Abstract
Vascular endothelial growth factor (VEGF) is a key mediator of angiogenesis, whose effect on cancer growth and development is well characterized. Alternative splicing of VEGF leads to several different isoforms, which are differentially expressed in various tumor types and have distinct functions in tumor blood vessel formation. Many cancer therapies aim to inhibit angiogenesis by targeting VEGF and preventing intracellular signaling leading to tumor vascularization; however, the effects of targeting specific VEGF isoforms have received little attention in the clinical setting. In this work, we investigate the effects of selectively targeting a single VEGF isoform, as compared with inhibiting all isoforms. We utilize a molecular-detailed whole-body compartment model of VEGF transport and kinetics in the presence of breast tumor. The model includes two major VEGF isoforms, VEGF121 and VEGF165, receptors VEGFR1 and VEGFR2, and co-receptors Neuropilin-1 and Neuropilin-2. We utilize the model to predict the concentrations of free VEGF, the number of VEGF/VEGFR2 complexes (considered to be pro-angiogenic), and the receptor occupancy profiles following inhibition of VEGF using isoform-specific anti-VEGF agents. We predict that targeting VEGF121 leads to a 54% and 84% reduction in free VEGF in tumors that secrete both VEGF isoforms or tumors that overexpress VEGF121, respectively. Additionally, 21% of the VEGFR2 molecules in the blood are ligated following inhibition of VEGF121, compared with 88% when both isoforms are targeted. Targeting VEGF121 reduces tumor free VEGF and is an effective treatment strategy. Our results provide a basis for clinical investigation of isoform-specific anti-VEGF agents.
Electronic supplementary material
The online version of this article (doi:10.1208/s12248-012-9363-4) contains supplementary material, which is available to authorized users.
Key words: angiogenesis, cancer drug target, computational model, pharmacokinetic model, systems biology
INTRODUCTION
Vascular endothelial growth factor (VEGF) is a potent promoter of angiogenesis, the formation of new blood vessels from pre-existing vasculature. Angiogenesis is required for cancer growth and development, and given the key role of VEGF in this process, cancer therapies targeting VEGF have gained prominence. Therapeutics such as antibodies against VEGF and its receptors, small molecule tyrosine kinase inhibitors, and peptides have been developed. These anti-angiogenic agents inhibit tumor growth and development by blocking VEGF-mediated signaling that promotes cell proliferation, migration, and adhesion, ultimately leading to vascularization.
The VEGF family consists of five ligands (VEGF-A through VEGF-D and placental growth factor, PlGF), three receptors (VEGFR1, VEGFR2, and VEGFR3), and two co-receptors, called neuropilins (NRP1 and NRP2). VEGF-A, often referred to as VEGF, is a well-studied member of this signaling family, and alternative splicing of VEGF produces numerous isoforms: VEGF121, VEGF121b, VEGF145, VEGF145b, VEGF165, VEGF165b, VEGF183, VEGF189, and VEGF206. The VEGFxxx species are considered pro-angiogenic, while VEGFxxxb molecules are generally regarded as endogenous anti-angiogenic species (1–5) or weakly angiogenic isoforms (6). The VEGF isoforms have unique binding profiles with the receptors and co-receptors. The molecular interactions of VEGF121 and VEGF165, in particular, have been widely studied. Co-receptors NRP1 and NRP2 form ternary complexes with VEGF and VEGFR, either through direct binding to VEGF165 (7) or through coupling to VEGFR1 (8). In addition, VEGF165 contains a heparin-binding domain (9), enabling it to bind to glycosaminoglycan (GAG) chains in the extracellular matrix (ECM) and cellular basement membranes (10). In contrast, VEGF121 is freely diffusible in the tissue interstitium (10).
In addition to having different binding profiles, VEGF isoforms perform unique functions in tumor vascularization. Experimental studies performed in mice using tumors that express the VEGF120, VEGF164, and VEGF188 isoforms individually (mouse orthologs of 121, 165, and 189) have different vascular structures from wild-type mice where the three isoforms are expressed together. Tumors that only express VEGF120 have lower microvessel density than wild-type mice, while the vascularity of VEGF164-expressing tumors closely resembles wild-type (11). Additionally, tumors that only express VEGF188 generate hypervascular intratumoral capillary networks. Based on these results, Grunstein and co-workers propose a gradient model of VEGF signaling in the tumor where individual isoforms contribute to tumor vascularization: VEGF120 recruits systemic, peripheral vessels; VEGF188 promotes angiogenesis within the tumor; and VEGF164 generates intermediate results (11). Tozer et al. found that vascular volume is highest in VEGF120 tumors, whereas VEGF188 tumors have significantly longer vessels (12). In human melanoma xenografts, VEGF165-overexpressing tumors are highly vascularized while VEGF121 tumors are characterized by sparse vessels and necrotic areas (13). These studies and others also investigate a correlation between VEGF isoform expression and tumor progression (14–17). Another study finds that VEGF121-expressing breast tumor xenografts are more evenly vascularized, leading to better oxygenation, compared with tumors that overexpress VEGF165 or fibroblast growth factor-1 (18). Given the differences observed in tumors that overexpress particular isoforms, it is possible that targeting specific VEGF isoforms may be an effective cancer therapy.
Four VEGF-neutralizing agents have either gained approval by the Food and Drug Administration (FDA) or are currently in clinical trials: bevacizumab (Avastin; Genentech), pegaptanib (Macugen; Eyetech/Pfizer), ranibizumab (Lucentis; Genentech), and aflibercept (VEGF-Trap; Regeneron). Bevacizumab, the recombinant humanized monoclonal antibody to VEGF, is the only VEGF inhibitor approved for the treatment of cancer (colorectal cancer, non-small cell lung cancer, glioblastoma, and kidney cancer). For these indications, bevacizumab works alone or in combination with other drugs such as chemotherapy agents and interferon. Additionally, it is used to treat wet age-related macular degeneration (AMD). Both pegaptanib, an RNA aptamer, and ranibizumab, a monoclonal antibody fragment, are FDA-approved for the treatment of AMD; however, the latter is used more frequently due to a better ability to maintain and restore vision. Pegaptanib is also being applied in glioma, in combination with radiation therapy (19). Lastly, aflibercept is a soluble decoy receptor that was recently approved for treatment of AMD and is currently in clinical trials for other eye diseases and several cancer types. Bevacizumab, ranibizumab, and aflibercept are able to bind all VEGF-A isoforms. In addition, aflibercept also binds placental growth factor. In contrast, pegaptanib selectively binds VEGF165, while having low affinity for other isoforms (20). VEGF165 was selected as the target because this isoform is generally thought to be the predominant VEGF-A isoform in the eye (21,22). Thus, there is a precedent for isoform-specific VEGF inhibition.
Mathematical models applying systems biology tools have proved useful in providing insight into the complex VEGF interactions, testing hypotheses not easily accessible via experiments, and designing therapeutics (23,24). The recent action by the FDA to revoke approval of bevacizumab for breast cancer demonstrates the need to better understand the effects of VEGF-neutralizing agents in order to develop effective treatment strategies. We aim to address this need by predicting the potential therapeutic effects of isoform-specific VEGF antibodies, thus elucidating their mechanism of action.
