDX-1 degradation by proteasome (47). The molecular mechanism underlying the enhanced ubiquitination of PDX-1 by SSTR5 is currently unknown. Given that SSTR5 exerts its cellular functions through affecting a wide variety of kinase-signaling pathways such as MAPK (66), phosphorylation, and sumoylation are the posttranslational modifications that contribute to regulation of PDX-1 expression. A number of signaling pathways involved in regulation of PDX-1 expression have been identified, including AKT (65), phosphatidylinositol 3-kinase (67), protein kinase C (68), c-Jun N-terminal kinase (69), and p38 (Ref. 60 in summary). It is thus reasonable to speculate that SSTR5 may regulate PDX-1 ubiquitination, stability, and subsequent expression via a phosphorylation-dependent mechanism. The missing signaling events that connect SSTR5 with PDX-1 warrant further studies.
In summary, PDX-1 expression was shown to be subject to the negative regulation by SSTR5 at both transcriptional and posttranslational levels as evidenced by SSTR5 inhibition of PDX-1 mRNA expression and enhancement of PDX-1 ubiquitination. Our studies demonstrate that SSTR5 may mediate the inhibitory effects of SST on insulin expression/secretion and cell proliferation via down-regulating PDX-1, a potential novel mechanism by which SST exerts its physiological actions.
Materials and Methods
Reagents
An enhanced chemiluminescence detection kit was purchased from Amershan Biosciences Corp (Piscataway, NJ). Lipofectamine 2000 was purchased from Invitrogen (Carlsbad, CA). Mercodia Ultrasensitive Mouse Insulin kit was purchased from Mercodia AB (Uppsala, Sweden). Anti-HA antibody (12CA5) was purchased from Boehringer-Mannheim (Indianapolis, IN). Anti-Flag (M2) and anti-β-actin antibodies were purchased from Sigma (St. Louis, MO). Antibodies against insulin, PCNA, cyclin E, CDK4, p53, p21, and p27 were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Anti-GFP antibody was purchased from Invitrogen. Rabbit anti-PDX-1 polyclonal antibody (no. 6751) was previously described (33). SSTR5 agonist PRL-1980 was a gift from Dr. David Coy (Tulane University, New Orleans, LA). All other chemical reagents were purchased from Sigma unless otherwise noted.
Plasmids
Mouse SSTR5 shRNA and a scramble shRNA were generated by annealing and ligating the nucleotides gatccccGCCAAGATGAAGACAGTTAttcaagagaTAACTGTCTTCATCTTGGCtttttggaaa and gatccccTTCTCCGAACGTGTCACGTttcaagagaACGTGACACGTTCGGAGAAtttttggaaa, respectively, into pSUPER vector at BglII and HindIII sites according to a Dharmacon protocol (Dharmacon, Lafayette, CO). GFP plasmid was obtained from CLONTECH Laboratories, Inc. (Mountain View, CA). Flag-PDX-1 was previously described (70).
Cells, transfection, immunoprecipitation, Western blotting, cell proliferation, and insulin assays
HEK293, MIN6, and β-TC6 cells were grown and maintained in DMEM supplemented with 10% fetal bovine serum and penicillin/streptomycin. Transient transfection was performed using Lipofectamine 2000 according to the manufacture's instruction. Immunoprecipitation was performed as previously described (71). Western blotting was performed using an enhanced chemiluminescence detection kit according to the manufacturer's protocols. Insulin concentration was measured by performing ELISA assays using a Mercodia Ultrasensitive Mouse Insulin ELISA kit.
