Abstract
In humans, painful stimuli can arrive to the brain at 20–22 weeks of gestation. Therefore several researchers have devoted their efforts to study fetal analgesia during prenatal surgery, and during painful procedures in premature babies. Aim of this paper is to gather from scientific literature the available data on the signals that the human fetus and newborns produce, and that can be interpreted as signals of pain. Several signs can be interpreted as signals of pain. We will describe them in the text. In infants, these signs can be combined to create specific and sensible pain assessment tools, called pain scales, used to rate the level of pain.
Key words: analgesic drug, fetus, newborn, pain, pain scale
INTRODUCTION
Infants can undergo painful events several times a day. In a neonatal intensive care unit, they undergo intubations, tracheal aspirations, blood samplings, vein incannulations, and other painful procedures (1); they can also undergo surgery, lumbar puncture, chest air leak drainage, and much more. Even healthy babies may receive painful interventions, such as circumcision and blood sampling for metabolic screening purposes, in the first few days of life. The recent development of fetal surgery has raised the problem of fetal pain and analgesia (2,3), making it important to recognize pain even in fetuses.
Fetuses and infants generate signals that can be decoded to recognize and grade the level of pain. The aim of this paper is to provide a survey of the most recent literature about the tools available to recognize and assess these signals.
RECOGNIZING PAIN IN THE FETUS AND NEWBORNS
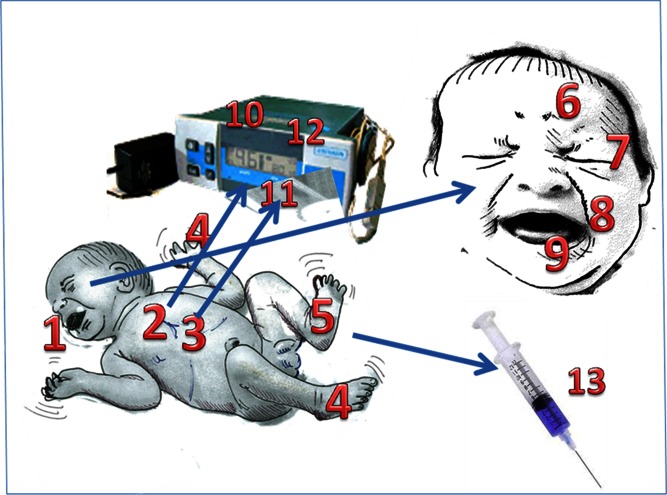
Pain induces reactions in the living organism (Fig. 1). Avoidance (e.g., the sudden withdrawal of an arm from a painful stimulus), aggression (e.g., the increase in muscular tone, blood pressure, and heart rate mediated by stress hormones or changes in posture or facial expressions), and alarm (e.g., crying) are the three main categories into which these reactions can be divided. These reactions consist of both symptoms (conscious expressions) and signs (unconscious or involuntary reactions to pain) (4). Symptoms are peculiar to those who can express their feeling, namely adults and children. In contrast, signs do not need words and therefore are present, even in fetuses and infants. Pain signs can share features common to those associated with anger or stress. However, the context of the signals and the use of pain scales can help us to discriminate the causes of these signs.
Fig. 1.
Signs of pain in infants. Whole body: 1 crying, 2 tachicardia, 3 polypnea, 4 hands and feet movements, 5 plantar/palmar sweating. Face: 6 brow bulge, 7 eyes squeezing, 8 nasolabial furrow, 9 mouth open wide. Lab/monitor signs: 10 heart rate, 11 oxygen saturation, 12 blood pressure, 13 adrenalin, cortisol, endorphines
Facial Expression
Facial expression is an important indicator of pain in neonates (5–7). It includes several features, such as brow bulge, eye squeeze, nasolabial furrow, and open mouth (8,9). Nevertheless, the variability in facial expression during non-painful episodes (10) can mimic pain, and this reduces the specificity of these signs as pain indicators. Facial expressions are integrated into several pain scales, such as the Neonatal Facial Coding System (NFCS), which uses ten discrete facial actions (11,12). Even fetal face expressions in reaction to sudden noises and stress can be recorded and monitored (13). The assessment of facial expression may in practice be hampered by a limited view of the fetal or neonatal face.
Body Movements
The activity of arms and legs—or more subtly the clenching of fists or toes—is an indicator of pain, (14). Nevertheless, pain assessment based only on body movements can be misleading. Some sick babies are severely hypotonic or hyporeactive, though still able to feel pain. Sudden body movements are an indicator of pain in the fetus during stressful procedures (15–18).
