Abstract
The study examined the sensitivity of DPI in vitro performance to formulation and device changes. Rotahaler/Rotacaps was selected as the reference DPI drug product, and Aerolizer was selected as the test device. Since the test device was recognized to have much greater efficiency of dispersion, simple modifications to both formulation and device were made in an effort to provide a closer match to the in vitro performance of the reference product. The modifications included varying the drug and lactose particle sizes and/or lactose fine particle content in the test formulations, as well as lowering the specific resistance of the test device. These modifications were intended to address variables important for drug product performance for a defined experimental design and were not intended to mimic the extensive formulation and device design strategies that are employed in an industrial setting. Formulation and device modifications resulted in a modified test product that approached the reference product in the in vitro performance.
KEY WORDS: device modifications, dry powder inhaler, formulation, generic, in vitro performance
INTRODUCTION
Dry powder inhalers (DPIs) for delivery of locally acting drugs to the lungs are increasingly important in the US marketplace. Factors accounting for this increase include environmental concerns with CFCs (1,2) and recognition of patient convenience compared to metered dose inhalers. All DPIs currently marketed in the USA utilize breath-actuated (“passive”) devices. In coming years, patents will expire on innovator DPIs, and interest will increase in the development of generic versions of these orally inhaled drug products.
The current thinking for establishing bioequivalence (BE) of DPIs in the USA is based on the aggregate weight of evidence approach. This approach includes device and formulation comparability, in vitro equivalence in a number of tests, and in vivo equivalence in systemic exposure and local delivery (3). In vitro tests used to support BE of DPIs include emitted dose (ED) and aerodynamic particle size distribution (APSD). The in vitro performance of DPIs depends significantly on the formulation and device properties. The physicochemical properties of drug substance and drug carrier (lactose), such as particle size, shape, density, surface area, roughness, and morphology (4–9), have been shown to affect particle interactions, stability, ease of dispersion, and deaggregation. The internal geometry of a DPI device (e.g., the dimensions and shape of the air channels) is known to play an important role in determining the device resistance. Thus, it influences the air flow rate through an inhaler device, as well as the level and nature of forces for the powder fluidization and deaggregation of drug particles from carriers (3). For the above reasons, to attain a close match between two DPIs with respect to ED and APSD, it is critical to have an adequate understanding of how these formulation and device variables interact and affect the in vitro performance of DPIs.
As a step toward a better understanding of the type and magnitude of changes that can be made to provide a better in vitro match between test and reference DPIs, this study examined the sensitivity of DPI in vitro performance to several formulation and device changes. The study selected a commercially available DPI device (Rotahaler) paired with a commercial capsule containing an albuterol sulfate formulation (Rotacaps) as a model “reference” product. It also selected another commercially available DPI device (Aerolizer) as a model “test” device. This model test DPI device was considered as an experimental platform, which could be modified to produce in vitro characteristics comparable to the reference product. Both test and reference DPI devices are unit dose, capsule-based devices. Based on USP/PF limits (10), both Rotahaler and Aerolizer are low resistance DPIs. However, the specific resistance of the Aerolizer device is greater than that of the Rotahaler device (11,12). The authors recognize that the knowledge, skills, and expertise available in the industry and effort expended for formulation and device optimization extend far beyond the modest efforts expended for the present studies. The data should, therefore, be interpreted in the context of the limited scope of the project and the intention to examine the ease with which using a very simple strategy similar performance from two devices can be approached.
Part I of the study focused on evaluating the effects of formulation variables on the in vitro performance of Aerolizer. Several “test” formulations were prepared to investigate the dependence of the in vitro performance of Aerolizer on key formulation variables, focusing on particle sizes of the drug (albuterol sulfate) and carrier (lactose) as well as lactose fine particle content. The results of this investigation would provide useful information on the extent to which these formulation variables could be modified to provide a closer match of ED and APSD between test and reference DPIs.
Part II of the study examined the effect of device modifications on the device resistance and subsequently the in vitro performance of modified Aerolizer devices with the formulations selected based on the Part I study results. Two modifications were made to the Aerolizer device to lower its specific resistance, in order to provide a better match to that of Rotahaler. The results of this examination would serve as an example to illustrate the importance of the device component in attaining in vitro comparability of test and reference DPIs.
MATERIALS AND METHODS
Rotahaler and Rotacaps
Rotahaler (GSK) was obtained from Australia (Homepharmacy.com.au). Obtaining Ventolin Rotacaps was not feasible. Asthalin Rotacaps (Cipla, Mumbai, India) were obtained. The package insert for Asthalin Rotacaps states that each capsule contains albuterol sulfate equivalent to 200 μg albuterol blended with 25 mg lactose, i.e., equivalent to 0.80 % albuterol base.
Albuterol
Albuterol sulfate (Spectrum, Gardena, CA) was micronized using an air jet mill (Model Trost Gem-T, Glen Mills, Clifton, NJ) with nitrogen inlet pressure greater than 80 psig to yield a distribution of particles having a measured D50 of 3.2 μm. The 3.2 μm batch was further milled to produce a batch with D50 of 1.9 μm.
Lactose
Milled inhalation grade lactose from two suppliers was used to prepare Formulations A to D. Respitose ML003 (lot 10250079), DMV International, Veghel, the Netherlands, is an intermediate size lactose. Lactohale LH 200 (batch 609313) alpha-lactose monohydrate, Friesland Foods Domo, Zwolle, the Netherlands, is a coarse lactose.(13) Lactohale LH 300 (batch 615225), Friesland Foods Domo, is a micronized lactose.
Preparation of Test Formulation Blends
Four test formulations (Formulations A to D) listed in Table I were prepared as follows. Albuterol sulfate–lactose blends were prepared containing albuterol sulfate with a target weight of 241 μg equivalent to 0.8 % w/w albuterol base (target 200 μg). Each blend consisted of albuterol sulfate (3.2 or 1.9 μm), and Respitose ML003, Lactohale LH 200, or Lactohale LH 200 +8.0 % Lactohale LH 300. The two grades of lactose (Lactohale LH 200 and Lactohale LH 300) were pre-blended for 30 min using a Turbula mixer (Glen Mills, Clifton, NJ), prior to blending with the drug. Drug/lactose pre-blends were prepared in 20-mL vials, and then in 120-mL bottles using a spatula by geometric dilution. The final two additions of lactose were blended for 5 min (first mixing) and 30 min (second mixing), with an approximate head space of 72 mL using a mixer setting of 22 RPM. These multiple blending steps were used to maximize the blend uniformity. Test formulation capsules were prepared by filling no. 3 Quali-V HPMC capsules (Qualicaps, Whitsett, NC) with 25 mg of powder.