Quantitative RT-PCR (qRT-PCR)
Total RNA were prepared using TriZol reagent (Invitrogen) from SSTR5 and PDX-1 cotransfected HEK293 cells and pSuper-shSSTR5 transfected β-TC-6 cells. qRT-PCR was carried out by using 100 ng of total RNA. A volume of 10 μl of 2× QuantiTect SYBR Green RT-PCR Master Mix (QIAGEN, Chatsworth, CA), 0.2 μl QuantiTect RT Mix (QIAGEN), 1 μl of 10 μm forward and reverse primers, and 6.8 μl of RNase-free water were added to each sample for analysis by absolute quantification. qRT-PCR was performed in 96-well plates with an Applied Biosystems 7300 Sequence Detection. The mRNA levels of PDX-1 in the samples were normalized against glyceraldehyde 3-phosphate dehydrogenase (GAPDH). PDX-1 and GAPDH were measured in triplicate. The primers used are as follows: mouse PDX-1: 5′-CCCCAGTTTACAAGCTCGCT-3′ and 5′-CTCGGTTCCATTCGGGAAAGG-3′; human PDX-1: 5′-ATCTCCCCATACGAAGTGCC-3′ and 5′-CGTGAGCTTTGGTGGATTTCAT-3′ and GAPDH: 5′-ATGCCATCACTGCCACCCAGAACG-3′ and 5′-GCCAGTGAGCTTCCCGTTCA-3′. The cDNA was prepared from the total RNA isolated with TriZol Reagent, using qScript cDNA SuperMix (Quanta Biosciences, Gaithersburg, MD) according to the manufacturer's protocol. PCR was performed on 1-μl aliquots from each cDNA reaction, using PDX-1 and GAPDH primer sets for 45 cycles. The PCR products were subjected to electrophoresis on a 2% agarose gel.
Animals, tissue collection, and immunohistochemistry staining
SSTR5 knockout (SSTR5−/−) mice were generated as previously described (51). Mice were housed in a facility with a 12-h light, 12-h dark cycle and fed standard chow. Mice were cared for in compliance with the “Guide for Care and Use of Laboratory Animals” prepared by the Institute of Laboratory Animal Resources, Commission of Life Science, National Research Council. The pancreas were removed from WT and SSTR5−/− mice after appropriate euthanasia. The tissue samples were immediately immersed in 4% (vol/vol) paraformaldehyde. Islet expressions of PDX-1, insulin, and PCNA in WT and SSTR5−/− mice were measured by performing immunohistochemistry staining as previously described (51) using an anti-PDX-1 (1:200), antiinsulin (1:75), and anti-PCNA (1:100) antibody, respectively. Simultaneous fluorescence microscopy observation and photography were carried out using an Olympus IX70 microscope (Olympus Corp., Lake Success, NY).
Sample collection and processing and immunohistochemistry staining
Informed consent from 22 patients with pancreatic neuroendocrine tumor (PNET) was obtained under an IRB-approved protocol. Human PNET specimens were fixed in 4% (vol/vol) paraformaldehyde for 24 h and embedded in paraffin. Tissue sections were cut, and slides were deparaffinized in xylene. Sections were rehydrated through graded alcohol. Slides were placed in a humidified chamber overlaid with anti-SSTR5 P335L monoclonal antibody (15 μg/ml) or anti-PDX-1 antibody (1:200) overnight at 4 C. After washing with PBS, culture slides and sections were incubated with a Cy3- or fluorescein isothiocyanate (FITC)-conjugated antimouse IgG second antibody for 1 h at room temperature. Simultaneous fluorescence microscopy observation and photography were carried out using an Olympus IX70 microscope.
Statistic analysis
The unpaired Student's t test was used for statistical analyses of PDX-1 mRNA expression levels and insulin levels, with P < 0.05 indicating significance.
Acknowledgments
We thank Dr. David Coy for RPL-1980. We also thank Katie Elsbury for editorial assistance and Priscilla Massey for administrative assistance.
This work was supported by National Institutes of Health Grant NIDDK R01-DK46441, the Vivian Smith Foundation, the MD Anderson Foundation (to F.C.B.), and the Caroline Wiess Law Fund for Molecular Medicine (to G.Z.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CDK4
- Cyclin-dependent kinase 4
- GAPDH
- glyceraldehyde 3-phosphate dehydrogenase
- GFP
- green fluorescent protein
- GLP-1
- glucagon-like peptide 1
- HA
- hemagglutinin
- HEK
- human embryonic kidney
- PCNA
- proliferating cell nuclear antigen
- PDX-1
- pancreatic and duodenal homeobox-1
- PNET
- pancreatic neuroendocrine tumor
- qRT-PCR
- quantitative RT-PCR
- shRNA
- small hairpin RNA
- SST
- somatostatin
- SSTR5
- somatostatin receptor subtype 5
- WT
- wild type.