Crying
While crying is a sign of pain (19,20), it can also be generated by other stimuli, such as hunger or anger. Therefore, it cannot be used as the sole indicator of pain. In the 1960s, it was suggested that newborns could cry in different ways depending on the cause (21–23). However, recent studies (24–26) showed that its features depend not upon the cause of the distress but rather its intensity. Thus, the presence of crying is not a direct sign of pain (27,28). Nevertheless, some crying features are typical of pain, such as the high-pitched cry in response to painful event (29). A pain scale combining several crying features has recently been developed and validated (30–32). Fetal crying has been described using US scans (33,34).
Behavioral States
In neonates, behavioral states vary from quiet sleep to wakefulness or crying. These states are useful in the assessment of pain. For example, while chronic pain can provoke an excess of sleep or of irritability, a sudden awakening, or crying, are indicators of acute pain. Some pain scales include the evaluation of babies' capability to rest or to sleep (35–37). Even in the fetus, it is possible to evaluate the behavioral states and their changes after stress (38,39).
Physiological Indicators
Variations in heart rate, blood pressure, oxygen saturation, and breathing patterns are the most frequently used physiological indicators of pain (40). Pain causes an increase in heart rate and blood pressure, a decrease in oxygen saturation, and more rapid, shallow, or irregular breathing. For example, heart rate and blood pressure variations can be used to determine the need for analgesia and sedatives during the administration of neuromuscular blocking agents.
Other pain indicators are intracranial pressure (41,42), palmar sweating (43), and heart rate variability (27). Emotional sweating in the palms and soles as a result of neurophysiological arousal with increased activity in the sympathetic nervous system is a reliable marker of pain (44–46). Other biological markers of pain include changes in thresholds for the dorsal cutaneous flexion reflex or abdominal skin reflex (47), cerebral blood flow (48), and processed EEG (49). A limitation of physiological indicators is that variations might also be caused by the underlying illness, rendering them less specific for pain (19,40).
Biological Markers of Pain
Stress hormone (cortisol, adrenaline, and beta-endorphins) measurement in serum or saliva perioperatively, during heel-stick and during mechanical ventilation, has been described (50,51). Several studies showed that during painful fetal interventions, there is an evident increase of stress hormones in fetal blood (52–54). Nevertheless, although useful for research purposes, its clinical utility is limited as it is not feasible to wait for laboratory results before adjusting analgesia.
RATING PAIN
Scales are needed to rate the level of pain. In older, cooperative subjects, it is possible to use a Visual Analogue Scale where the patient simply points to a place on a scale that describes the level of the perceived pain. However, such tools cannot be applied to neonates or infants. Adding to the complexity of the situation is that crying or body movements alone are not sufficiently specific or reliable markers of pain. Thus, some researchers have developed methods for combining several signs to facilitate the identification and rating of the pain. The most frequently employed scales are described in Table I.
Table I.
Pain Scales
| DAN (55) | Term and preterm babies | Face expression | Acute pain |
| Arm movements | |||
| Crying | |||
| NIPS (56) | Term and preterm babies | Facial expression | Acute pain |
| Crying | |||
| Breathing pattern | |||
| Arms/legs movements | |||
| State of arousal | |||
| ABC (32) | Term and preterm | Pitch of the first cry emitted after the stimulus | Acute pain |
| Crying rythmicity | |||
| Constance of crying intensity | |||
| PIPP (57) | Term and preterm babies | Gestational age | Acute pain |
| Behavioral state | |||
| Rate of heartbeat increase | |||
| Rate of oxygen saturation decrease | |||
| Brow bulge | |||
| Eye squeeze | |||
| Nasolabial furrow | |||
| CRIES (58) | Term and preterm babies | Crying | Prolonged pain |
| Oxygen requirement | |||
| Vital signs (heart rate and breathing) | |||
| Face expression | |||
| Sleeping pattern | |||
| EDIN (39) | Term and preterm babies | Face activity | Prolonged pain |
| Sleep pattern | |||
| Consolability | |||
| Interaction with nurses | |||
| Body movements | |||
| COMFORT (59) | Term and preterm babies; babies in the first 3 years of life | Alertness | Acute and prolonged pain |
| Calmness/agitation | |||
| Respiratory response | |||
| Physical movement | |||
| Blood pressure | |||
| Heart rate | |||
| Muscle tone | |||
| Facial tension | |||
| NFCS (60) | Term and preterm babies; Babies in the first 18 months of life | Brow lowering | Acute and prolonged pain |
| Eyes squeezed shut | |||
| Deepening of the nasolabial furrow | |||
| Open lips | |||
| Vertical mouth stretch | |||
| Horizontal mouth stretch | |||
| Taut tongue | |||
| Chin quiver | |||
| Lip pursing | |||
| Tongue protrusion | |||
| FLACC (61) | 2 Months–7 years | Facial expression | Prolonged pain |
| Leg movement | |||
| Activity | |||
| Cry | |||
| Consolability |
Pain Assessment Tools
Various neonatal pain scales are based on babies' changes in posture or behavior, and sometimes include the data generated by monitoring of the vital parameters. These scales can distinguish pain from other phenomena. Studies have confirmed that these tools are more reliable than pain assessments based upon such one-dimensional indicators as crying or facial movements. Moreover, pain scales can rate pain intensity.