We have previously developed a compartment model of VEGF transport and kinetics in the human body and applied the model to predict how free VEGF levels respond to anti-VEGF treatment (25,26). However, we did not consider the effects of targeting single VEGF isoforms, as compared with inhibiting all isoforms simultaneously. Utilizing isoform-specific antibodies may be an effective treatment strategy, since experimental data shows that the VEGF isoforms differentially contribute to vascular patterning in tumors, leading to variations in tumor growth, as described above. Therefore, we utilize our model of VEGF kinetics and transport to predict the concentrations of free VEGF and the VEGF/VEGFR2 complexes, and the receptor occupancy profiles following isoform-specific anti-VEGF therapy. In addition, we investigate how free VEGF is affected by isoform-specific anti-VEGF agents in tumors that preferentially secrete different VEGF isoform(s).
METHODS
Computational Model
We utilize a molecular-detailed compartment model of VEGF kinetics and transport in the human body. The model includes normal tissue (represented by skeletal muscle), blood, and tumor (parameterized as a breast tumor; however, the model is applicable to any solid tumor). We include the two major VEGF isoforms VEGF121 and VEGF165, receptors VEGFR1 and VEGFR2, and co-receptors NRP1 and NRP2. VEGF is secreted by parenchymal cells (muscle fibers in the normal compartment and tumor cells in the diseased compartment), and the molecular interactions are illustrated in Fig. 1. VEGF transport between compartments occurs via microvascular permeability, and we simulate lymphatic drainage from the interstitial space of the normal tissue into the blood (tumor lymphatics are assumed to be non-functional, based on experimental evidence (27,28)). Additionally, free VEGF is subjected to protein degradation in the tissue compartments and is cleared from the blood. VEGF receptors and co-receptors are localized on the abluminal and luminal endothelial surfaces, as well as on parenchymal cells. Receptor density is based on in vitro and in vivo experimental data from quantitative flow cytometry (25,29). The full model has been described in our previous papers (25), and the complete set of model equations and parameters are given in Electronic supplementary material 1.
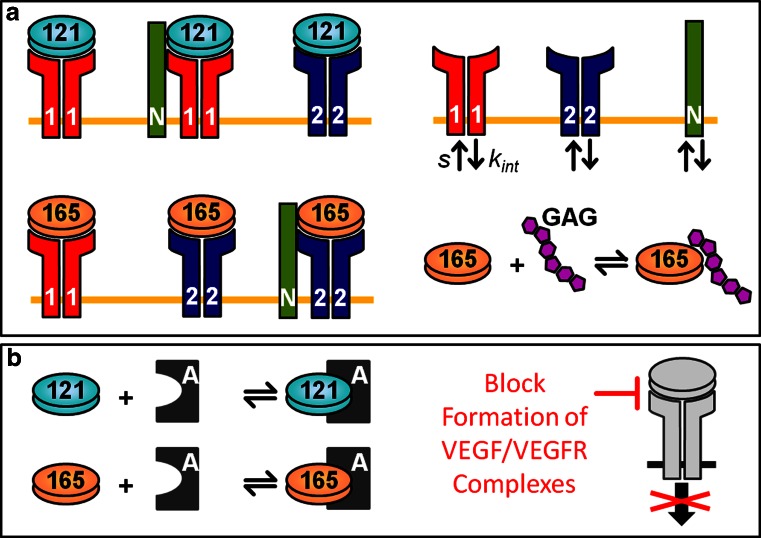
Fig. 1.
Schematic of VEGF interactions and neutralization. a The compartment model simulates the molecular interactions of two VEGF isoforms (VEGF121 and VEGF165), receptors (VEGFR1 and VEGFR2), and co-receptors (NRP1 and NRP2). VEGF receptors and co-receptors are expressed on the abluminal and luminal endothelial surfaces, muscle fibers, and tumor cells and are internalized (k int) and inserted into the cell membrane (s). VEGF121 binds to VEGFR1 and VEGFR2 and can form a ternary complex with the coupled VEGFR1–NRP1 complex. VEGF165 binds to VEGFR1 and can form the ternary complex VEGF165–VEGFR2–NRP by binding to VEGFR2 or one of the NRPs. In addition, VEGF165 can be sequestered by GAG chains in the ECM and cellular basement membranes. b VEGF-neutralizing agents bind to the isoforms to block the formation of VEGF/VEGFR complexes and inhibit intracellular angiogenic signaling
Numerical Implementation
The model predicts the concentration of 89 species and is described by 89 non-linear ordinary differential equations (ODEs), which include 24 for the normal compartment, 27 for the blood, and 38 for the tumor compartment. The ODEs and parameters were implemented in MATLAB (v7.11.0.584 R2010b, Mathworks) using the SimBiology toolbox. The steady-state and dynamic solutions were calculated using the Sundials solver, where 10−9 and 10−20 were used for the absolute and relative tolerances, respectively. The model is provided in SBML format in Electronic supplementary material 2, and instructions for its use are given in the notes section of the model.
Simulation of VEGF-Targeting Agents
We investigate the effect of isoform-specific anti-VEGF agents (denoted by anti-VEGF121 and anti-VEGF165). For comparison, we also simulate the effect of a VEGF antibody that sequesters both isoforms (pan anti-VEGF). In each simulation, the system is first allowed to reach a steady state, and then the anti-VEGF agent is administered via an intravenous infusion (i.e., into the blood compartment). A single dose of 10 mg/kg is given, which is within the range clinically used when treating various forms of cancer (30). The kinetic parameters of the anti-VEGF agent are based on bevacizumab (31–33); however, the parameters can easily be changed to simulate other VEGF antibodies or VEGF-neutralizing macromolecules.
We use the change in free VEGF at 3 weeks following administration of the anti-VEGF compared with the pre-treatment, steady-state level, as an indication of the response to therapy. This comparison is calculated using the fold-change,
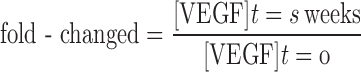 |
1 |
The fold-change indicates whether free VEGF increases (fold-change > 1), decreases (fold-change < 1), or is unchanged (fold-change = 1) following anti-VEGF treatment. This parameter is calculated for all compartments; however, we propose that the anti-VEGF has a therapeutic effect when tumor free VEGF decreases following treatment, relative to the pre-treatment level.
RESULTS
Targeting VEGF121 Is Most Effective in Reducing Tumor Free VEGF
Blood
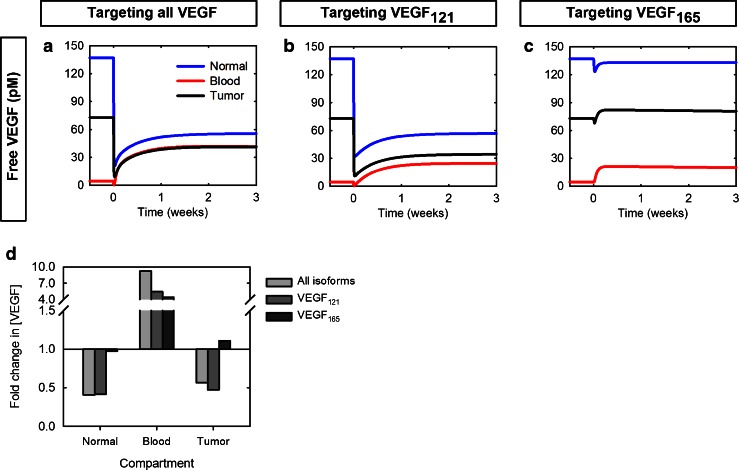
We have previously shown that, for a specific set of parameters, inhibiting both VEGF isoforms leads to a transient decrease in free VEGF in the blood followed by a 9.2-fold increase above pre-treatment levels (25). The model predicts that isoform-specific anti-VEGF agents also lead to an increase in free VEGF in the blood (Fig. 2a–c); however, targeting VEGF165 results in a relatively small increase in the concentration of free VEGF, compared with the other agents, as indicated by the fold-change (Fig. 2d). Anti-VEGF165 treatment induces a fold-change of 4.3, compared with 5.4 or 9.2 when targeting VEGF121 or both isoforms, respectively.