References
- 1. Krulich L, Dhariwal AP, McCann SM. 1968. Stimulatory and inhibitory effects of purified hypothalamic extracts on growth hormone release from rat pituitary in vitro. Endocrinology 83:783–790 [DOI] [PubMed] [Google Scholar]
- 2. Bodei L, Ferone D, Grana CM, Cremonesi M, Signore A, Dierckx RA, Paganelli G. 2009. Peptide receptor therapies in neuroendocrine tumors. J Endocrinol Invest 32:360–369 [DOI] [PubMed] [Google Scholar]
- 3. Florio T. 2008. Molecular mechanisms of the antiproliferative activity of somatostatin receptors (SSTRs) in neuroendocrine tumors. Front Biosci 13:822–840 [DOI] [PubMed] [Google Scholar]
- 4. Modlin IM, Pavel M, Kidd M, Gustafsson BI. 2010. Review article: somatostatin analogues in the treatment of gastroenteropancreatic neuroendocrine (carcinoid) tumours. Aliment Pharmacol Ther 31:169–188 [DOI] [PubMed] [Google Scholar]
- 5. Pyronnet S, Bousquet C, Najib S, Azar R, Laklai H, Susini C. 2008. Antitumor effects of somatostatin. Mol Cell Endocrinol 286:230–237 [DOI] [PubMed] [Google Scholar]
- 6. Patel YC. 1999. Somatostatin and its receptor family. Front Neuroendocrinol 20:157–198 [DOI] [PubMed] [Google Scholar]
- 7. Strowski MZ, Blake AD. 2008. Function and expression of somatostatin receptors of the endocrine pancreas. Mol Cell Endocrinol 286:169–179 [DOI] [PubMed] [Google Scholar]
- 8. Fagan S, Azizzadeh A, Moldovan S, Ray MK, Adrian TE, Ding X, Coy DH, Brunicardi FC. 1998. Insulin secretion is inhibited by subtype five somatostatin receptor in the mouse. Surgery 124:254–259 [PubMed] [Google Scholar]
- 9. Reubi JC, Horisberger U, Essed CE, Jeekel J, Klijn JG, Lamberts SW. 1988. Absence of somatostatin receptors in human exocrine pancreatic adenocarcinomas. Gastroenterology 95:760–763 [DOI] [PubMed] [Google Scholar]
- 10. Player A, Gillespie J, Fujii T, Fukuoka J, Dracheva T, Meerzaman D, Hong KM, Curran J, Attoh G, Travis W, Jen J. 2003. Identification of TDE2 gene and its expression in non-small cell lung cancer. Int J Cancer 107:238–243 [DOI] [PubMed] [Google Scholar]
- 11. Rosenberg L, Lipsett M, Yoon JW, Prentki M, Wang R, Jun HS, Pittenger GL, Taylor-Fishwick D, Vinik AI. 2004. A pentadecapeptide fragment of islet neogenesis-associated protein increases β-cell mass and reverses diabetes in C57BL/6J mice. Ann Surg 240:875–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zatelli MC, Tagliati F, Taylor JE, Rossi R, Culler MD, degli Uberti EC. 2001. Somatostatin receptor subtypes 2 and 5 differentially affect proliferation in vitro of the human medullary thyroid carcinoma cell line tt. J Clin Endocrinol Metab 86:2161–2169 [DOI] [PubMed] [Google Scholar]
- 13. Qiu CZ, Wang C, Huang ZX, Zhu SZ, Wu YY, Qiu JL. 2006. Relationship between somatostatin receptor subtype expression and clinicopathology, Ki-67, Bcl-2 and p53 in colorectal cancer. World J Gastroenterol 12:2011–2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Patel SG, Zhou G, Liu SH, Li M, Jeong JW, Demayo FJ, Gingras MC, Gibbs RA, Fisher WE, Brunicardi FC.2009. Microarray analysis of somatostatin receptor 5-regulated gene expression profiles in murine pancreas. World J Surg 33:630–637 [DOI] [PubMed] [Google Scholar]
- 15. Ohlsson H, Karlsson K, Edlund T. 1993. IPF1, a homeodomain-containing transactivator of the insulin gene. EMBO J 12:4251–4259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Leonard J, Peers B, Johnson T, Ferreri K, Lee S, Montminy MR. 1993. Characterization of somatostatin transactivating factor-1, a novel homeobox factor that stimulates somatostatin expression in pancreatic islet cells. Mol Endocrinol 7:1275–1283 [DOI] [PubMed] [Google Scholar]