Table I.
Characterization of Particle Sizes by Laser Diffraction for the Reference and Test Formulations and Lactose
| Formulation | Drug particle size (D50), μm | Lactose brand and grade | Drug concentrationa (RSD %),b albuterol base, % w/w | Volume diameter (SDc), μm | Lactose fine content,d % | |||
|---|---|---|---|---|---|---|---|---|
| D10 | D50 | D90 | (D90–D10)/D50 | Mean (SD) | ||||
| Reference (Rotacaps) | – | Milled | 0.820 (3.76) | 2.78 (0.09) | 39.9 (1.10) | 97.3 (1.85) | 2.37 (0.02) | 20.9 (0.48) |
| A | 1.9 | Respitose ML003, milled | 0.797 (0.925) | 3.48 (0.05) | 34.4 (0.32) | 102 (0.32) | 2.86 (0.02) | 22.9 (0.25) |
| B | 1.9 | Lactohale LH 200, milled | 0.773 (0.984) | 6.67 (0.11) | 73.2 (0.39) | 161 (0.37) | 2.11 (0.01) | 12.4 (0.10) |
| C | 3.2 | Respitose ML003, milled | 0.785 (0.887) | 3.85 (0.04) | 35.3 (0.43) | 107 (0.94) | 2.91 (0.01) | 22.0 (0.21) |
| D | 3.2 | Lactohale LH 200 + LH 300, millede | 0.729 (0.967)f | 3.13 (0.02) | 58.7 (1.53) | 144 (3.37) | 2.40 (0.01) | 24.0 (0.31) |
| Lactose only | Respitose ML003, milled | 4.00 (0.07) | 35.7 (0.40) | 106 (2.94) | 2.84 (0.05) | 20.8 (0.19) | ||
| Lactose only | Lactohale LH 200, milled | 9.97 (0.08) | 74.9 (0.30) | 158 (1.89) | 1.97 (0.02) | 10.0 (0.07) | ||
| Lactose only | Lactohale LH 300, micronized | 0.77 (0.01) | 3.54 (0.03) | 8.91 (0.03) | 2.30 (0.05) | 93.0 (0.08) | ||
aThe mean of six measurements (25 mg per sample) in terms of albuterol base
bRelative standard deviation
cStandard deviation
dPercentage below 10 μm
e8.0 % Lactohale LH 300
fFormulation D was formulated 8.9 % below target albuterol concentration. For exploratory purposes, performance of the lower concentration formulation was studied in an attempt to offset the greater dispersion efficiency of the test product
Solid-State Characterization of Albuterol Sulfate, Lactose, Asthalin Rotacaps™, and Test Formulations
Laser diffraction was conducted using a Sympatec HELOS instrument (Clausthal-Zellerfeld, Germany) with a RODOS dry powder dispersion unit and a VIBRI vibrating feeder to disperse the powders into the laser beam. D10, D50 (volume median diameter), D90, and fine particle content (percentage below 10 μm) for each powder were determined. The VIBRI feed rate was 40 %. Powders were dispersed using approximate RODOS pressures of 1 bar and 20 mbar (primary and secondary, respectively). A range of lens attachments (R1–R5) were used for capturing the particle size distribution (PSD) of commercial reference product, lactose, micronized albuterol sulfate, and albuterol sulfate–lactose blends. Experiments were conducted in triplicate.
Scanning electron microscopy was conducted (Research Triangle Institute, Research Triangle Park, NC) using a Hitachi field emission instrument (Pleasanton, CA). A sample of formulation was distributed onto a conductive carbon substrate adhered to a stub, and the sample was then coated with a thin layer of evaporated carbon to enhance conductivity.
Analytical Method
The analytical method for assay of albuterol for content uniformity and cascade impactor tests was liquid chromatography with mass-selective detection (14). The method was precise, accurate, and linear over the range of 2 (LLOQ) to 200 ng/mL. Albuterol-d3 (C/D/N Isotopes, Pointe-Claire, Quebec) was used as an internal standard. Samples were recovered and diluted with 0.1 % formic acid. Samples were analyzed, using a Shimadzu system consisting of a SIL-20 AC autosampler, CTO-20A column oven, LC-20 AD pump, DGU-20A3 degasser, 10A VP system controller, and thermo surveyor MSQ. The column was an Agilent Zorbax Eclipse XDB-C18, 2.1 × 30 mm, 1.8 μm analytical column. Mobile phase was 0.1 % formic acid (93 %) and methanol (7 %). Column temperature and mobile phase flow rate were 60°C and 0.5 mL/min, respectively. Sample run time was 1 min. Mass spectrometry settings include the following: AP-ESI source, SIM mode, positive polarity, channel 1 mass to charge ratio of 240.3 m/z, channel 2 mass to charge ratio of 243.0 m/z, probe temperate of 350°C, needle potential of 3,000 V, cone potential of 50 V, dwell time of 0.15 s, time span of 0.1 min, and time range of 0.1–1.0 min.
Cascade Impactor Measurements
Single actuation cascade impaction studies were conducted with the Andersen eight-stage Nonviable Cascade Impactor (ACI; Thermo Scientific, Waltham, MA), or the Westech eight-stage ACI (Westech Instruments, Marietta, GA). The cascade impactors were fitted with a mouthpiece adaptor, USP Induction Port, and were configured for 28.3, 60, and 90 LPM flow rates. A preseparator was used for the 28.3 and 60 LMP configuration. A preseparator was not used for the 90 LPM configuration because the 90 LPM preseparator available at the time the studies were initiated was not validated for a 10-μm effective cutoff diameter (ECD). An airflow volume of 2.0 L was used for all studies. Plates were coated with silicon oil. Experiments were conducted in triplicate.
Mass median aerodynamic diameter (MMAD) and geometric standard deviation (GSD) were calculated based upon the inverse normal of the cumulative % under the stated aerodynamic diameter versus the log of the ECD (15). Linear regression of the three data points closest to 50 % of the cumulative particle mass that entered the cascade impactor was performed to compute the MMAD and GSD (16).
Airflow Resistance
Resistances were determined using the USP <601> Sampling Apparatus B for delivered dose uniformity of DPIs (17). The inhaler devices containing an unpunctured capsule were attached to the apparatus in line with a Copley TPK flow controller. Uniform flow rates were established and the pressure was measured distal to the device using the P1 function of the flow controller. The pressure gradient was measured at flow rates ranging from 20 to 100 LPM. Specific resistances were determined from the slopes of the square root of the pressure drop versus flow rate plots.