Fig. 2.
Effect of targeting VEGF isoforms on free VEGF concentration in the body. Free VEGF concentration profiles following a single intravenous injection of 10 mg/kg anti-VEGF given at time 0. Three cases were considered: a pan anti-VEGF targeting both VEGF isoforms. b anti-VEGF121 agent. c anti-VEGF165 agent. d the fold-change in free VEGF concentration following targeting all isoforms simultaneously (light gray), VEGF121 (dark gray), or VEGF165 (black)
Tissue Compartments
We also predicted that targeting both VEGF isoforms results in a depletion of free VEGF relative to the pre-treatment level in the normal tissue and tumor of 0.4- and 0.6-fold, respectively (25). Targeting VEGF121 produces similar effects as targeting both isoforms and is most effective in reducing tumor free VEGF, where the fold-change is predicted to be 0.5 (Fig. 2d). In comparison, targeting VEGF165 does not significantly influence free VEGF levels in these compartments (Fig. 2a–c). Following anti-VEGF165 treatment, the fold-change is 0.97 and 1.1 in the normal tissue and tumor, respectively (Fig. 2d).
Receptor Occupancy Is Influenced by Isoform-Specific Anti-VEGF Treatment
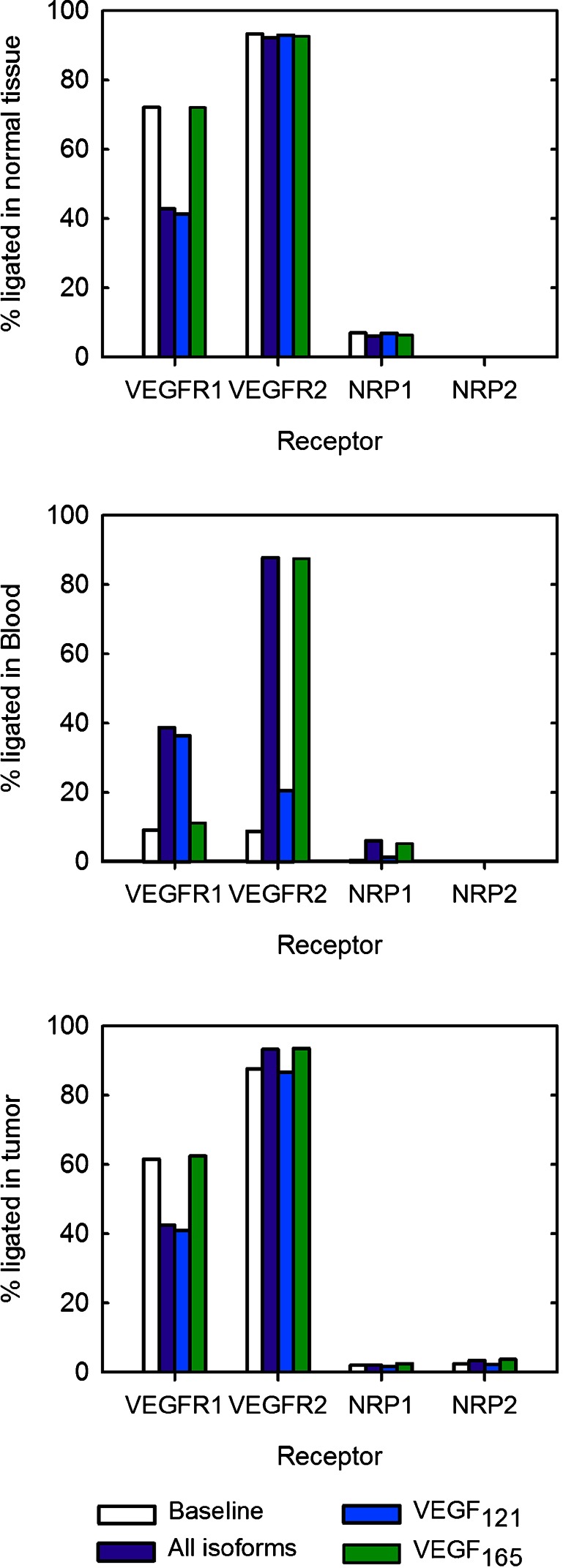
In addition to investigating the effect of anti-VEGF treatment on free VEGF levels, we have used receptor occupancy as a measure of the angiogenic state of the body. Here, we compare the baseline receptor occupancy predicted at steady state prior to anti-VEGF treatment to the occupancy 3 weeks after treatment (Fig. 3).
Fig. 3.
Effect of targeting specific VEGF isoforms on receptor occupancy. The percentage of ligated receptors. From top to bottom: normal tissue, blood, and tumor
Normal Tissue
Targeting all isoforms and targeting VEGF121 individually produce similar effects, and VEGFR1 occupancy is reduced to ∼40%, as compared with the baseline value when 72% of VEGFR1 molecules are ligated. In contrast, anti-VEGF165 treatment does not significantly influence VEGFR1 occupancy from the steady-state levels. VEGFR2 and NRP1 occupancy are nearly unchanged for all anti-VEGF agents.
Blood
Anti-VEGF treatment increases occupancy for all receptors, irrespective of the isoform(s) targeted. This is due to the increase in free VEGF following anti-VEGF treatment, described above. Following treatment with the pan anti-VEGF or anti-VEGF121 agent, VEGFR1 occupancy becomes four times greater than the baseline value. It is interesting to note that targeting both isoforms or VEGF165 individually dramatically increases VEGFR2 occupancy, where the percentage of ligated VEGFR2 molecules increases tenfold, while anti-VEGF121 treatment increases VEGFR2 occupancy 2.3-fold. The fraction of ligated NRP1 increases from the baseline value of 0.4% to 6% when targeting both isoforms. When targeting VEGF121 or VEGF165, the fraction of ligated NRP1 increases to 1.3% or 5%, respectively.
Tumor
Targeting all isoforms or VEGF121 individually results in a decrease in the occupancy of VEGFR1 from 62% to approximately 41%. In contrast, anti-VEGF165 treatment does not significantly change VEGFR1 occupancy. All of the anti-VEGF agents produce a similar effect on the percentage of VEGFR2 that is bound to the ligand, where the occupancy ranges from 87% to 94%, depending on the isoform(s) targeted by the anti-VEGF. Similarly, the fractions of ligated neuropilins remain relatively unchanged, where 2–4% of NRPs are bound to VEGF.
In summary, the various anti-VEGF agents differentially influence receptor occupancy. In the tissue compartments, VEGFR1 occupancy is decreased following treatment with the pan anti-VEGF or VEGF121 agent, while VEGFR2 occupancy is only slightly different from the baseline value. In the blood, the occupancies of both VEGFR1 and VEGFR2 vary with anti-VEGF treatment, and a larger percentage of receptors are ligated following anti-VEGF treatment.