Pain scales for acute pain are numerous but are scarcely used for two reasons. Firstly, they are difficult to use (62) because a caregiver cannot simultaneously perform a clinical procedure and measure the parameters of the pain scale. Secondly, the results of these assessments are complete after the painful event has occurred. Thus, their utility is limited to a research or training environment. We report in Table II the ABC scale, one of the most easy to use.
Table II.
ABC Scale
| Parameters | Score | |
|---|---|---|
| Acuteness of the first cry | Absent | 0 |
| Present | 2 | |
| Burst Rhythmicity | Absent | 0 |
| Present | 2 | |
| Constancy of the crying intensity | Absent | 0 |
| Intermediate | 1 | |
| Costant | 2 | |
Pain scales for chronic (prolonged) pain are few. However, these scales have a utility in that they help the practitioner modulate an analgesic therapy after surgery or during prolonged intubations. They include the N-PASS (Neonatal Pain, Agitation and Sedation Scale) (63), the EDIN (Échelle Douleur Inconfort Nouveau-Né [Neonatal Pain and Discomfort Scale], see Table III) (39) and the DEGR (Douleur Enfant Gustave Roussy) (64).
Table III.
EDIN Scale. Scoring Method: Nurses Observe the Infant for Several Hours During and Between Caring and Feeding, and Test the Efficacy of Consoling. They then Score Each EDIN Item and Calculate the Total EDIN Score as the Sum of the Five Items
| Indicator | Description | Score |
|---|---|---|
| Facial activity | Relaxed facial activity | 0 |
| Transient grimaces with frowning, lip purse, and chin quiver or tautness | 1 | |
| Frequent grimaces, lasting grimaces | 2 | |
| Permanent grimaces resembling crying or blank face | 3 | |
| Body movements | Relaxed body movements | 0 |
| Transient agitation, often quiet | 1 | |
| Frequent agitation but can be calmed down | 2 | |
| Permanent agitation with contraction of fingers and toes and hypertonia of limbs or infrequent, slow movements, and prostration | 3 | |
| Quality of sleep | Falls asleep easily | 0 |
| Falls asleep with difficulty | 1 | |
| Frequent, spontaneous arousals, independent of nursing, restless sleep | 2 | |
| Spontaneous arousals, independent of nursing, restless sleep | 3 | |
| Sleepless | 4 | |
| Quality of contact with nurses | Smiles, attentive to voice | 0 |
| Transient apprehension during interactions with nurses | 1 | |
| Difficulty communicating with nurses. Cries in response to minor stimulation | 2 | |
| Refuses to communicate with nurses. No interpersonal rapport. Moans without stimulation | 3 | |
| Consolability | Quiet, total relaxation | 0 |
| Calms down quickly in response to stroking or voice, or with sucking | 1 | |
| Calms down with difficulty | 2 | |
| Disconsolate. Sucks desperately | 3 |
SOME CRITICISMS
Recognition of the existence of neonatal pain is relatively recent scientific acquisition. It was not accepted up to the end of the 1980s. Consequently, pharmacologic analgesia was rarely provided to babies during surgery (65). As late as 1999, it was stated that “[p]ain experience is placed at approximately 12 months of age” (66). Today, clinicians estimate that most neonatal intensive care unit procedures are painful, but many neonates do not receive appropriate analgesic therapy. Despite the accumulating evidence that neonatal procedural pain is harmful, analgesic treatment for painful procedures is limited (1). However, with recent changes in the practice of neonatal medicine, there is growing recognition of the importance of treating the pain that such treatments may induce. These shifts in clinical care have led to a growing recognition of the need for new observational and measurement tools (67).