RESULTS
Part I
Solid-State Characterization of Asthalin Rotacaps and Test Formulations
Among all DPI formulation design variables, the current study focused on the drug and lactose particle sizes as well as lactose fine particle content (<10 μm). These formulation properties have been shown to have significant effect on the in vitro performance of DPIs (8,9).
For the reference formulation, scanning electron micrographs showed a rough surface of the particles in the formulation, indicative of milled rather than spray-dried lactose (data not shown) (18). Laser diffraction revealed particles ranging in size from less than 10 to approximately 100 μm, D50 of 39.9 μm, and 21 % lactose fine particle content (Table I). Because the concentration of drug in the formulation is under 1 %, the observed sizes reflect predominantly lactose particles. The mean drug concentration was within 3 % of the target concentration of 0.80 %, as determined by albuterol assay.
Test Formulations A and C were prepared with albuterol sulfate of 1.9 or 3.2 μm particle size, respectively (Table I). Respitose ML003 (D50 = 35.7 μm) was used for these two test formulations to match the approximate D50 of lactose (39.9 μm) in the reference formulation. The lactose fine particle content of Formulations A (23 %) and C (22 %) was comparable to that of the reference formulation (21 %). Formulation B was prepared with the coarser Lactohale LH 200, resulting in a D50 of 73.2 μm, nearly twice that of the reference formulation and a considerably lower lactose fine particle content (12 %). Another formulation, Formulation D (3.2 μm albuterol sulfate) was prepared by addition of Lactohale LH 300 to Lactohale LH 200 to achieve a similar lactose fine particle content (24 %) as the reference formulation, Formulations A and C. The addition of Lactohale LH 300 to Lactohale LH 200 also resulted in the D50 of Formulation D (58.7 μm), which was smaller than the D50 of Formulation B but higher than the D50 of the reference product, Formulations A and C.
Mean albuterol concentrations of Test Formulations A, B, and C were within 3.4 % of the 0.8 % w/w target concentration (albuterol base). Mean albuterol concentration of Formulation D was 8.9 % lower than the target concentration. The RSD of the albuterol content of each of the test formulations was about one fourth that of the reference formulation (Table I).
Cascade Impaction Testing of Rotahaler with Asthalin Rotacaps
The cascade impaction testing of Rotahaler/Rotacaps was conducted at 28.3, 60, and 90 LPM (Table II). Relative to the 28.3 LPM flow rate, the emitted dose increased about 10 % and appeared to reach a plateau, signifying flow rate independence at the two higher flow rates. Such an ED dependence of Ventolin Rotahaler with Ventolin Rotacaps on flow rate has been previously observed (19,20). Relative to ED, a disproportionately greater increase in FPM and FPF was observed with flow rate increase, which may be explained by more efficient deaggregation and dispersion of drug from carrier (13).
Table II.
Cascade Impactor Measurements for Test and Reference Products [Mean (SD)]
| Flow rate, LPM | Emitted dose, μg | Emitted dose, %a | Throat + preseparator deposition, μg | Impactor-sized mass, μg | Fine particle mass, μgb | Fine particle fraction, %c | MMAD, μm | GSD, μm |
|---|---|---|---|---|---|---|---|---|
| Rotahaler with Asthalin Rotacaps (reference) | ||||||||
| 28.3 | 112 (9.5) | 55.8 (4.8) | 82.0 (9.0) | 25.1 (5.4) | 16.1 (3.5) | 14.4 (2.8) | 5.14 (0.15) | 1.63 (0.05) |
| 60 | 125 (15.2) | 62.5 (7.6) | 83.7 (9.1) | 37.7 (6.5) | 26.2 (4.9) | 20.9 (2.4) | 3.72 (0.15) | 2.04 (0.04) |
| 90d | 123 (17.3) | 61.4 (8.6) | 16.6 (1.2) | 69.4 (13.5) | 29.0 (6.3) | 23.4 (2.0) | 3.49 (0.11) | 2.55 (0.20) |
| Aerolizer with Asthalin Rotacaps | ||||||||
| 60 | 174 (8.1) | 86.8 (4.1) | 118 (2.0) | 52.4 (5.9) | 42.8 (6.1) | 24.6 (2.4) | 2.94 (0.15) | 1.98 (0.04) |
| Aerolizer with Formulation A | ||||||||
| 60 | 158 (8.0) | 78.7 (2.5) | 65.3 (0.3) | 89.6 (7.7) | 69.6 (6.8) | 44.0 (2.2) | 2.51 (0.11) | 2.07 (0.00) |
| Aerolizer with Formulation B | ||||||||
| 60 | 166 (3.0) | 85.5 (1.2) | 63.6 (1.3) | 101 (1.8) | 89.6 (1.6) | 54.0 (0.3) | 1.87 (0.09) | 3.14 (0.88) |
| Aerolizer with Formulation C | ||||||||
| 28.3 | 117 (27.3) | 59.5 (14.0) | 44.5 (7.0) | 67.7 (19.0) | 42.1 (14.1) | 35.0 (4.7) | 4.34 (0.06) | 1.60 (0.02) |
| 60 | 155 (8.3) | 78.4 (3.7) | 60.0 (2.5) | 90.1 (6.5) | 63.8 (9.8) | 41.1 (4.2) | 3.60 (0.16) | 1.84 (0.04) |
| 90d | 173 (4.7) | 88.1 (1.1) | 45.2 (1.7) | 113 (2.9) | 71.4 (2.2) | 41.3 (1.1) | 2.94 (0.13) | 2.01 (0.27) |
| Aerolizer with Formulation D | ||||||||
| 28.3 | 87.9 (11.6) | 48.4 (6.6) | 46.7 (5.6) | 37.1 (5.6) | 20.5 (3.3) | 23.2 (1.0) | 4.86 (0.09) | 1.62 (0.01) |
| 60 | 138 (3.1) | 75.3 (0.8) | 71.2 (3.8) | 62.6 (1.9) | 42.2 (2.7) | 30.7 (2.2) | 4.11 (0.07) | 1.68 (0.01) |
| 90d | 156 (13.8) | 84.3 (6.8) | 46.4 (3.5) | 92.2 (8.9) | 45.5 (4.5) | 29.2 (0.6) | 3.38 (0.02) | 1.78 (0.03) |
| Modified Aerolizer A with Formulation C | ||||||||
| 60 | 137 (14.8) | 70.0 (7.9) | 55.9 (3.9) | 77.5 (11.1) | 53.0 (12.8) | 38.1 (5.3) | 3.72 (0.14) | 1.80 (0.07) |
| Modified Aerolizer A with Formulation D | ||||||||
| 60 | 102 (21.0) | 55.8 (11.6) | 52.4 (8.6) | 46.3 (12.4) | 27.6 (11.3) | 26.4 (5.9) | 4.22 (0.36) | 1.90 (0.38) |
| Modified Aerolizer B with Formulation C | ||||||||
| 60 | 151 (18.8) | 76.3 (9.8) | 59.5 (3.7) | 87.0 (15.3) | 59.9 (12.9) | 39.3 (4.6) | 3.69 (0.06) | 1.85 (0.03) |
aEmitted dose as a % of nominal dose (200 μg)
bFine particle mass is defined as the mass of drug ≤stage 2 (4.7 μm cut-point, 28.3 LPM configuration), ≤stage 1 (4.4 μm cut-point, 60 LPM), and ≤stage (−0) (5.2 μm cut-point, 90 LPM configuration)
cFine particle fraction, % expresses fine particle mass as a % of emitted dose
dA preseparator was not used in the 90.0 LPM configuration. In the 90 LPM configuration, much more drug was deposited on the top stage and the casing relative to the lower flow rates. Coincident with increased drug on the casing, more drug could have been deposited on the lower stages, increasing the FPF and FPM
Cascade Impaction Testing of Aerolizer with Asthalin Rotacaps and Test Formulations
Table II summarizes the results of cascade impaction testing of Aerolizer with Asthalin Rotacaps and with Formulations A, B, C, and D.