- 17. Olson LK, Redmon JB, Towle HC, Robertson RP. 1993. Chronic exposure of HIT cells to high glucose concentrations paradoxically decreases insulin gene transcription and alters binding of insulin gene regulatory protein. J Clin Invest 92:514–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miller CP, McGehee RE, Jr, Habener JF. 1994. IDX-1: a new homeodomain transcription factor expressed in rat pancreatic islets and duodenum that transactivates the somatostatin gene. EMBO J 13:1145–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marshak S, Totary H, Cerasi E, Melloul D. 1996. Purification of the β-cell glucose-sensitive factor that transactivates the insulin gene differentially in normal and transformed islet cells. Proc Natl Acad Sci USA 93:15057–15062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Macfarlane WM, Smith SB, James RF, Clifton AD, Doza YN, Cohen P, Docherty K. 1997. The p38/reactivating kinase mitogen-activated protein kinase cascade mediates the activation of the transcription factor insulin upstream factor 1 and insulin gene transcription by high glucose in pancreatic β-cells. J Biol Chem 272:20936–20944 [DOI] [PubMed] [Google Scholar]
- 21. Khoo C, Yang J, Weinrott SA, Kaestner KH, Naji A, Schug J, Stoffers DA. 2012. Research resource: the pdx1 cistrome of pancreatic islets. Mol Endocrinol 26:521–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ashizawa S, Brunicardi FC, Wang XP. 2004. PDX-1 and the pancreas. Pancreas 28:109–120 [DOI] [PubMed] [Google Scholar]
- 23. Kaneto H, Miyatsuka T, Shiraiwa T, Yamamoto K, Kato K, Fujitani Y, Matsuoka TA. 2007. Crucial role of PDX-1 in pancreas development, β-cell differentiation, and induction of surrogate β-cells. Curr Med Chem 14:1745–1752 [DOI] [PubMed] [Google Scholar]
- 24. Jonsson J, Carlsson L, Edlund T, Edlund H. 1994. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature 371:606–609 [DOI] [PubMed] [Google Scholar]
- 25. Stoffers DA, Zinkin NT, Stanojevic V, Clarke WL, Habener JF. 1997. Pancreatic agenesis attributable to a single nucleotide deletion in the human IPF1 gene coding sequence. Nat Genet 15:106–110 [DOI] [PubMed] [Google Scholar]
- 26. Ahlgren U, Jonsson J, Jonsson L, Simu K, Edlund H. 1998. β-cell-specific inactivation of the mouse Ipf1/Pdx1 gene results in loss of the β-cell phenotype and maturity onset diabetes. Genes Dev 12:1763–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brissova M, Shiota M, Nicholson WE, Gannon M, Knobel SM, Piston DW, Wright CV, Powers AC. 2002. Reduction in pancreatic transcription factor PDX-1 impairs glucose-stimulated insulin secretion. J Biol Chem 277:11225–11232 [DOI] [PubMed] [Google Scholar]
- 28. Johnson JD, Ahmed NT, Luciani DS, Han Z, Tran H, Fujita J, Misler S, Edlund H, Polonsky KS. 2003. Increased islet apoptosis in Pdx1+/− mice. J Clin Invest 111:1147–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Al-Quobaili F, Montenarh M. 2008. Pancreatic duodenal homeobox factor-1 and diabetes mellitus type 2 (review). Int J Mol Med 21:399–404 [PubMed] [Google Scholar]
- 30. Leys CM, Nomura S, Rudzinski E, Kaminishi M, Montgomery E, Washington MK, Goldenring JR. 2006. Expression of PDX-1 in human gastric metaplasia and gastric adenocarcinoma. Hum Pathol 37:1162–1168 [DOI] [PubMed] [Google Scholar]