Still being debated is fetal pain. Despite the evidence that prematurely born fetuses can in fact experience pain, some authors argue that the amniotic fluid has sedative capacities (68), and therefore, pain is not felt by the fetus. Nonetheless, there are several reasons (69) to doubt that the amniotic fluid has any sedative effects: for instance the sudden crying of almost all babies at birth, as well as the reactions to external stimuli when fetuses are still in the womb, testifies that they are far from being sedated. Therefore, most authors have argued that fetal pain can be experienced during the third trimester (70) second trimester (71) or after 20 weeks of gestational age (72). Given the uncertainty about the gestational age when pain can be experienced, the precautionary principle points to a need to use analgesic treatments (73–75) since when the neural connections between peripheral receptors, the thalamus, and the cortical subplate the basis for pain perception appear at about 20–22 weeks from conception (76,77). Unfortunately, no pain scales exist to date for the evaluation of fetal pain. Consequently, there exists no specific tool for rating the pain or to help discriminate between reactions attributable to pain versus due to reactions to causes other than pain.
CONCLUSION
It is possible to interpret pain expressions in the first phases of life. This should be a tenet in medicine schools, in order to provide the opportune analgesic drugs when necessary, and to avoid excesses or deficiencies in analgesic treatment. Ultimately, regardless of age, the patient has the right to analgesia and that right should be recognized and guaranteed, even when the need for analgesia cannot be expressed by the patient.
REFERENCES
- 1.Simons SH, van Dijk M, Anand KS, Roofthooft D, van Lingen RA, Tibboel D. Do we still hurt newborn babies? A prospective study of procedural pain and analgesia in neonates. Arch Pediatr Adolesc Med. 2003;157(11):1058–64. doi: 10.1001/archpedi.157.11.1058. [DOI] [PubMed] [Google Scholar]
- 2.Bebbington MW, Danzer E, Johnson MP, Adzick NS. Open fetal surgery for myelomeningocele. Prenat Diagn. 2011;31(7):689–94. doi: 10.1002/pd.2805. [DOI] [PubMed] [Google Scholar]
- 3.Dwinnell SJ, Coad S, Butler B, Albersheim S, Wadsworth LD, Wu JK, Delisle MF. In utero diagnosis and management of a fetus with homozygous α-thalassemia in the second trimester: a case report and literature review. J Pediatr Hematol Oncol. 2011;33(8):e358–60. doi: 10.1097/MPH.0b013e31821b368c. [DOI] [PubMed] [Google Scholar]
- 4.Craig KD, Gilbert-MacLeod CA, Lilley CM. Crying as an indicator of pain in infants. In: Barr RG, Hopkins B, Green JA, editors. Crying as a sign, symptom and a signal. Cambridge: Cambridge University Press; 2000. pp. 23–40. [Google Scholar]
- 5.Craig KD, Whitfield MF, Grunau RVE, Linton J, Hadjistavropoulos HD. Pain in the preterm neonate: behavioural and physiological indices. Pain. 1993;52:287–299. doi: 10.1016/0304-3959(93)90162-I. [DOI] [PubMed] [Google Scholar]
- 6.Craig KD. The facial display of pain. In: Finley GA, McGrath PJ, editors. Measurement of pain in infants and children. Progress in pain research and management. Seattle: IASP Press; 1998. pp. 103–122. [Google Scholar]
- 7.Grunau RVE, Craig KD. Facial activity as a measure of neonatal pain expression. In: Tyler DC, Krane EJ, editors. Advances in pain research and therapy. New York: Raven; 1990. pp. 147–155. [Google Scholar]
- 8.Schiavenato M, Butler-O'Hara M, Scovanner P. Exploring the association between pain intensity and facial display in term newborns. Pain Res Manag. 2011;16(1):10–2. doi: 10.1155/2011/873103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grunau RVE, Johnston CC, Craig KD. Neonatal facial and cry responses to invasive and noninvasive procedures. Pain. 1990;42:295–305. doi: 10.1016/0304-3959(90)91142-6. [DOI] [PubMed] [Google Scholar]