Screening Characterization (60 LPM)
At 60 LPM, the ED for Aerolizer/Rotacaps (174 μg) was much higher than that from Rotahaler/Rotacaps (125 μg). The fine particle mass (FPM) for Aerolizer/Rotacaps (42.8 μg) was higher than that for Rotahaler/Rotacaps (26.2 μg). However, the fine particle fraction (FPF) of Rotahaler/Rotacaps, expressed as a percentage (%) of the ED, is close to that of the Aerolizer/Rotacaps due to the much lower ED from the Rotahaler/Rotacaps. Consistent with the greater FPM of Aerolizer/Rotacaps, the MMAD of this test product (2.94 μm) is lower than that of Rotahaler/Rotacaps (3.72 μm).
The EDs for Aerolizer/Formulations A (158 μg) and B (166 μg) were higher than the ED for Rotahaler/Rotacaps. The FPMs for Aerolizer/Formulations A (69.6 μg) and B (89.6 μg) were also higher than the FPM for Rotahaler/Rotacaps. The MMADs for Aerolizer/Formulations A (2.51 μm) and B (1.87 μm) were smaller than the MMAD for Rotahaler/Rotacaps. Note that Formulations A and B both used albuterol sulfate with the same D50 (1.9 μm), but differed in the carrier size (i.e., D50 of 35.7 μm for Formulation A and 74.9 μm for Formulation B). Aerolizer/Formulation C, differing from Aerolizer/Formulation A only in its larger drug particle size (D50 of 3.2 μm), produced a similar ED (155 μg) to, but a smaller mean FPM (63.8 μg) and a larger MMAD (3.60 μm) than Aerolizer/Formulation A.
Formulation D, which contained a mean albuterol sulfate concentration 8.9 % lower than the target concentration, was developed later in screening based on Aerolizer/Formulation C results in attempt to provide a better match to the in vitro performance of Rotahaler/Rotacaps. In comparison to Formulations A–C, the lower albuterol concentration in Formulation D might contribute to lowering the ED (138 μg) and FPM (42.2 μg) of Aerolizer/Formulation D and thus provided a closer match to the reference product. However, the MMAD of Aerolizer/Formulation D (4.11 μm) was larger than that of Aerolizer/Formulation C.
As a result, of the four Aerolizer/Test Formulation combinations (Table II), Formulation D most closely approached the reference product in ED, FPM, FPF, and throat + preseparator deposition. Formulation C was closer in MMAD. Therefore, Aerolizer/Formulations C and D were selected for full characterization.
Full Characterization (28.3, 60, and 90 LPM)
The ED for Aerolizer/Formulations C and D increased with increasing flow rate, consistent with published data for Foradil Aerolizer (21). Flow dependence of the ED for these two test products was much greater than that of Rotahaler/Rotacaps. The ED for Rotahaler/Rotacaps, Aerolizer/Formulation C, and Formulation D increased 10 %, 47 %, and 77 %, respectively, over the 28.3–90 LPM range. The FPM and FPF for Aerolizer/Formulations C and D also increased with increasing flow rate, although both trended towards a plateau between 60 and 90 LPM. However, the FPM and FPF values at 90 LPM could be biased due to the lack of a preseparator at 90 LPM.
Part II
Aerolizer Modifications
Following Part I of the study, two changes were made to the Aerolizer device to obtain specific resistances of test devices closer to the reference device. One modification was to the mouthpiece design (Modified Aerolizer A) and the other modification was to the capsule chamber (Modified Aerolizer B).
Modified Aerolizer A
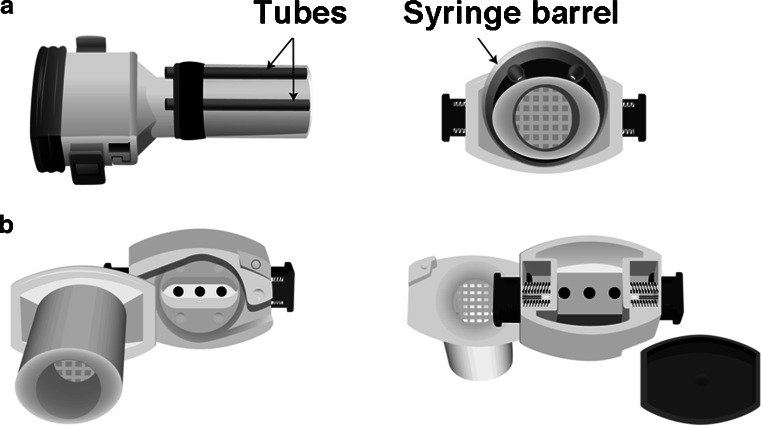
Two coaxial airflow openings were introduced surrounding the mouthpiece (Fig. 1a). The Aerolizer was fitted with a cut syringe barrel surrounding the mouthpiece, which was used as a sleeve to insert two tubes. The ends of the tubes were aligned with the end of the mouthpiece. The syringe barrel and two tubes were sealed to the Aerolizer with Flexbar ReproRubber. The two coaxial airflow openings increased the functional cross-sectional area of the mouthpiece, reduced airflow resistance of the inhaler, and decreased the air flow through the device.