- 31. Sakai H, Eishi Y, Li XL, Akiyama Y, Miyake S, Takizawa T, Konishi N, Tatematsu M, Koike M, Yuasa Y. 2004. PDX-1 homeobox protein expression in pseudopyloric glands and gastric carcinomas. Gut 53:323–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miyatsuka T, Kaneto H, Shiraiwa T, Matsuoka TA, Yamamoto K, Kato K, Nakamura Y, Akira S, Takeda K, Kajimoto Y, Yamasaki Y, Sandgren EP, Kawaguchi Y, Wright CV, Fujitani Y. 2006. Persistent expression of PDX-1 in the pancreas causes acinar-to-ductal metaplasia through Stat3 activation. Genes Dev 20:1435–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang XP, Li ZJ, Magnusson J, Brunicardi FC. 2005. Tissue MicroArray analyses of pancreatic duodenal homeobox-1 in human cancers. World J Surg 29:334–338 [DOI] [PubMed] [Google Scholar]
- 34. Jonmarker S, Glaessgen A, Culp WD, Pisa P, Lewensohn R, Ekman P, Valdman A, Egevad L. 2008. Expression of PDX-1 in prostate cancer, prostatic intraepithelial neoplasia and benign prostatic tissue. APMIS 116:491–498 [DOI] [PubMed] [Google Scholar]
- 35. Koizumi M, Doi R, Toyoda E, Masui T, Tulachan SS, Kawaguchi Y, Fujimoto K, Gittes GK, Imamura M. 2003. Increased PDX-1 expression is associated with outcome in patients with pancreatic cancer. Surgery 134:260–266 [DOI] [PubMed] [Google Scholar]
- 36. Liu T, Gou SM, Wang CY, Wu HS, Xiong JX, Zhou F. 2007. Pancreas duodenal homeobox-1 expression and significance in pancreatic cancer. World J Gastroenterol 13:2615–2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu S, Rao D, Nemunaitis J, Senzer N, Dawson D, Gingras MC, Wang Z, Gibbs R, Norman M, Templeton N, DeMayo F, Stehling K, Fisher W, Brunicardi FC. 22 June 2011. A novel therapeutic strategy for pancreatic neoplasia using a novel RNAi platform targeting PDX-1. Nat Proc 10101/npre.2011.6047.1 [Google Scholar]
- 38. Gidekel Friedlander SY, Chu GC, Snyder EL, Girnius N, Dibelius G, Crowley D, Vasile E, DePinho RA, Jacks T. 2009. Context- dependent transformation of adult pancreatic cells by oncogenic K-Ras. Cancer Cell 16:379–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu SH, Patel S, Gingras MC, Nemunaitis J, Zhou G, Chen C, Li M, Fisher W, Gibbs R, Brunicardi FC. 2011. PDX-1: demonstration of oncogenic properties in pancreatic cancer. Cancer 117:723–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhou G, Gingras MC, Liu SH, Li D, Li Z, Catania RL, Stehling KM., Li M, Paganelli G, Gibbs RA, Demayo FJ, Fisher WE, Brunicardi FC. 2011. The hypofunctional effect of P335L single nucleotide polymorphism on SSTR5 function. World J Surg 35:1715–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li Y, Cao X, Li LX, Brubaker PL, Edlund H, Drucker DJ. 2005. J β-Cell Pdx1 expression is essential for the glucoregulatory, proliferative, and cytoprotective actions of glucagon-like peptide-1. Diabetes 54:482–491 [DOI] [PubMed] [Google Scholar]
- 42. Movassat J, Beattie GM, Lopez AD, Hayek A. 2002. Exendin 4 up-regulates expression of PDX 1 and hastens differentiation and maturation of human fetal pancreatic cells. J Clin Endocrinol Metab 87:4775–4781 [DOI] [PubMed] [Google Scholar]
- 43. Tirone TA, Norman MA, Moldovan S, DeMayo FJ, Wang XP, Brunicardi FC. 2003. Pancreatic somatostatin inhibits insulin secretion via SSTR-5 in the isolated perfused mouse pancreas model. Pancreas 26:e67–e73 [DOI] [PubMed] [Google Scholar]