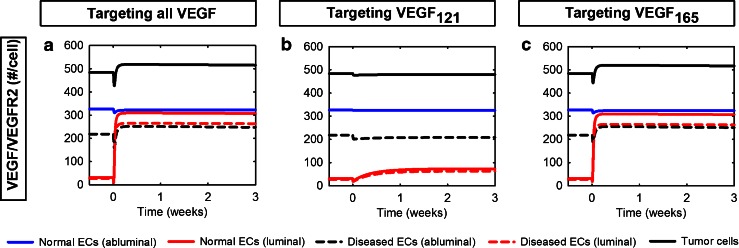
Targeting VEGF121 Effectively Inhibits the Formation of the Pro-angiogenic Complex VEGF/VEGFR2 in the Body
VEGF is able to stimulate proliferation and regulate vessel permeability leading to capillary sprouting by binding to and activating its receptors. The VEGF/VEGFR2 complex in particular is considered to be pro-angiogenic. Therefore, in addition to investigating the receptor occupancy profiles, we have predicted the number of VEGF/VEGFR2 molecules/cell following anti-VEGF treatment. The number of VEGF/VEGFR2 molecules per cell responds in a similar manner whether targeting both VEGF isoforms or VEGF165 individually (Fig. 4). In both cases, the number of VEGF/VEGFR2 molecules on the abluminal endothelial surface in the normal compartment changes by less than 2%, and the concentration of luminal VEGF/VEGFR2 molecules increases nearly tenfold in the blood. Additionally, the number of VEGF/VEGFR2 molecules on tumor cells increases 15% and 7% on tumor ECs and tumor cells, respectively. Anti-VEGF121 treatment does not impact VEGF/VEGFR2 levels in the normal tissue. However, in the tumor compartment, the number of VEGF/VEGFR2 molecules decreases 5% and less than 1% for abluminal diseased endothelial and tumor cells, respectively. In the blood, treatment with the anti-VEGF121 agent leads to a 1.3-fold increase in the concentration of VEGF/VEGFR2 molecules on the luminal endothelial surfaces, which is much lower than the tenfold increase induced by the other agents. These results show that targeting VEGF121 effectively inhibits the formation of the pro-angiogenic complex VEGF/VEGFR2 in the body.
Fig. 4.
Formation of VEGF/VEGFR2 complexes in response to isoform-specific anti-VEGF treatment. The number of VEGF/VEGFR2 complexes in the normal tissue, blood, and tumor is calculated following isoform-specific VEGF treatments: a Pan anti-VEGF. b Anti-VEGF121 agent. c Anti-VEGF165 agent
Isoform-Specific Agents Have Different Effects on Free VEGF in Tumors That Preferentially Secrete One or Both VEGF Isoforms
We have previously shown that, when targeting all VEGF isoforms, a therapeutic effect (reduction in tumor free VEGF at 3 weeks post-treatment) is observed when VEGF121 comprises more than 25% of the total VEGF secreted by the tumor (25). We now investigate the effect of isoform-specific anti-VEGF treatment on the level of free VEGF in tumors that preferentially secrete a single VEGF isoform. We predict the dynamic concentration profiles of free VEGF (Electronic supplementary material 3, Figure S1) and summarize the results by estimating the fold-change of free VEGF in the body at 3 weeks following anti-VEGF treatment for isoform-specific tumors (Fig. 5) and for tumors that secrete different ratios of VEGF121 and VEGF165 (Fig. 6). In all simulation cases, the total tumor VEGF secretion rate was held constant.
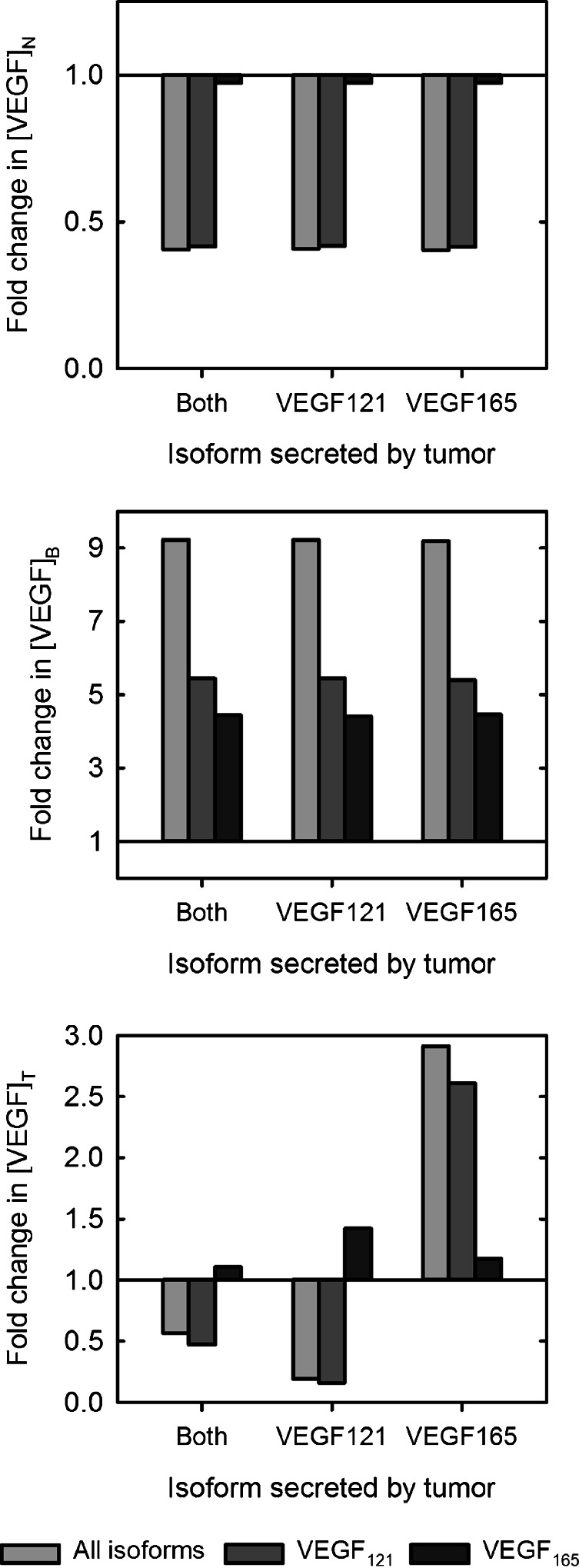
Fig. 5.