Fig. 1.
a Modified Aerolizer A and b Modified Aerolizer B
Modified Aerolizer B
Two concurrent modifications were made in attempt to lower specific resistance (Fig. 1b). The device was operated with the backside cover removed, whereas the cover is in place in standard operation. Additionally, three 3/32″ holes were drilled into the back of the capsule chamber. These modifications were also made in an attempt to potentially alter the flow characteristics that govern capsule spinning aerodynamics, and therefore could alter the emitted dose and fine particle fraction. This was consistent with the observation that when holes drilled in the unit were too large, the device failed to function, i.e., the capsule would not spin at one or more airflow rates. For this reason, this type of modification was limited in terms of specific resistance matching between test and reference devices.
Airflow Resistances of Rotahaler and Modified Aerolizer Devices
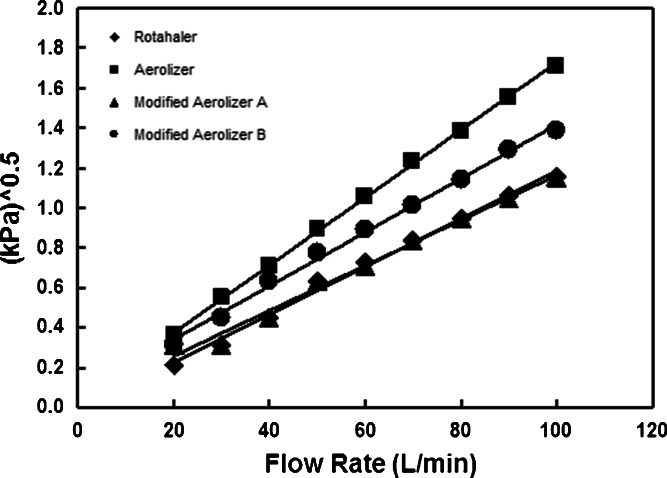
Observed specific resistance values for Rotahaler and Aerolizer (Fig. 2) are in agreement with previously observed values (11). The addition to the test product of two coaxial airflow openings surrounding the mouthpiece (Modified Aerolizer A) reduced specific resistance equal to that of the reference product. The modifications made to the Aerolizer (Modified Aerolizer B) lowered specific resistance to a value intermediate between the Aerolizer and Rotahaler devices, while maintaining the spinning movement of a capsule within the device.
Fig. 2.
Specific resistance for the reference and test devices. The specific resistance of Rotahaler, Aerolizer, Modified Aerolizer A, and Modified Aerolizer B are 0.012, 0.017, 0.011, and 0.014 kPa0.5 min/L, respectively. The means of triplicate measurements were plotted at each flow rate
Cascade Impaction Testing of Modified Aerolizer A with Formulations C and D, and Modified Aerolizer B with Formulation C
Based on the Part I study results, Modified Aerolizer A and B products were studied only with Formulations C and/or D. The APSD characterization of Modified Aerolizers A and B was conducted only at 60 LPM (Table II).
Modified Aerolizer A/Formulation C showed lower ED than Aerolizer/Formulation C. Similarly, Modified Aerolizer A/Formulation D showed lower ED than Aerolizer/Formulation D. Modified Aerolizer A/Formulation C relative to Aerolizer/Formulation C also exhibited decreased ISM and FPM, but only a slight increase in MMAD. A similar result was observed for Modified Aerolizer A/Formulation D relative to Aerolizer/Formulation D, resulting in an MMAD somewhat less favorable relative to the reference product.
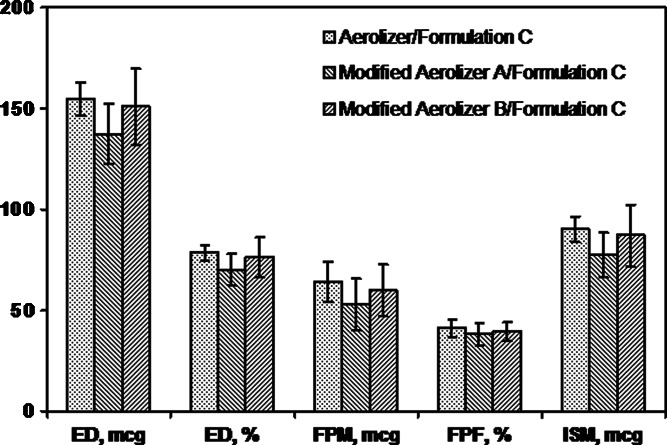
Modified Aerolizer B/Formulation C exhibited comparable aerodynamic performance in all measures reported in Table II to Aerolizer/Formulation C at 60 LPM. Thus, this observation indicated that for Formulation C, the means by which device resistance was lowered in Modified Aerolizer B did not result in significantly different in vitro performance relative to Aerolizer at 60 LPM.
DISCUSSION
Asthalin Rotacaps with Rotahaler and Aerolizer
Based on cascade impaction testing (Table II), Aerolizer/Rotacaps showed a considerable increase in ED, ED%, FPM, and ISM, and a large decrease in MMAD, in comparison to Rotahaler/Rotacaps. The APSD result suggested that Aerolizer was more efficient than Rotahaler with respect to fluidization and deaggregation of the reference DPI formulation. This difference in the in vitro performance of Aerolizer and Rotahaler could be due to differences in the specific resistance and mode of aerosolization of these two DPIs.
Aerolizer with Test Formulations
The scanning electron micrographs suggested that milled lactose was used in the reference formulation. Thus, two sources of milled lactose were selected. They were used alone or in combination with two different size batches of albuterol sulfate to prepare Formulations A, B, C, and D, in order to provide different combinations of the albuterol sulfate particle size, lactose particle size, and fine particle content. In vitro characterization of Aerolizer with these test formulations provided information on the extent to which changes in these formulation variables affected the in vitro performance of Aerolizer and its comparability to the reference product.
Formulation B was designed to have the lower abundance of lactose fine particles (Table I) and thus to provide a test formulation of lower dispersion. Based on “active sites,” “agglomerates,” or alternative hypotheses (22–24), Aerolizer/Formulation B would be expected to have a lower FPM than Aerolizer/Formulation A and provide a closer FPM match to the reference product. However, during the formulation screening, a greater FPM of Aerolizer/Formulation B was observed at 60 LPM relative to Aerolizer/Formulation A. Since the same batch of drug with D50 of 1.9 μm was used to prepare both Formulations A and B, this result suggested some other physicochemical aspects of lactose other than the fine lactose content that might influence drug–lactose interactions could play a more dominant role in determining FPM. Further characterization of Respitose ML003 and Lactohale LH 200 would be needed, in order to identify and understand the key differences (e.g., surface roughness and amorphous content) between these two sources of lactose that resulted in the greater FPM of Aerolizer/Formulation B.