- 44. Fujitani Y, Fujitani S, Boyer DF, Gannon M, Kawaguchi Y, Ray M, Shiota M, Stein RW, Magnuson MA, Wright CV. 2006. Targeted deletion of a cis-regulatory region reveals differential gene dosage requirements for Pdx1 in foregut organ differentiation and pancreas formation. Genes Dev 20:253–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wu KL, Gannon M, Peshavaria M, Offield MF, Henderson E, Ray M, Marks A, Gamer LW, Wright CV, Stein R. 1997. Hepatocyte nuclear factor 3β is involved in pancreatic β-cell-specific transcription of the pdx-1 gene. Mol Cell Biol 17:6002–6013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gao N, LeLay J, Vatamaniuk MZ, Rieck S, Friedman JR, Kaestner KH. 2008. Dynamic regulation of Pdx1 enhancers by Foxa1 and Foxa2 is essential for pancreas development. Genes Dev 22:3435–3448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Boucher MJ, Selander L, Carlsson L, Edlund H. 2006. Phosphorylation marks IPF1/PDX1 protein for degradation by glycogen synthase kinase 3-dependent mechanisms. J Biol Chem 281:6395–6403 [DOI] [PubMed] [Google Scholar]
- 48. Humphrey RK, Yu SM, Flores LE, Jhala US. 2010. Glucose regulates steady-state levels of PDX1 via the reciprocal actions of GSK3 and AKT kinases. J Biol Chem 285:3406–3416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lebrun P, Montminy MR, Van Obberghen E. 2005. Regulation of the pancreatic duodenal homeobox-1 protein by DNA-dependent protein kinase. J Biol Chem 280:38203–38210 [DOI] [PubMed] [Google Scholar]
- 50. Kishi A, Nakamura T, Nishio Y, Maegawa H, Kashiwagi A. 2003. Sumoylation of Pdx1 is associated with its nuclear localization and insulin gene activation. Am J Physiol Endocrinol Metab 284:E830–E840 [DOI] [PubMed] [Google Scholar]
- 51. Wang XP, Norman M, Yang J, Liu SH, Magnusson J, DeMayo FJ, Brunicardi FC. 2005. The effect of global SSTR5 gene ablation on the endocrine pancreas and glucose regulation in aging mice. J Surg Res 129:64–72 [DOI] [PubMed] [Google Scholar]
- 52. Wang XP, Norman MA, Yang J, Cheung A, Moldovan S, Demayo FJ, Brunicardi FC. 2004. Double-gene ablation of SSTR1 and SSTR5 results in hyperinsulinemia and improved glucose tolerance in mice. Surgery 136:585–592 [DOI] [PubMed] [Google Scholar]
- 53. Wang XP, Yang J, Norman MA, Magnusson J, DeMayo FJ, Brunicardi FC. 2005. SSTR5 ablation in islet results in alterations in glucose homeostasis in mice. FEBS Lett 579:3107–3114 [DOI] [PubMed] [Google Scholar]
- 54. De Martino MC, Hofland LJ, Lamberts SW. 2010. Somatostatin and somatostatin receptors: from basic concepts to clinical applications. Prog Brain Res 182:255–280 [DOI] [PubMed] [Google Scholar]
- 55. Kaltsas GA, Papadogias D, Makras P, Grossman AB. 2005. Treatment of advanced neuroendocrine tumours with radiolabelled somatostatin analogues. Endocr Relat Cancer 12:683–699 [DOI] [PubMed] [Google Scholar]
- 56. Zhou G, Gingras MC, Liu SH, Sanchez R, Edwards D, Dawson D, Christensen K, Paganelli G, Gibbs R, Fisher W, Brunicardi FC. 2011. SSTR5 P335L monoclonal antibody differentiates pancreatic neuroendocrine neuroplasms with different SSTR5 genotypes. Surgery 150:1136–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Todisco A, Campbell V, Dickinson CJ, DelValle J, Yamada T. 1994. Molecular basis for somatostatin action: inhibition of c-fos expression and AP-1 binding. Am J Physiol 267:G245–G253 [DOI] [PubMed] [Google Scholar]
- 58. Todisco A, Seva C, Takeuchi Y, Dickinson CJ, Yamada T. 1995. Somatostatin inhibits AP-1 function via multiple protein phosphatases. Am J Physiol 269:G160–G166 [DOI] [PubMed] [Google Scholar]