Response to isoform-specific treatment in tumors that preferentially secrete various VEGF isoform(s). The fold-change in free VEGF concentration in isoform-specific tumors following targeting all isoforms simultaneously (light gray), VEGF121 (dark gray), or VEGF165 (black). From top to bottom: normal tissue (subscript N), blood (subscript B), and tumor (subscript T)
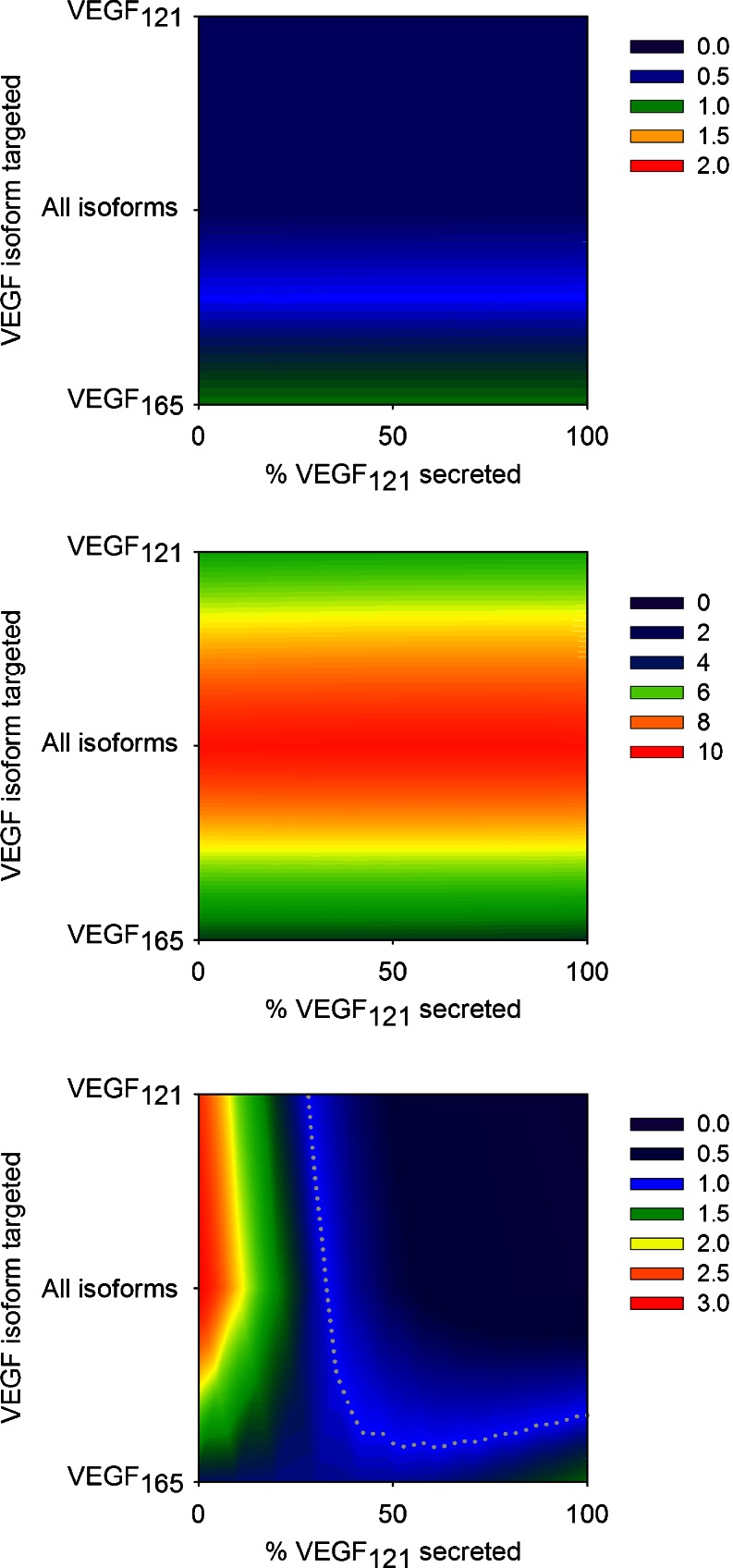
Fig. 6.
The effect of the tumor isoform secretion ratio on the response to isoform-specific treatment. The fold-change in free VEGF concentration is calculated as a function of the anti-VEGF agent and the tumor isoform secretion ratio. Three VEGF-neutralizing agents were examined: pan anti-VEGF, anti-VEGF121, and anti-VEGF165. The relative amount of VEGF121 secreted by the tumor was varied from zero to 100%. From top to bottom: normal tissue (subscript N), blood (subscript B), and tumor (subscript T). The gray dotted line in the bottom panel is the isocline for a fold-change of 1. Note that the scale is different for each panel
Isoform-Specific Tumors
Normal Tissue
When targeting all VEGF isoforms, free VEGF in the normal tissue is reduced from the pre-treatment level for all tumor types (fold-change is 0.4). Targeting VEGF121 induces a similar reduction in free VEGF in the normal tissue, where the fold-change is also predicted to be 0.4 for all tumor types (Fig. 5). In contrast, free VEGF levels in the normal tissue are only reduced by 3% when VEGF165 is targeted, even when the tumor secretes only VEGF165. This is because VEGF165 constitutes only 20% of free VEGF in the normal tissue at steady state for all tumor types. Therefore, targeting the VEGF165 isoform does not significantly influence free VEGF levels in this compartment.
Blood
None of the three VEGF antibodies examined in this study result in a depletion of free VEGF in the blood (Fig. 5). However, across all tumor types, targeting VEGF165 results in the smallest increase in free VEGF in the blood, where the fold-change is approximately 4.4, compared with 9.2 and 5.4 for the pan anti-VEGF and anti-VEGF121 agents, respectively.
Tumor
Inhibiting both VEGF isoforms depletes free VEGF in tumors that secrete both isoforms and in VEGF121-secreting tumors, where the fold-change is predicted to be 0.6 and 0.2, respectively. Anti-VEGF121 treatment reduces free VEGF relative to the pre-treatment level in tumors that secrete both isoforms and in VEGF121-secreting tumors in a similar fashion. In tumors that only secrete VEGF165, inhibiting both VEGF isoforms increases tumor free VEGF 2.9-fold. The anti-VEGF121 agent has a similar effect in VEGF165 tumors, where the fold-change in tumor free VEGF is 2.6. Inhibiting VEGF165 individually results in an increase in tumor free VEGF, and the fold-change ranges from 1.1 to 1.4, depending on the tumor type.
Tumors with Various VEGF Isoform Secretion Ratios
We also examined the effect of varying the tumor VEGF isoform secretion ratio, where the relative amount of VEGF121 secreted by tumor cells ranged from zero to 100% (Fig. 6). We find that the tumor VEGF isoform secretion ratio does not influence VEGF inhibition in the normal tissue, regardless of the isoform(s) being targeted. Similarly, the model predicts that the effect of VEGF neutralization in the blood does not depend on the tumor VEGF isoform secretion ratio. In contrast, the fold-change in tumor free VEGF is highly sensitive to the tumor isoform secretion ratio. When VEGF121 comprises at least 25% of total VEGF secreted, the anti-VEGF121 agent has a therapeutic effect. We previously predicted this response for the pan anti-VEGF (25). No tumor VEGF isoform secretion ratio allows a reduction in tumor free VEGF with anti-VEGF165 treatment.
We also investigated the effect of varying the dose of anti-VEGF administered, for a range of isoform secretion ratios. We examined how anti-VEGF dosages ranging from 1 to 25 mg/kg influenced the fold-change in free VEGF in the body (Electronic supplementary material 3, Figures S2–S4). The model predicts that decreasing doses of the pan anti-VEGF and anti-VEGF121 agents are less effective in reducing interstitial free VEGF. That is, lower doses lead to a higher fold-change. This is because the decrease in free VEGF in the normal tissue and tumor immediately after the intravenous injection is less pronounced for smaller doses, and tumor VEGF returns to its pre-treatment level more rapidly. Interestingly, for 1 mg/kg of the anti-VEGF165 agent, the fold-change in tumor free VEGF is slightly less than one in tumors whose isoform secretion ratio is shifted toward VEGF165. Additionally, lower doses of the anti-VEGF agents lead to a smaller increase in the concentration of free VEGF in the blood, compared with higher doses. For all anti-VEGF agents, increasing the dosage above 10 mg/kg does not largely impact the fold-change in VEGF in the body, for the full range of tumor isoform secretion ratios.
Altogether, these results suggest anti-VEGF121 treatment acts to reduce tumor free VEGF in tumors that secrete both VEGF isoforms and in VEGF121-secreting tumors, for the dosage levels prescribed to treat cancer.