Based on the cascade impaction results at 60 LPM, the larger drug particles in Formulations C and D (D50 of 3.2 μm) constituted one of the contributing factors to a smaller FPM and a greater MMAD of Aerolizer/Formulations C and D, and hence helped produce a closer match of Aerolizer/Formations C and D to the reference product than Aerolizer/Formulations A and B. As mentioned previously, the lower albuterol concentration in Formulation D appeared to further improve the in vitro matching of Aerolizer/Formulation D to the reference product with respect to ED and FPM. However, the MMAD of Aerolizer/Formulation D was higher than that of Aerolizer/Formulation C and the reference product. Since both Formulations C and D contained drug particles with the same D50 and the similar level of fine lactose contents (Table I), the difference in MMAD between Aerolizer/Formulation C and Aerolizer/Formulation D were likely attributed to differences in physicochemical properties of lactose. This result again highlighted the importance of physicochemical properties of drug carriers other than the particle size distribution in determining the in vitro performance of DPIs.
Although greater flow dependence was observed in ED for Aerolizer/Formulations C and D than for the reference product, viewed broadly, flow dependence in ISM, FPM, FPF, and MMAD were generally similar (Table II). Each of these measures required further lowering to more closely match the reference product. Thus, attempts were further made to reduce efficiency of these two investigational products through device modifications.
Device Modifications
The specific resistance of the Aerolizer was over 40 % greater than that of the Rotahaler (0.017 vs 0.012 kPa1/2 min/L). The higher resistance device requires a greater inspiratory effort (pressure drop) for a given flow rate, and is generally associated with greater turbulence, shear, and/or impaction, resulting in more efficient dispersion (20,25). Therefore, reducing the specific resistance of the Aerolizer device would be expected to reduce the dispersion of the investigational formulations. As indicated in the Part II, Results section, two types of device modifications were introduced to lower the specific resistance to more closely match that of the reference product.
Using the unmodified devices, Aerolizer/Formulation D was closest in performance to the reference drug at 60 LPM. Of the formulations tested in the two modified devices, Formulation D in Modified Aerolizer A trended closer to the in vitro performance of the reference product at 60 LPM (Table II) for most of measures such as ED (%), ISM, FPM, and FPF. However, MMAD remained higher than the reference product and was unchanged from the unmodified Aerolizer/Formulation D (Table II).
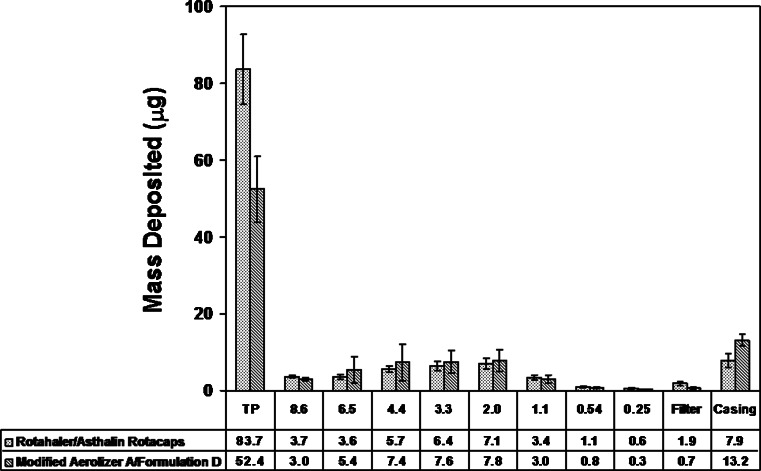
Formulation A was initially prepared in an effort to match the Rotacaps formulation, assuming a drug particle size near 1.9 μm. Table III provides mean aerodynamic measures and point estimates of the test/reference ratio at 60 LPM for Aerolizer/Formulation A and for Modified Aerolizer A/Formulation D, the product that trended closest in performance to the Reference Product. A comparison of cascade impactor deposition profiles for the Modified Aerolizer A/Formulation D with the Reference Product (Fig. 3) suggested the trend towards aerodynamic particle size comparability at 60 LPM.
Table III.
Mean Values and Point Estimates for the Reference Product at 60 LPM, a Screening Test Product, and Modified Aerolizer A/Formulation D
| Emitted dose, μg | Throat + preseparator deposition, μg | Impactor-sized mass, μg | Fine particle dose, μg | Fine particle fraction, % | MMAD, μm | |
|---|---|---|---|---|---|---|
| Aerolizer/form A | 158 | 65.3 | 89.6 | 69.6 | 44.0 | 2.51 |
| Reference product | 125 | 83.7 | 37.7 | 26.2 | 20.9 | 3.72 |
| Point estimate | 1.26 | 0.78 | 2.38 | 2.66 | 2.11 | 0.67 |
| Modified Aerolizer A/formulation D | 102 | 52.4 | 46.3 | 27.6 | 26.4 | 4.22 |
| Reference product | 125 | 83.7 | 37.7 | 26.2 | 20.9 | 3.72 |
| Point estimate | 0.81 | 0.63 | 1.23 | 1.05 | 1.26 | 1.13 |
Fig. 3.
Comparison of mean (SD) drug (micrograms) on cascade impactor deposition sites at 60 LPM. Effective cutoff diameters (micrometers) are listed for stages 1 (8.6 μm) to 6 (0.25 μm)
Impact of Formulation Versus Device
The two device modifications were expected to influence in vitro performance as a result of the changes in airflow pathways and device resistance. Examination of the performance of Formulation C in the Aerolizer, Modified Aerolizer A, and Modified Aerolizer B at 60 LPM (Figs. 4 and 5) revealed small to moderate mean differences between products. For all measures, mean differences were less than 17 %. For the Modified Aerolizer devices prepared ad hoc, product variability tended to be greater than for the Aerolizer product (Table II). Results show that for Formulation C, despite a significant difference in device-specific resistance, the three devices tested had modest effect on performance at 60 LPM.
Fig. 4.
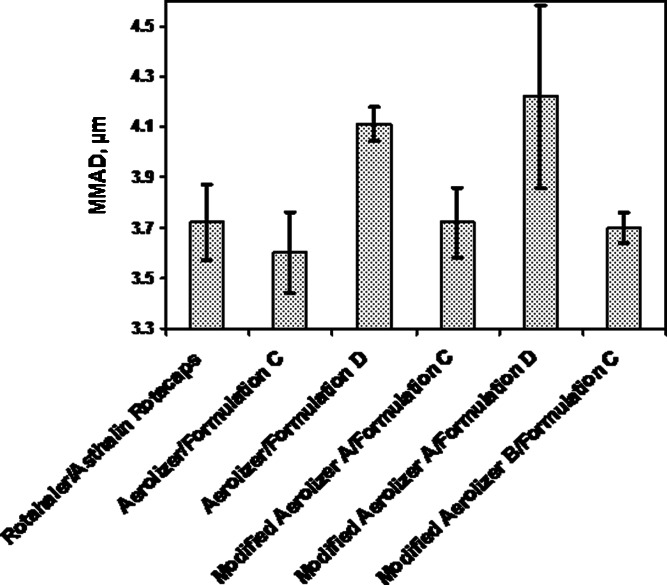
Mean (SD) MMAD and device deposition for selected test products relative to the reference product at 60 LPM
Fig. 5.