- 10.Barr RG. “Is this infant in pain?”: caveats from the clinical setting. J Pain. 1992;1:187–190. [Google Scholar]
- 11.Grunau RE, Oberlander T, Holsti L, Whitfield MF. Bedside application of the Neonatal Facial Coding System in pain assessment of premature neonates. Pain. 1998;76:277–286. doi: 10.1016/S0304-3959(98)00046-3. [DOI] [PubMed] [Google Scholar]
- 12.Peters JW, Koot HM, Grunau RE, et al. Neonatal Facial Coding System for assessing postoperative pain in infants: item reduction is valid and feasible. Clin J Pain. 2003;19:353–363. doi: 10.1097/00002508-200311000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Bellieni CV, Severi F, Bocchi C, Caparelli N, Bagnoli F, Buonocore G, Petraglia F. Blink-startle reflex habituation in 30-34-week low-risk fetuses. J Perinat Med. 2005;33(1):33–7. doi: 10.1515/JPM.2005.005. [DOI] [PubMed] [Google Scholar]
- 14.Bischoff A. Not neglecting analgesia! How children show pain. MMW Fortschr Med. 2011;153(35):20. doi: 10.1007/BF03371765. [DOI] [PubMed] [Google Scholar]
- 15.Zimmermann M. Pain in the fetus: neurobiological, psychophysiological and behavioral aspects. Schmerz. 1991;5(3):122–30. doi: 10.1007/BF02528097. [DOI] [PubMed] [Google Scholar]
- 16.Petrikovsky BM. Predicting ambulation by checking leg withdrawal in fetuses with spina bifida. Am J Obstet Gynecol. 2002;187(1):256. doi: 10.1067/mob.2002.124953. [DOI] [PubMed] [Google Scholar]
- 17.Peisker N, Preissel AK, Ritzmann M, Schuster T, Thomes R, Henke J. Fetal responses to different methods of electrocution of pregnant sows. Berl Munch Tierarztl Wochenschr. 2008;121(9-10):317–28. [PubMed] [Google Scholar]
- 18.Petrikovsky BM, Kaplan GP. Fetal responses to inadvertent contact with the needle during amniocentesis. Fetal Diagn Ther. 1995;10(2):83–5. doi: 10.1159/000264210. [DOI] [PubMed] [Google Scholar]
- 19.van Dijk M, de Boer JB, Koot HM, et al. The association between physiological and behavioralpain measures in 0 to 3-year-old infants after major surgery. J Pain Symptom Manag. 2001;22:600–609. doi: 10.1016/S0885-3924(01)00288-3. [DOI] [PubMed] [Google Scholar]
- 20.Barr RG, Hopkins B, Green JA. Crying as a sign, symptom and a signal: evolving concepts of crying behavior. In: Barr RG, Hopkins B, Green JA, editors. Crying as a sign, symptom and a signal. Cambridge: Cambridge University Press; 2000. pp. 1–7. [Google Scholar]
- 21.Wolff PH. The natural history of crying and other vocalizations in early infancy. In: Foss B, editor. Determinants of infant behavior. London: Mathuen; 1969. pp. 81–109. [Google Scholar]
- 22.Valanne EH, Vuorenkoski V, Partanen TJ, Lind J, Wasz-Hockert O. The ability of human mothers to identify the hunger cry signals of their own new-born infants during the lying-in period. Experientia. 1967;23(768–769):24. doi: 10.1007/BF02154167. [DOI] [PubMed] [Google Scholar]
- 23.Wasz-Hockert O, Valanne E, Vuorenkoski V, Michelsson K, Sovijarvi A. Analysis of some types of vocalization in the newborn and in early infancy. Ann Paediatr Fenn. 1963;9:1–10. [PubMed] [Google Scholar]
- 24.Porter FL, Miller RH, Marshall RE. Neonatal pain cries: effect of circumcision on acoustic features and perceived urgency. Child Dev. 1986;57:790–802. doi: 10.2307/1130355. [DOI] [PubMed] [Google Scholar]
- 25.Murray AD. Infant crying as an elicitor of parental behavior: an examination of two models. Psychol Bull. 1979;86:191–215. doi: 10.1037/0033-2909.86.1.191. [DOI] [PubMed] [Google Scholar]