DISCUSSION
We have applied a compartment model of VEGF kinetics and transport to investigate the effects of targeting specific VEGF isoforms, as compared with inhibiting all VEGF isoforms simultaneously. The model predicts that the pan anti-VEGF and anti-VEGF121 produce similar effects in free VEGF levels and receptor occupancy in all compartments. However, there are two primary distinctions between these treatments. Firstly, targeting VEGF121 does not produce the increase in the concentration of VEGF/VEGFR2 molecules in the blood and tumor that is predicted to occur when targeting both VEGF isoforms. Secondly, the percentage of VEGFR2 molecules in the blood that are ligated following anti-VEGF121 treatment is 21%, compared with 88% when the pan anti-VEGF agent is administered. Although both treatments result in an increase in plasma free VEGF, the smaller fraction of ligated VEGFR2 in the blood following anti-VEGF121 treatment indicates that the luminal endothelial surface is less poised to initiate intracellular signaling leading to proliferation, migration, and chemotaxis. Thus, the pro-angiogenesis signaling via VEGFR2 activation, both in the blood and the tumor, is diminished with anti-VEGF121 treatment. These anti-angiogenic effects occur to a lesser extent with the pan anti-VEGF agent and are not predicted to occur with anti-VEGF165 treatment. The primary reason for the lack of efficacy of anti-VEGF165 in reducing tumor free VEGF can be attributed to the relative fraction of unbound VEGF in the two isoforms. VEGF165 comprises just 7% of total tumor free VEGF prior to treatment. Thus, there is relatively little VEGF165 available for the antibody to neutralize, and targeting this isoform does not largely impact the level of free VEGF following anti-VEGF165 treatment. In addition, we have previously shown that allowing the anti-VEGF agent to bind VEGF sequestered in the extracellular matrix, a reservoir of VEGF165 comprising 25% of VEGF165 in the tumor, does not affect the level of tumor free VEGF at 3 weeks post-treatment (25). Based on these results, it may be of interest to target VEGF121, particularly since this isoform is involved in recruiting host vasculature (11).
Importance of the Relative Expression of VEGF Isoforms
The prediction that targeting VEGF121 inhibits VEGF-mediated angiogenesis is not immediately obvious. Some studies show that VEGF165 induces tumor vascularization (11,16,34), while other data highlight the importance of VEGF121 in tumor angiogenesis (35,36). However, these studies agree in concluding that the expression of VEGF isoforms differs among tumor types, and this contributes to tumor vascularization. Thus, the relative expression of the isoforms is an important factor to consider.
The impact of the relative isoform expression levels was demonstrated in our previous work (25) and is made clear in the present study when simulating the effect of anti-VEGF treatment on tumors that preferentially secrete individual VEGF isoforms. In tumors that secrete only VEGF121, it is possible to observe a depletion in tumor free VEGF with the pan anti-VEGF and anti-VEGF121 agents because, prior to treatment, there is a high level of free VEGF, which is predominantly in the form of VEGF121. In contrast, tumors that secrete VEGF165 have a low level of free VEGF at steady state, and even targeting VEGF165 does not result in a depletion of tumor free VEGF (due to the VEGF concentration gradients in the body and VEGF flow (transport) between compartments (25,26)). Thus, we propose that quantitative measurement of the relative expression of the VEGF isoforms in the tumor may aid in determining the appropriate VEGF antibody to use.
Quantitative data for the relative levels of VEGF isoforms is limited to mRNA expression, rather than protein concentration. These data reveal that VEGF121 is expressed at similar levels or at higher levels than VEGF165 depending on the tumor type (37–41). As VEGF gene expression has the potential to identify prospective patients for bevacizumab treatment in epithelial ovarian cancer (42), quantification of VEGF isoform protein levels in the tumor would also be useful in stratifying patients for isoform-specific anti-VEGF treatment. The interstitial fluid represents the microenvironment of a particular tissue sample and may contain factors locally secreted by parenchymal cells. In the case of tumor tissues, this would include VEGF secreted by tumor cells. Thus, there is a need for isolation of tumor interstitial fluid (43) and quantitative measurement of its VEGF protein concentration.
In our current model, we assume that tumors predominantly express the VEGF121 and VEGF165 isoforms. Recent experimental data reveal the importance of the VEGFxxxb isoforms, which are shown to be either weakly angiogenic (3,5,6) or to have anti-angiogenic effects (1). Therefore, it is of interest to expand the model to include the VEGFxxxb isoforms. Additionally, given the potential role of VEGF189 in tumor angiogenesis, it may be important to include this isoform as well (16). The difficulty is that very little if any quantitative information is available on the relative secretion of these isoforms. However, as these data become available, we can expand the model in order to understand the effect that other VEGF isoforms have on the distribution of VEGF and the response to anti-VEGF therapy.
Qualitative Comparison to Experimental Data
It is important to compare our model predictions with available experimental data. However, there are a limited number of studies that explore the effect of isoform-specific VEGF agents. An aptamer that specifically inhibits VEGF165 was found to decrease vascularization and reduce tumor weight in an experimental model of Wilms tumor (44). Similarly, pegaptanib, an aptamer that neutralizes VEGF164/165 was found to decrease tumor blood vessel density in intercerebral glioma (19). In that study, the anti-VEGF165 agent still allowed the formation of tumor satellites; however, the satellites were suppressed with combined pegaptanib and irradiation. Pegaptanib also reduces vascular density and prevents disease progression in a model of T cell-dependent colitis (45). It is interesting to note that neutralizing VEGF with VEGF trap did not produce the same effects (45). Pegaptanib was also shown to increase VEGF concentration above the pre-treatment level in the aqueous humor of the eye following intravitreal injection in AMD patients (46). In the same study, treatment with ranibizumab, which targets all VEGF isoforms, leads to a reduction in VEGF levels in the humor (46). Although the aqueous humor has different transport and geometric properties than the tumor, it is worth noting that, in this setting, the VEGF-targeting agents have differential effects on VEGF concentration. Additionally, in a study targeting VEGF121, Tian and coworkers found that an antibody directed toward VEGF121 blocked VEGF-induced growth in a gastric carcinoma cell line (47). Finally, we have identified one study that examines the effect of VEGF neutralization in tumors that overexpress a particular VEGF isoform. Guo et al. found that VEGF121- and VEGF165-expressing tumors responded similarly to a VEGF antibody that recognizes all isoforms in the presence or absence of estrogen treatment (48).
These data do not conclusively support the model predictions that targeting VEGF121 is more effective than inhibiting VEGF165. However, since our model predicts that the efficacy of the anti-VEGF agents depends on the relative expression of the VEGF isoforms, it is possible that the experimental models utilize tumor xenografts whose VEGF isoform expression ratio does not correspond to the conditions reflected in our model. Thus, it is difficult to directly compare the experimental results to our model predictions, as they were performed in different animal models or cellular contexts under various experimental conditions, and our model may not account for some of these features. Still, our computational studies complement the experimental work and highlight the therapeutic potential of isoform-specific VEGF neutralizing agents.
CONCLUSIONS
VEGF-neutralizing agents inhibit tumor angiogenesis by blocking activation of VEGF receptors and impeding intracellular signaling that promotes proliferation and vessel growth. Our model predicts that isoform-specific anti-VEGF agents have differential effects on tumor free VEGF. Specifically, we find that targeting VEGF121 is an effective treatment strategy, particularly in tumors that secrete both VEGF121 and VEGF165, or tumors that over-express VEGF121. Current VEGF inhibitors tested as anti-cancer therapeutics typically do not target individual isoforms; however, we hope that our results will motivate and provide a basis for clinical investigation of isoform-specific anti-VEGF agents.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(PDF 101 kb)
(XML 318 kb)
(PDF 405 kb)
Acknowledgments
The authors thank Gang Liu and Spyridon Stamatelos for helpful discussions. This work was supported by National Institutes of Health grant R01 CA138264 (ASP) and fellowship F32 CA154213 (SDF), and the UNCF/Merck Postdoctoral Fellowship (SDF).