Mean (SD) aerodynamic measures for the Aerolizer and two Aerolizer modifications tested with Formulation C at 60 LPM
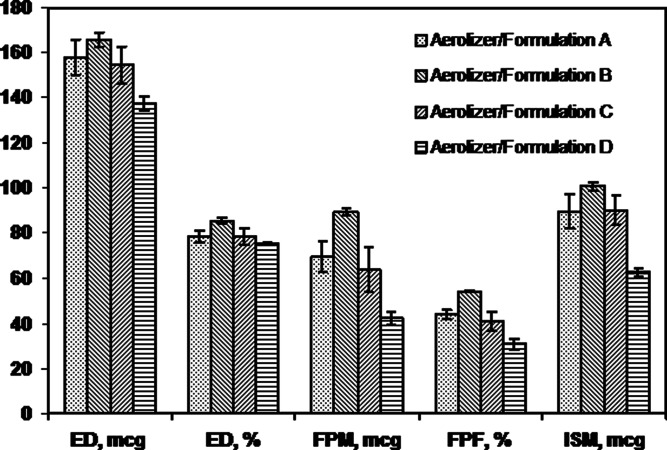
Figures 4 and 6 indicate the dependence of product performance on formulation when the four formulations were tested in the same device. As expected, in vitro product performance is affected appreciably by formulation.
Fig. 6.
Mean (SD) ACI measures for the Aerolizer tested with four test formulations
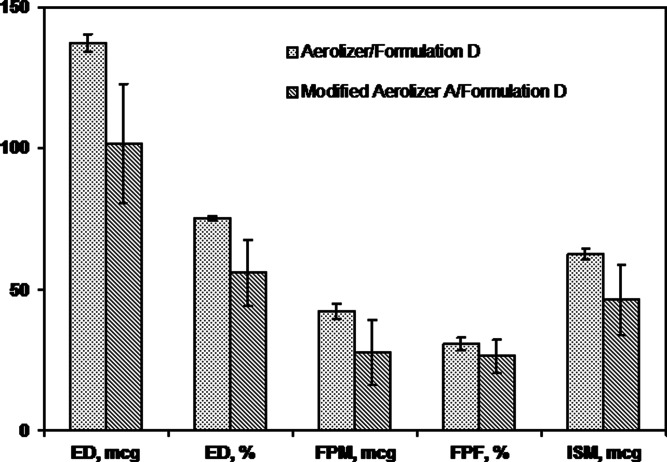
When Formulation D was tested in the unmodified Aerolizer or Modified Aerolizer A at 60 LPM (Figs. 4 and 7), differences between products were greater than differences for Formulation C tested with these two devices. Mean differences ranged from about 14–26 % were observed for ED, ED%, ISM, and FPM, even though data for the Modified Aerolizer A/Formulation D were much more variable than for Aerolizer/Formulation D. Thus, device modification had a greater impact on performance with Formulation D than with Formulation C. Furthermore, Rotahaler versus Aerolizer device differences tested with Rotacaps at 60 LPM revealed even greater differences in mean ACI measures, ranging from 18 to 63 % (Table II). The above results suggested device characteristics also can have an effect on DPI performance, but the magnitude seems to be formulation-dependent.
Fig. 7.
Mean (SD) ACI measures for the Aerolizer and Modified Aerolizer A tested with Formulation D
The above observations indicate that in addition to device resistance, further characterization of fluid mechanics or flow characteristics of these devices and their interactions with formulation properties are needed (26–29). Such information would be helpful in designing modifications of both device and formulation in order to achieve in vitro comparability of test and reference DPIs.
CONCLUSIONS
A test product recognized to have much greater efficiency of dispersion was modified in an effort to bring its in vitro performance closer to that of a relatively inefficient reference product. This was not intended to be a strategy for product development but represented a simple approach to the objective of product matching for research purposes. Changes were made that produced a product that trended towards the reference product in a number of measures. The current study suggests that a simple approach to the development of a test DPI product that meets in vitro equivalence criteria will be challenging, and that the drug and excipient particle sizes, lactose fine particle content and device-specific resistance are important design variables, but may not be sufficient to attain in vitro equivalence. Additional studies will be needed to identify and evaluate other product design variables that may be critical to achieving a better in vitro match between test and reference DPI products. Undoubtedly, a comprehensive approach to device design and formulation development in the context of specific products would have greater potential to achieve the objective of achieving performance equivalence. This would require significant investment of time and resources which may explain the absence of such products from the marketplace at this time.
ACKNOWLEDGMENTS
We would like to thank Maureen Stewart, Visual Information Specialist, CDER, FDA, Silver Spring, MD, for preparing the illustrations of the two Modified Aerolizers.
Footnotes
This article represents the personal opinions of the authors and does not necessarily represent the views or policies of the US Food and Drug Administration.