- 26.Gustafson GE, Harris KL. Women's responses to young infants' cries. Dev Psychol. 1990;26:144–152. doi: 10.1037/0012-1649.26.1.144. [DOI] [Google Scholar]
- 27.Oberlander T, Saul JP. Methodological considerations for the use of heart rate variability as a measure of pain reactivity in vulnerable infants. Clin Perinatol. 2002;29(3):427–43. doi: 10.1016/S0095-5108(02)00013-1. [DOI] [PubMed] [Google Scholar]
- 28.Hadjistavropoulos HD, Craig KD, Grunau RE, Whitfield MF. Judging pain in infants: behavioural, contextual, and developmental determinants. Pain. 1997;73(3):319–24. doi: 10.1016/S0304-3959(97)00113-9. [DOI] [PubMed] [Google Scholar]
- 29.Bellieni CV, Sisto R, Cordelli DM, Buonocore G. Cry features reflect pain intensity in term newborns: an alarm threshold. Pediatr Res. 2004;55(1):142–6. doi: 10.1203/01.PDR.0000099793.99608.CB. [DOI] [PubMed] [Google Scholar]
- 30.Sisto R, Bellieni CV, Perrone S, Buonocore G. Neonatal pain analyzer: development and validation. Med Biol Eng Comput. 2006;44(10):841–5. doi: 10.1007/s11517-006-0101-x. [DOI] [PubMed] [Google Scholar]
- 31.Bellieni C, Maffei M, Ancora G, Cordelli D, Mastrocola M, Faldella G, Ferretti E, Buonocore G. Is the ABC pain scale reliable for premature babies? Acta Paediatr. 2007;96(7):1008–10. doi: 10.1111/j.1651-2227.2007.00355.x. [DOI] [PubMed] [Google Scholar]
- 32.Bellieni CV, Bagnoli F, Sisto R, Neri L, Cordelli D, Buonocore G. Development and validation of the ABC pain scale for healthy full-term babies. Acta Paediatr. 2005;94(10):1432–6. doi: 10.1080/08035250510039919. [DOI] [PubMed] [Google Scholar]
- 33.Gingras JL, Mitchell EA, Grattan KE. Fetal homologue of infant crying. Arch Dis Child Fetal Neonatal Ed. 2005;90(5):F415–8. doi: 10.1136/adc.2004.062257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jakobovits A. Paradigms of fetal ethology. Orv Hetil. 2006;147(11):509–15. [PubMed] [Google Scholar]
- 35.Cignacco E, Mueller R, Hamers JP, Gesler P. Pain assessment in the neonate using the Bernese Pain Scale for Neonates. Early Hum Dev. 2004;78:125–131. doi: 10.1016/j.earlhumdev.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 36.Pillai M, James D. Are the behavioural states of the newborn comparable to those of the fetus? Early Hum Dev. 1990;22(1):39–49. doi: 10.1016/0378-3782(90)90024-D. [DOI] [PubMed] [Google Scholar]
- 37.Legido A, Valencia I, Smith JD. Fetal neurological evaluation. Rev Neurol. 2004;39(5):454–64. [PubMed] [Google Scholar]
- 38.Pokela M. Pain relief can reduce hypoxemia in distressed neonates during routine treatment procedures. Pediatrics. 1994;93:379–383. [PubMed] [Google Scholar]
- 39.Debillon T, Zupan V, Ravault N, Magny JF, Dehan M. Development and initial validation of the EDIN scale, a new tool for assessing prolonged pain in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2001;85:F36–F41. doi: 10.1136/fn.85.1.F36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sweet SD, McGrath PJ. Physiological measures of pain. In: Finley GA, McGrath PJ, editors. Measurement of pain in infants and children. Progress in pain research and management. Seattle: IASP Press; 1998. pp. 59–81. [Google Scholar]
- 41.Stevens BJ, Johnston CC, Horton L. Multidimensional pain assessment in premature neonates: a pilot study. J Obstet Gynecol Neonatal Nurs. 1993;22(6):531–41. doi: 10.1111/j.1552-6909.1993.tb01838.x. [DOI] [PubMed] [Google Scholar]
- 42.Bellieni CV, Burroni A, Perrone S, Cordelli DM, Nenci A, Lunghi A, Buonocore G. Intracranial pressure during procedural pain. Biol Neonate. 2003;84(3):202–5. doi: 10.1159/000072303. [DOI] [PubMed] [Google Scholar]