References
- 1.Rennel ES, Harper SJ, Bates DO. Therapeutic potential of manipulating VEGF splice isoforms in oncology. Futur Oncol. 2009;5(5):703–712. doi: 10.2217/fon.09.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bates DO, Cui T-G, Doughty JM, Winkler M, Sugiono M, Shields JD, et al. VEGF165b, and inhibitory splice variant of vascular endothelial growth factor, is down-regulated in renal cell carcinoma. Cancer Res. 2002;62:4123–4131. [PubMed] [Google Scholar]
- 3.Nowak DG, Woolard J, Amin EM, Konopatskaya O, Saleem MA, Churchill AJ, et al. Expression of pro- and anti-angiogenic isoforms of VEGF is differentially regulated by splicing and growth factors. J Cell Sci. 2008;121:3487–3495. doi: 10.1242/jcs.016410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dokun AO, Annex BH. The VEGF165b "ICE-o-form" puts a chill on the VEGF story. Circul Res. 2011;109:246–247. doi: 10.1161/CIRCRESAHA.111.249953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manetti M, Guiducci S, Romano E, Ceccarelli C, Bellando-Randone S, Conforti ML, et al. Overexpression of VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, leads to insufficient angiogenesis in patients with systemic sclerosis. Circul Res. 2011;109:e14–e26. doi: 10.1161/CIRCRESAHA.111.242057. [DOI] [PubMed] [Google Scholar]
- 6.Catena R, Larzabal L, Larrayoz M, Molina E, Hermida J, Agorreta J, et al. VEGF121b and VEGF165b are weakly angiogenic isoforms of VEGF-A. Molecular Cancer. 2010;9:320. doi: 10.1186/1476-4598-9-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soker S, Miao H-Q, Nomi M, Takashima S, Klagsbrun M. VEGF165 mediates formation of complexes containing VEGFR-2 and neuropilin-1 that enhance VEGF165-receptor binding. J Cell Biochem. 2002;85(2):357–368. doi: 10.1002/jcb.10140. [DOI] [PubMed] [Google Scholar]
- 8.Fuh G, Garcia KC, De Vos AM. The interaction of neuropilin-1 with vascular endothelial growth factor and its receptor flt-1. J Biol Chem. 2000;275:26690–26695. doi: 10.1074/jbc.M003955200. [DOI] [PubMed] [Google Scholar]
- 9.Park JE, Keller G-A, Ferrara N. The vascular endothelial growth factor (VEGF) isoforms: differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol Biol Cell. 1993;4:1317–1326. doi: 10.1091/mbc.4.12.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Houck K, Leung DW, Rowland AM, Winer J, Ferrara N. Dual regulation of vascular endothelial growth factor bioavailability by genetic and proteolytic mechanisms. J Biol Chem. 1992;268(36):26031–26037. [PubMed] [Google Scholar]
- 11.Grunstein J, Masbad JJ, Hickey R, Giordano F, Johnson RS. Isoforms of vascular endothelial growth factor act in a coordinate fashion to recruit and expand tumor vasculature. Mol Cell Biol. 2000;20(19):7292–1. doi: 10.1128/MCB.20.19.7282-7291.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tozer GM, Akerman S, Cross NA, Barber PR, Bjorndahl MA, Greco O, et al. Blood vessel maturation and response to vascular-disrupting therapy in single vascular endothelial growth factor-A isoform-producing tumors. Cancer Res. 2008;68:2301–2311. doi: 10.1158/0008-5472.CAN-07-2011. [DOI] [PubMed] [Google Scholar]
- 13.Yu JL, Rak JW, Klement G, Kerbel RS. Vascular endothelial growth factor isoform expression as a determinant of blood vessel patterning in human melanoma xenografts. Cancer Res. 2002;62:1838–1846. [PubMed] [Google Scholar]
- 14.Yuan A, Yu C-J, Kuo S-H, Chen W-J, Lin F-Y, Luh K-T, et al. Vascular endothelial growth factor 189 mRNA isoform expression specifically correlates with tumor angiogenesis, patient survival, and postoperative relapse in non-small-cell lung cancer. J Clin Oncol. 2001;19(2):432–441. doi: 10.1200/JCO.2001.19.2.432. [DOI] [PubMed] [Google Scholar]
- 15.Cheng S-Y, Nagane M, Huang H-JS, Cavenee WK. Intracerebral tumor-associated hemorrhage caused by overexpression of the vascular endothelial growth factor isoforms VEGF121 and VEGF165 but not VEGF189. Proc Natl Acad Sci U S A. 1997;94(22):12081–12087. doi: 10.1073/pnas.94.22.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan A, Lin C-Y, Chou C-H, Shih C-M, Chen C-Y, Cheng H-W, et al. Functional and structural characteristics of tumor angiogenesis in lung cancers overexpressing different VEGF isoforms assessed by DCE- and SSCE-MRI. PLoS One. 2011;6(1):e16062. doi: 10.1371/journal.pone.0016062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tokunaga T, Oshika Y, Abe Y, Ozeki Y, Sadahiro S, Kijima H, et al. Vascular endothelial growth factor (VEGF) mRNA isoform expression pattern is correlated with liver metastasis and poor prognosis in colon cancer. Br J Cancer. 1998;77(6):998–1002. doi: 10.1038/bjc.1998.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fenton BM, Paoni SF, Liu W, Cheng S-Y, Hu B, Ding I. Overexpression of VEGF121, but not VEGF165 or FGF-1, improves oxygenation in MCF-7 breast tumors. Br J Cancer. 2004;90(2):430–435. doi: 10.1038/sj.bjc.6601539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verhoeff JJC, Stalpers LJA, Claes A, Hovinga KE, Musters GD, Vandertop WP, et al. Tumour control by whole brain irradiation of anti-VEGF-treated mice bearing intracerebral glioma. Eur J Cancer. 2009;45(17):3074–3080. doi: 10.1016/j.ejca.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Ruckman J, Green LS, Beeson J, Waugh S, Gillette WL, Henninger DD, et al. 2′-Fluoropyrimidine RNA-based aptamers to the 165-amino acid form of vascular endothelial growth factor (VEGF165). Inhibition of receptor binding and VEGF-induced vascular permeability through interactions requiring the exon 7-encoded domain. J Biol Chem. 1998;273:20556–20567. doi: 10.1074/jbc.273.32.20556. [DOI] [PubMed] [Google Scholar]
- 21.Ng EWM, Shima DT, Calias P, Cunningham ET, Jr, Guyer DR, Adamis AP. Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat Rev Drug Discov. 2006;5:123–132. doi: 10.1038/nrd1955. [DOI] [PubMed] [Google Scholar]
- 22.Ishida S, Usui T, Yamashiro K, Kaji Y, Amano S, Ogura Y, et al. VEGF164-mediated inflammation is required for pathological, but not physiological, ischemia-induced retinal neovascularization. J Exp Med. 2003;198(3):483–489. doi: 10.1084/jem.20022027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arrell DK, Terzic A. Network systems biology for drug discovery. Clin Pharmacol Ther. 2010;8(1):120–125. doi: 10.1038/clpt.2010.91. [DOI] [PubMed] [Google Scholar]
- 24.Laubenbacher R, Hower V, Jarrah A, Torti SV, Shulaev V, Mendes P, et al. A systems biology view of cancer. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer. 2009;1796(2):129–139. doi: 10.1016/j.bbcan.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finley SD, Engel-Stefanini MO, Imoukhuede PI, Popel AS. Pharmacokinetics and pharmacodynamics of VEGF-neutralizing antibodies. BMC Syst Biol. 2011;5:193. doi: 10.1186/1752-0509-5-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stefanini MO, Wu FTH, Mac Gabhann F, Popel AS. Increase of plasma VEGF after intravenous administration of bevacizumab is predicted by a pharmacokinetic model. Cancer Res. 2010;70(23):9886–9894. doi: 10.1158/0008-5472.CAN-10-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Padera TP, Kadambi A, Di Tomaso E, Carreira CM, Brown EB, Boucher Y, et al. Lymphatic metastasis in the absence of functional intratumor lymphatics. Science. 2002;296(5574):1883–1886. doi: 10.1126/science.1071420. [DOI] [PubMed] [Google Scholar]
- 28.Leu AJ, Berk DA, Lymboussaki A, Alitalo K, Jain RK. Absence of functional lymphatics within a murine sarcoma: a molecular and functional evaluation. Cancer Res. 2000;60:4324–4327. [PubMed] [Google Scholar]
- 29.Imoukhuede PI, Popel AS. Quantification and cell-to-cell variation of vascular endothelial growth factor receptors. Exp Cell Res. 2011;317(7):955–965. doi: 10.1016/j.yexcr.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Genentech, Inc. Avastin prescribing information [cited September2011]; Available from: http://www.avastin.com/avastin/hcp/overview/about/dosing/index.html.