REFERENCES
- 1.US Environmental Protection Agency (EPA) Protection of stratospheric ozone; labeling. Fed Regist. 1993;58:8136–69. [Google Scholar]
- 2.US Department of Health and Human Services (DHHS). Food and Drug Administration (FDA) Use of ozone-depleting substances; essential use determinations. Fed Regist. 1999;64:47719–41. [Google Scholar]
- 3.Lee SL, Adams WP, Li BV, Conner DP, Chowdhury BA, Yu LX. in vitro considerations to support bioequivalence of locally acting drugs in dry powder inhalers for lung diseases. AAPS J. 2009;11:414–23. doi: 10.1208/s12248-009-9121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fults KA, Miller IF, Hickey AJ. Effect of particle morphology on emitted dose of fatty acid-treated disodium cromoglycate powder aerosols. Pharm Dev Technol. 1997;2:67–79. doi: 10.3109/10837459709022610. [DOI] [PubMed] [Google Scholar]
- 5.Dunbar CA, Hickey AJ, Holzner P. Dispersion and characterization of pharmaceutical dry powder aerosols. Kona. 1998;16:7–45. [Google Scholar]
- 6.Gonda I. Targeting by deposition. In: Hickey AJ, editor. Pharmaceutical inhalation aerosol technology. 2. New York: Marcel Dekker; 2004. pp. 65–88. [Google Scholar]
- 7.Telko MJ, Hickey AJ. Dry powder inhaler formulation. Respir Care. 2005;50:1209–27. [PubMed] [Google Scholar]
- 8.Donovan MJ, Smyth HD. Influence of size and surface roughness of large lactose carrier particles in dry powder inhaler formulations. Int J Pharm. 2010;402:1–9. doi: 10.1016/j.ijpharm.2010.08.045. [DOI] [PubMed] [Google Scholar]
- 9.Guenette E, Barrett A, Kraus D, Brody R, Harding L, Magee G. Understanding the effect of lactose particle size on the properties of DPI formulations using experimental design. Int J Pharm. 2009;380:80–8. doi: 10.1016/j.ijpharm.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 10.USP Advisory Panel on Aerosols Recommendations of the USP advisory panel on aerosols on the USP general chapters on aerosols <601> and uniformity of dosage units <905>. Pharm Forum. 1994;20:7477–7503. [Google Scholar]
- 11.Frijlink HW, De Boer AH. Dry powder inhalers for pulmonary drug delivery. Expert Opin Drug Deliv. 2004;1:67–86. doi: 10.1517/17425247.1.1.67. [DOI] [PubMed] [Google Scholar]
- 12.Clark AR, Hollingworth AM. The relationship between powder inhaler resistance and peak inspiratory conditions in healthy volunteers—implications for in vitro testing. J Aerosol Med. 1993;6:99–110. doi: 10.1089/jam.1993.6.99. [DOI] [PubMed] [Google Scholar]
- 13.Martin GP, Zeng XM, Tee SK, Abu Ghoush A, Marriott C. Effects of particle size and adding sequence of fine lactose on the deposition of salbutamol sulphate from a dry powder formulation. Int J Pharm. 1999;182:133–44. doi: 10.1016/S0378-5173(99)00021-6. [DOI] [PubMed] [Google Scholar]
- 14.Menzeleev R, Bovet J-M, Hickey AJ. Unit dose level particle size distribution characterization of inhalation products: a new analytical approach using LC-MSD as a platform. In: Dalby RN, Byron PR, Peart J, Suman JD, Farr SL, editors. Respiratory drug delivery IX. River Grove: Davis Healthcare International Publishing; 2004. pp. 453–6. [Google Scholar]
- 15.<601> Aerosols . Metered-dose inhalers and dry powder inhalers, data analysis. Rockville: U.S. Pharmacopeia 31/National Formulary 26, United States Pharmacopeial Convention; 2008. [Google Scholar]
- 16.Berg RL, Svensson JO, Asking L. MMAD based on dose to impactor rather than on delivered dose. In: Dalby RN, Byron PR, Peart J, Farr SL, editors. Respiratory drug delivery VIII. Godalming: Davis Horwood International; 2002. pp. 339–42. [Google Scholar]
- 17.<601> Aerosols . Metered-dose inhalers and dry powder inhalers, sampling the delivered dose from dry powder inhalers. Rockville: U.S. Pharmacopeia 31/National Formulary 26, United States Pharmacopeial Convention; 2008. [Google Scholar]
- 18.Saleem I, Smyth H, Telko M. Prediction of dry powder inhaler formulation performance from surface energetics and blending dynamics. Drug Dev Ind Pharm. 2008;34:1002–10. doi: 10.1080/03639040802154905. [DOI] [PubMed] [Google Scholar]
- 19.Hindle M, Byron PR. Dose emissions from marketed dry powder inhalers. Int J Pharm. 1995;116:169–77. doi: 10.1016/0378-5173(94)00287-F. [DOI] [Google Scholar]
- 20.Byron PR. Drug delivery devices: issues in drug development. Proc Am Thorac Soc. 2004;1:321–8. doi: 10.1513/pats.200403-023MS. [DOI] [PubMed] [Google Scholar]
- 21.Chew NYK, Chan HK. in vitro aerosol performance and dose uniformity between the Foradile (R) Aerolizer (R) and the Oxis (R) Turbuhaler (R) J Aerosol Med. 2001;14:495–501. doi: 10.1089/08942680152744703. [DOI] [PubMed] [Google Scholar]
- 22.Bridson RH, Robbins PT, Chen Y, Westerman D, Gillham CR, Roche TC, et al. The effects of high shear blending on alpha-lactose monohydrate. Int J Pharm. 2007;339:84–90. doi: 10.1016/j.ijpharm.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 23.Jones MD, Hooton JC, Dawson ML, Ferrie AR, Price R. An investigation into the dispersion mechanisms of ternary dry powder inhaler formulations by the quantification of interparticulate forces. Pharm Res. 2008;25:337–48. doi: 10.1007/s11095-007-9467-1. [DOI] [PubMed] [Google Scholar]
- 24.Shur J, Harris H, Jones MD, Kaerger JS, Price R. The role of fines in the modification of the fluidization and dispersion mechanism within dry powder inhaler formulations. Pharm Res. 2008;25:1931–40. doi: 10.1007/s11095-008-9538-y. [DOI] [PubMed] [Google Scholar]
- 25.Louey MD, Van Oort M, Hickey AJ. Aerosol dispersion of respirable particles in narrow size distributions produced by jet-milling and spray-drying techniques. Pharm Res. 2004;21:1200–6. doi: 10.1023/B:PHAM.0000033007.27278.60. [DOI] [PubMed] [Google Scholar]
- 26.Coates MS, Chan HK, Fletcher DF, Raper JA. Influence of air flow on the performance of a dry powder inhaler using computational and experimental analyses. Pharm Res. 2005;22:1445–53. doi: 10.1007/s11095-005-6155-x. [DOI] [PubMed] [Google Scholar]
- 27.Coates MS, Chan HK, Fletcher DF, Raper JA. Effect of design on the performance of a dry powder inhaler using computational fluid dynamics. Part 2: air inlet size. J Pharm Sci. 2006;95:1382–92. doi: 10.1002/jps.20603. [DOI] [PubMed] [Google Scholar]
- 28.Coates MS, Fletcher DF, Chan HK, Raper JA. Effect of design on the performance of a dry powder inhaler using computational fluid dynamics. Part 1: grid structure and mouthpiece length. J Pharm Sci. 2004;93:2863–76. doi: 10.1002/jps.20201. [DOI] [PubMed] [Google Scholar]
- 29.Coates MS, Fletcher DF, Chan HK, Raper JA. The role of capsule on the performance of a dry powder inhaler using computational and experimental analyses. Pharm Res. 2005;22:923–32. doi: 10.1007/s11095-005-4587-y. [DOI] [PubMed] [Google Scholar]