- 43.Munsters J, Wallström L, Agren J, Norsted T, Sindelar R. Skin conductance measurements as pain assessment in newborn infants born at 22-27 weeks gestational age at different postnatal age. Early Hum Dev. 2011;88(1):21–6. doi: 10.1016/j.earlhumdev.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 44.Eriksson M, Storm H, Fremming A, Schollin J. Skin conductance compared to a combined behavioural and physiological pain measure in newborn infants. Acta Paediatr. 2008;97(1):27–30. doi: 10.1111/j.1651-2227.2007.00586.x. [DOI] [PubMed] [Google Scholar]
- 45.Hellerud BC, Storm H. Skin conductance and behaviour during sensory stimulation of preterm and term infants. Early Hum Dev. 2002;70(1–2):35–46. doi: 10.1016/S0378-3782(02)00070-1. [DOI] [PubMed] [Google Scholar]
- 46.Gjerstad AC, Wagner K, Henrichsen T, Storm H. Skin conductance versus the modified COMFORT sedation score as a measure of discomfort in artificially ventilated children. Pediatrics. 2008;122(4):e848–53. doi: 10.1542/peds.2007-2545. [DOI] [PubMed] [Google Scholar]
- 47.Abdulkader HM, Freer Y, Fleetwood-Walker SM, McIntosh N. Bodily progression of motor responses to increasing mechanical force stimulation in the newborn infant and the effect of heel prick. Neonatology. 2008;94(1):38–44. doi: 10.1159/000112948. [DOI] [PubMed] [Google Scholar]
- 48.Mainous RO, Looney S. A pilot study of changes in cerebral blood flow velocity, resistance, and vital signs following a painful stimulus in the premature infant. Adv Neonatal Care. 2007;7(2):88–104. doi: 10.1097/01.anc.0000267914.96844.ce. [DOI] [PubMed] [Google Scholar]
- 49.Slater R, Fabrizi L, Worley A, Meek J, Boyd S, Fitzgerald M. Premature infants display increased noxious-evoked neuronal activity in the brain compared to healthy age-matched term-born infants. NeuroImage. 2010;52(2):583–9. doi: 10.1016/j.neuroimage.2010.04.253. [DOI] [PubMed] [Google Scholar]
- 50.Herrington CJ, Olomu IN, Geller SM. Salivary cortisol as indicators of pain in preterm infants: a pilot study. Clin Nurs Res. 2004;13(1):53–68. doi: 10.1177/1054773803259665. [DOI] [PubMed] [Google Scholar]
- 51.Guinsburg R, Kopelman BI, Anand KJ, de Almeida MF, Peres CA, Miyoshi MH. Physiological, hormonal, and behavioral responses to a single fentanyl dose in intubated and ventilated preterm neonates. J Pediatr. 1998;132(6):954–9. doi: 10.1016/S0022-3476(98)70390-7. [DOI] [PubMed] [Google Scholar]
- 52.Giannakoulopoulos X, Teixeira J, Fisk N, Glover V. Human fetal and maternal noradrenaline responses to invasive procedures. Pediatr Res. 1999;45(4 Pt 1):494–9. doi: 10.1203/00006450-199904010-00007. [DOI] [PubMed] [Google Scholar]
- 53.Teixeira J, Fogliani R, Giannakoulopoulos X, Glover V, Fisk NM. Fetal haemodynamic stress response to invasive procedures. Lancet. 1996;347(9001):624. doi: 10.1016/S0140-6736(96)91327-6. [DOI] [PubMed] [Google Scholar]
- 54.Giannakoulopoulos X, Sepulveda W, Kourtis P, Glover V, Fisk NM. Fetal plasma cortisol and beta-endorphin response to intrauterine needling. Lancet. 1994;344(8915):77–81. doi: 10.1016/S0140-6736(94)91279-3. [DOI] [PubMed] [Google Scholar]
- 55.Carbajal R, Paupe A, Hoenn E, Lenclen R, Olivier MM. APN: evaluation behavioural scale of acute pain in newborn infants. Arch Pediatr. 1997;4:623–8. doi: 10.1016/S0929-693X(97)83360-X. [DOI] [PubMed] [Google Scholar]
- 56.Lawrence J, Alcock D, McGrath P, et al. The development of a tool to assess neonatal pain. Neonatal Netw. 1993;12:59–66. [PubMed] [Google Scholar]
- 57.Stevens B, Johnston C, Petryshen P, Taddio A. Premature infant pain profile: development and initial validation. Clin J Pain. 1996;12:13–22. doi: 10.1097/00002508-199603000-00004. [DOI] [PubMed] [Google Scholar]
- 58.Suraseranivongse S, Kaosaard R, Intakong P, et al. A comparison of postoperative pain scales in neonates. Br J Anesth. 2006;97:540–4. doi: 10.1093/bja/ael184. [DOI] [PubMed] [Google Scholar]