- 31.Gordon MS, Margolin K, Talpaz M, Sledge GW, Jr, Holmgren E, Benjamin R, et al. Phase I safety and pharmacokinetic study of recombinant human anti-vascular endothelial growth factor in patients with advanced cancer. J Clin Oncol. 2001;19(3):843–850. doi: 10.1200/JCO.2001.19.3.843. [DOI] [PubMed] [Google Scholar]
- 32.Hsei V, DeGuzman GG, Nixon A, Gaudreault J. Complexation of VEGF with bevacizumab decreases VEGF clearance in rats. Pharm Res. 2002;19(11):1753–1756. doi: 10.1023/A:1020778001267. [DOI] [PubMed] [Google Scholar]
- 33.Liang W-C, Wu X, Peale FV, Lee CV, Meng YG, Gutierrez J, et al. Cross-species vascular endothelial growth factor (VEGF)-blocking antibodies completely inhibit the growth of human tumor xenografts and measure the contribution of stromal VEGF. J Biol Chem. 2006;281:951–961. doi: 10.1074/jbc.M508199200. [DOI] [PubMed] [Google Scholar]
- 34.Guo P, Xu L, Pan S, Brekken RA, Yang S-T, Whitaker GB, et al. Vascular endothelial growth factor isoforms display distinct activities in promoting tumor angiogenesis at different anatomic sites. Cancer Res. 2001;61:8569–8577. [PubMed] [Google Scholar]
- 35.Catena R, Muniz-Medina V, Moralejo B, Javierre B, Best CJM, Emmert-Buck MR, et al. Increased expression of VEGF121/VEGF165-189 ratio results in a significant enhancement of human prostate tumor angiogenesis. Int J Cancer. 2007;120:2096–2109. doi: 10.1002/ijc.22461. [DOI] [PubMed] [Google Scholar]
- 36.Zhang H-T, Scot PAE, Morbidelli L, Peak S, Moore J, Turley H, et al. The 121 amino acid isoform of vascular endothelial growth factor is more strongly tumorigenic than other splice variants in vivo. Br J Cancer. 2000;83(1):63–68. doi: 10.1054/bjoc.2000.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stimpfl M, Tong D, Fasching B, Schuster E, Obermair A, Leodolter S, et al. Vascular endothelial growth factor splice variants and their prognostic value in breast and ovarian cancer. Clin Cancer Res. 2002;8(7):2253–2259. [PubMed] [Google Scholar]
- 38.Yuan A, Yu CJ, Luh KT, Lin FY, Kuo SH, Yang PC. Quantification of VEGF mRNA expression in non-small cell lung cancer using a real-time quantitative reverse transcription-PCR assay and a comparison with quantitative competitive reverse transcription-PCR. Lab Invest. 2000;2000(80):11. doi: 10.1038/labinvest.3780177. [DOI] [PubMed] [Google Scholar]
- 39.Cheung N, Wong MP, Yuen ST, Leung SY, Chung LP. Tissue-specific expression pattern of vascular endothelial growth factor isoforms in the malignant transformation of lung and colon. Hum Pathol. 1998;29(9):910–914. doi: 10.1016/S0046-8177(98)90195-2. [DOI] [PubMed] [Google Scholar]
- 40.Ljungberg B, Jacobsen J, Haggstrom-Rudolfssson S, Rasmuson T, Lindh G, Grankvist K. Tumor vascular endothelial growth factor (VEGF) mRNA in relation to serum VEGF protein levels and tumour progression in human renal cell carcinoma. Urol Res. 2003;31(5):335–340. doi: 10.1007/s00240-003-0346-x. [DOI] [PubMed] [Google Scholar]
- 41.Zygalaki E, Tsaroucha EG, Kaklamanis L, Lianidou ES. Quantitative real-time reverse transcription-PCR study of the expression of vascular endothelial growth factor (VEGF) splice variants and VEGF receptors (VEGFR-1 and VEGFR-2) in non-small cell lung cancer. Clin Chem. 2007;53(8):1433–1439. doi: 10.1373/clinchem.2007.086819. [DOI] [PubMed] [Google Scholar]
- 42.Hata K, Watanabe Y, Nakai H, Hata T, Hoshiai H. Expression of the vascular endothelial growth factor (VEGF) gene in epithelial ovarian cancer: an approach to anti-VEGF therapy. Anticancer Res. 2011;31(2):731–737. [PubMed] [Google Scholar]
- 43.Wiig H, Tenstad O, Iversen PO, Kalluri R, Bjerkvig R. Interstitial fluid: the overlooked component of the tumor microenvironment? Fibrogenesis Tissue Repair. 2010;3:12. doi: 10.1186/1755-1536-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang J, Moore J, Soffer S, Kim E, Rowe D, Manley CA, et al. Highly specific antiangiogenic therapy is effective in suppressing growth of experimental Wilms tumors. J Pediatr Surg. 2001;36:357–361. doi: 10.1053/jpsu.2001.20716. [DOI] [PubMed] [Google Scholar]
- 45.Chidlow JH, Jr, Glawe JD, Pattillo CB, Pardue S, Zhang S, Kevil CG. VEGF164 isoform specific regulation of T-cell-dependent experimental colitis in mice. Inflamm Bowel Dis. 2011;17:1501–1512. doi: 10.1002/ibd.21525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sawada O, Kawamura H, Kakinoki M, Sawada T, Ohji M. Vascular endothelial growth factor in aqueous humor before and after intravitreal injection of bevacizumab in eyes with diabetic retinopathy. Arch Ophthalmol. 2007;125(10):1363–1366. doi: 10.1001/archopht.125.10.1363. [DOI] [PubMed] [Google Scholar]
- 47.Tian XJ, Wu J, Meng L, Dong ZW, Shou CC. Expression of VEGF121 in gastric carcinoma MGC803 cell line. World J Gastroenterol. 2000;6(2):281–283. doi: 10.3748/wjg.v6.i2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guo P, Fang Q, Tao H-Q, Schafer CA, Fenton BM, Ding I, et al. Overexpression of vascular endothelial growth factor by MCF-7 breast cancer cells promotes estrogen-independent tumor growth in vivo. Cancer Res. 2003;63:4684–4691. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 101 kb)
(XML 318 kb)
(PDF 405 kb)