- 59. Grant M, Alturaihi H, Jaquet P, Collier B, Kumar U. 2008. Cell growth inhibition and functioning of human somatostatin receptor type 2 are modulated by receptor heterodimerization. Mol Endocrinol 22:2278–2292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Feanny MA, Fagan SP, Ballian N, Liu SH, Li Z, Wang X, Fisher W, Brunicardi FC, Belaguli NS. 2008. PDX-1 expression is associated with islet proliferation in vitro and in vivo. J Surg Res 144:8–16 [DOI] [PubMed] [Google Scholar]
- 61. Buteau J, Roduit R, Susini S, Prentki M. 1999. Glucagon-like peptide-1 promotes DNA synthesis, activates phosphatidylinositol 3-kinase and increases transcription factor pancreatic and duodenal homeobox gene 1 (PDX-1) DNA binding activity in β (INS-1)-cells. Diabetologia 42:856–864 [DOI] [PubMed] [Google Scholar]
- 62. Wang X, Cahill CM, Piñeyro MA, Zhou J, Doyle ME, Egan JM. 1999. Glucagon-like peptide-1 regulates the β cell transcription factor, PDX-1, in insulinoma cells. Endocrinology 140:4904–4907 [DOI] [PubMed] [Google Scholar]
- 63. Stoffers DA, Kieffer TJ, Hussain MA, Drucker DJ, Bonner-Weir S, Habener JF, Egan JM. 2000. Insulinotropic glucagon-like peptide 1 agonists stimulate expression of homeodomain protein IDX-1 and increase islet size in mouse pancreas. Diabetes 49:741–748 [DOI] [PubMed] [Google Scholar]
- 64. Sun Y, Zhang L, Gu HF, Han W, Ren M, Wang F, Gong B, Wang L, Guo H, Xin W, Zhao J, Gao L. 2008. Peroxisome proliferator-activated receptor-α regulates the expression of pancreatic/duodenal homeobox-1 in rat insulinoma (INS-1) cells and ameliorates glucose-induced insulin secretion impaired by palmitate. Endocrinology 149:662–671 [DOI] [PubMed] [Google Scholar]
- 65. Watanabe H, Saito H, Ueda J, Evers BM. 2008. Regulation of pancreatic duct cell differentiation by phosphatidylinositol-3 kinase. Biochem Biophys Res Commun 370:33–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Takeda S, Nakao A, Miyoshi K, Takagi H. 1998. Gene therapy for pancreatic cancer. Semin Surg Oncol 15:57–61 [DOI] [PubMed] [Google Scholar]
- 67. Rafiq I, da Silva Xavier G, Hooper S, Rutter GA. 2000. Glucose-stimulated preproinsulin gene expression and nuclear trans-location of pancreatic duodenum homeobox-1 require activation of phosphatidylinositol 3-kinase but not p38 MAPK/SAPK2. J Biol Chem 275:15977–15984 [DOI] [PubMed] [Google Scholar]
- 68. Furukawa N, Shirotani T, Araki E, Kaneko K, Todaka M, Matsumoto K, Tsuruzoe K, Motoshima H, Yoshizato K, Kishikawa H, Shichiri M. 1999. Possible involvement of atypical protein kinase C (PKC) in glucose-sensitive expression of the human insulin gene: DNA-binding activity and transcriptional activity of pancreatic and duodenal homeobox gene-1 (PDX-1) are enhanced via calphostin C-sensitive but phorbol 12-myristate 13-acetate (PMA) and Go 6976-insensitive pathway. Endocr J 46:43–58 [DOI] [PubMed] [Google Scholar]
- 69. Kawamori D, Kaneto H, Nakatani Y, Matsuoka TA, Matsuhisa M, Hori M, Yamasaki Y. 2006. The forkhead transcription factor Foxo1 bridges the JNK pathway and the transcription factor PDX-1 through its intracellular translocation. J Biol Chem 281:1091–1098 [DOI] [PubMed] [Google Scholar]
- 70. Liu S, Ballian N, Belaguli NS, Patel S, Li M, Templeton NS, Gingras MC, Gibbs R, Fisher W, Brunicardi FC. 2008. PDX-1 acts as a potential molecular target for treatment of human pancreatic cancer. Pancreas 37:210–220 [DOI] [PubMed] [Google Scholar]
- 71. Zhou G, Boomer JS, Tan TH. 2004. Protein phosphatase 4 is a positive regulator of hematopoietic progenitor kinase 1. J Biol Chem 279:49551–49561 [DOI] [PubMed] [Google Scholar]