- 59.Van Dijk M, De Boer JB, Koot HM, et al. The reliability and validity of the COMFORT scale as a postoperative pain instrument in 0 to 3-year-old infants. Pain. 2000;84:367–377. doi: 10.1016/S0304-3959(99)00239-0. [DOI] [PubMed] [Google Scholar]
- 60.Grunau RVE, Craig KD. Pain expression in neonates: facial action and cry. Pain. 1987;28:395–410. doi: 10.1016/0304-3959(87)90073-X. [DOI] [PubMed] [Google Scholar]
- 61.Merkel SI, Voepel-Lewis T, Shayevitz JR, The MS. The FLACC: a behavioral scale for scoring postoperative pain in young children. Pediatr Nurs. 1997;23(3):293–7. [PubMed] [Google Scholar]
- 62.Bellieni CV, Cordelli DM, Caliani C, Palazzi C, Franci N, Perrone S, Bagnoli F, Buonocore G. Inter-observer reliability of two pain scales for newborns. Early Hum Dev. 2007;83(8):549–52. doi: 10.1016/j.earlhumdev.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 63.Hummel P, Puchalski M, Creech S, The WMG. The N-PASS: Neonatal Pain, Agitation, and Sedation Scale—initial validity and reliability. J Perinatol. 2008;28(1):55–60. doi: 10.1038/sj.jp.7211861. [DOI] [PubMed] [Google Scholar]
- 64.Gauvain-Piquard A, Rodary C, Rezvani A, Serbouti S. The Development of the DEGR: a scale to assess pain in young children with cancer. Eur J Pain. 1999;3:165–176. doi: 10.1053/eujp.1999.0118. [DOI] [PubMed] [Google Scholar]
- 65.De Lima J, Lloyd-Thomas AR, Howard RF, et al. Infant and neonatal pain: anaesthetists' perceptions and prescribing patterns. BMJ. 1996;313:787. doi: 10.1136/bmj.313.7060.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Derbyshire SW. Locating the beginnings of pain. Bioethics. 1999;13(1):1–31. doi: 10.1111/1467-8519.00129. [DOI] [PubMed] [Google Scholar]
- 67.Allegaert K. The clinical pharmacology of short acting analgo-sedatives in neonates. Curr Clin Pharmacol. 2011;6(4):222–6. doi: 10.2174/157488411798375912. [DOI] [PubMed] [Google Scholar]
- 68.Mellor DJ, Diesch TJ, Gunn AJ, Bennet L. The importance of 'awareness' for understanding fetal pain. Brain Res Brain Res Rev. 2005;49(3):455–71. doi: 10.1016/j.brainresrev.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 69.Bellieni CV, Buonocore G. Is fetal pain a real evidence? J Matern Fetal Neonatal Med. 2011 (in press) [DOI] [PubMed]
- 70.Lee SJ, Ralston HJ, Drey EA, Partridge JC, Rosen MA. Fetal pain: a systematic multidisciplinary review of the evidence. JAMA. 2005;294(8):947–54. doi: 10.1001/jama.294.8.947. [DOI] [PubMed] [Google Scholar]
- 71.Anand KJS. Fetal pain? Pain Clin Updat. 2006;14(2):1–4. [Google Scholar]
- 72.Glover V. The fetus may feel pain from 20 weeks. Conscience. 2004–2005 Winter 25(3):35–7. [PubMed]
- 73.Fisk NM, Gitau R, Teixeira JM, Giannakoulopoulos X, Cameron AD, Glover VA. Effect of direct fetal opioid analgesia on fetal hormonal and hemodynamic stress response to intrauterine needling. Anesthesiology. 2001;95(4):828–35. doi: 10.1097/00000542-200110000-00008. [DOI] [PubMed] [Google Scholar]
- 74.Gaiser RR, Kurth CD. Anesthetic considerations for fetal surgery. Semin Perinatol. 1999;23:507e14. doi: 10.1016/S0146-0005(99)80029-9. [DOI] [PubMed] [Google Scholar]
- 75.Sutton LN. Fetal surgery for neural tube defects. Best Pract Rres Clin Obstet Gynecol. 2008;22(1):175–88. doi: 10.1016/j.bpobgyn.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hevner RF. Development of connections in the human visual system during fetal mid-gestation: a DiI-tracing study. J Neuropathol Exp Neurol. 2000;59:385–92. doi: 10.1093/jnen/59.5.385. [DOI] [PubMed] [Google Scholar]
- 77.Kanold PO, Luhmann HJ. The subplate and early cortical circuits. Annu Rev Neurosci. 2010;33:23–48. doi: 10.1146/annurev-neuro-060909-153244. [DOI] [PubMed] [Google Scholar]